Abstract
Chronic exposure to stress has many deleterious effects on behavior, which can often lead to self-medication with anxiolytics, antidepressants, or alcohol. We determined the effects of alcohol administration following a stressor on established behavioral, physiological, and neural responses to stress. Male Sprague-Dawley rats received: No alcohol / No stress (CON), Alcohol alone (ALC), Stress alone (STR), or Stress plus Alcohol (STR+ALC). For seven consecutive days, two cohorts received an oral dose of 2 g/kg of either 20% ethanol or saline. In Cohort 1, behavioral testing began after the final treatment (day-8). Memory was tested using the object recognition (OR) and Y-maze, anxiety on the plus maze, and depression on the forced swim task. Memory on OR and Y-maze tasks was impaired in the ALC and STR groups. This deficit was reversed in the STR+ALC group, which performed not differently from the CON group. Stress alone was associated with increased anxiety, which was alleviated with alcohol treatment. No treatment effects were found in the forced swim task. In Cohort 2, hippocampal GABAα4 was upregulated in the STR+ALC group and GluN2B was upregulated in the ALC and STR+ALC groups. The STR+ALC group in Cohort 1 showed enhanced corticosterone levels after forced swim. The STR+ALC group in Cohort 2 showed increased corticosterone levels on day-1 of treatment and a habituation by day-7. In conclusion, this study found a reversal of stress-induced deficits in cognition and anxiety when alcohol was given post-stress, and changes in neurotransmitter receptor expression may contribute to these behavioral effects.
Keywords: Stress, Alcohol, Memory, Anxiety, Corticosterone
Introduction
Alcohol consumption is believed to be a common way of coping with stressful life events. Alcohol abuse is often a response to fear, anxiety, and depression as a means of coping with the stress of these life events (review, Sinha, 2008). Thus, research has focused mainly on the relationship between alcohol consumption and states of anxiety or depression in order to understand coping mechanisms (Kushner et al., 2000; Sullivan et al., 2005). Despite the robust stress effect on cognition (Lupien et al., 2009) and the relationship to alcohol consumption (Barr et al., 2009), few studies have examined the effects of alcohol consumption after a stressor on cognition. After 21-days of stress, male rats show impairments in tasks assessing learning and memory such as object recognition, Y-maze, water maze, and radial arm maze (review Luine et al., 2007). Altering the stress response via enriched housing or drug treatment can mitigate the deleterious effects of chronic stress on memory (Wright and Conrad, 2008; Scullion et al., 2009). Therefore, there may be a link between stress management and memory function that may lead to excess alcohol intake during times of stress.
Changes in anxiety or depression are often associated with increased or prolonged stress and alcohol consumption. The link between stress and drug use has shown that initiation or continuance of psychoactive substance use is often attributed to an attempted reduction of negative affect (Koob and LeMoal, 2008). However, with prolonged use of alcohol, levels of anxiety and depression rise. Thus, in alcoholics it is difficult to determine if dependence is due to self-medication of a pre-existing mood disorder or if excessive drinking is maintained by negative reinforcement of alcohol-induced changes in mood (Allan, 1995). Studies using animal models have found a range of effects of restraint stress on alcohol consumption, demonstrating decreased (Sprague and Maickel, 1994), increased (Lynch et al., 1999), or no change (Roman et al., 2004) in voluntary alcohol intake following stress treatment. We recently reported that 1-hour of restraint stress followed by 1-hour access to alcohol increased consumption as compared to that of non-stressed rats. In addition, deficits in spatial memory associated with either alcohol or stress treatment alone were alleviated in rats with access to alcohol after the stressor (Gomez et al., 2012).
Alcohol can block stress-induced impairments in memory by altering the stress response system (Thatcher-Britton and Koob, 1986; Liu and Weiss, 2003; Scullion et al., 2009). Possible neurochemical mechanisms that may play a role in these alcohol effects on stress are the GABAergic and glutamatergic systems where alcohol is an indirect agonist of the former and antagonist of the latter (Crabbe et al., 2011; Orchinik et al., 1995; Martin and Wellman, 2011). Alterations of these two neurochemical systems may also underlie the interactive effects of alcohol and stress on memory. Both GABA and glutamate systems are important in learning and memory processes. Chronic abuse causes changes in receptor expression in order to compensate for the consistent activation or suppression of these neurotransmitters (Grant et al., 1990; Trevisan et al., 1994). Current research indicates that the GABAα4 subunit plays a significant role in alcohol anxiolytic effects (Gulinello et al. 2001). Moreover, the glutamatergic system plays a significant role in alcohol effects on memory and withdrawal. The GluN2B receptor function is altered by alcohol and may mediate some aspects of alcohol withdrawal symptomology (Follesa and Ticku, 1995; Hu et al., 1996).
Stress alters metabolism (Mezey et al., 1979; Ryabinin et al., 1995) and can affect the blood alcohol curve (Breslin et al., 1994). Moreover, intoxicating doses of alcohol increase stress hormone levels in human and animal models (Sinha, 2001; Patterson-Buckendahl et al., 2005). The rise of corticosterone by alcohol intake is sometimes enhanced when combined with a stressor (Trudeau et al., 1990; 1991). However, the enhancement or reduction is dependent on the type of stressor experienced and alcohol dose. Brick and Pohorecky (1982) found that ethanol treatment (0.5 g/kg) before foot-shock stress reduces corticosterone release but does not alter the response to restraint stress. Much like psychological stressors, chronic alcohol exposure leads to tolerance, both in stress hormone response and in behavioral effects (Spencer and McEwen, 1990; Boulouard et al., 2002).
The focus of the present study was to determine the behavioral, physiological, and neurochemical effects of alcohol exposure after a stressor. In these experiments we employed gastric gavage instead of voluntary consumption as in Gomez et al. (2012). This permitted control over the dosages on alcohol and the ability to more directly evaluate the effects of alcohol after the stress procedures. A dose of 2 g/kg produces blood alcohol levels which would be equivalent to human consuming 3-5 standard drinks dependent on weight and sex. Rats received 7-days of treatments followed by 3-days of behavioral testing or treatments alone and were sacrificed for measurement of hippocampal neurotransmitter receptor expression. Tissue was collected before and after behavioral testing because recent studies have shown changes in hippocampal morphology in rats exposed to such testing versus naive rats (Conrad et al., 2012, Eilam-Stock et al., 2012).
Methods and Materials
Subjects
Male Sprague-Dawley rats (Weight ≈ 220 g, Age ≈ 3 months, N = 64) obtained from Harlan Sprague-Dawely, Inc. (USA) were pair-housed and kept on a 12hr light cycle with lights on at 09:00. Standard rat chow and water was available ad libitum. Rats were randomly assigned to one of four conditions (n=8 per group); No Stress / No Alcohol Control (CON), Alcohol alone (ALC), Stress alone (STR), or combination of Stress and Alcohol (STR+ALC). Two cohorts were utilized, group assignments for Cohort 1 and Cohort 2 were identical. All procedures were approved by the Hunter College Institutional Animal Care and Use Committee.
Procedure
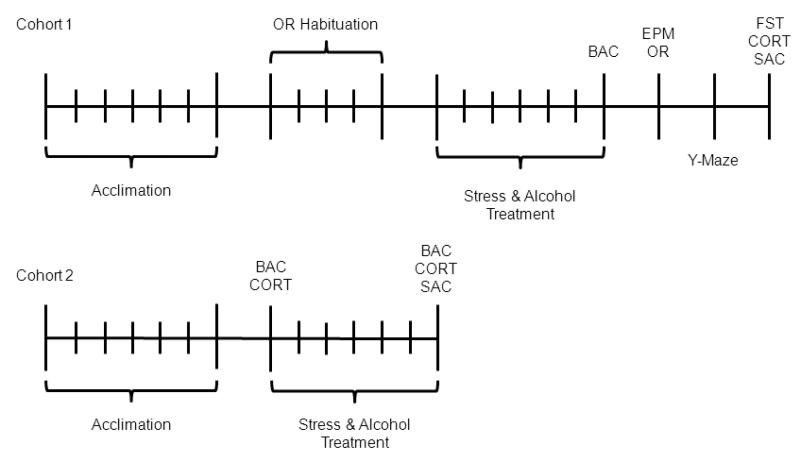
Cohort 1: After acclimation to the environment, habituation for object recognition (OR) was conducted for 5-days. On day-1, rats were exposed to the open field for 5-minutes (no objects). From day-2 through day-5 the OR task was run with various objects with increasing inter-trial delays each day (1m, 40m, 1h, and 4h). The object during testing were different than any used during habituation. At two points during OR habituation, all rats were administered 1.0 cc of saline via gastric gavage to reduce stress associated with the procedure. Alcohol and stress treatment started after the last OR habituation trial. For seven consecutive days, rats in the stress groups were restrained for 6-hours each day from 10:00-16:00. Each day, post-stress, alcohol (ALC and STR+ALC) or saline (CON and STR) was administered via gastric gavage at a dose of 2 g/kg. Following treatments, rats were put through a battery of behavioral tests with lights on for all tests. Rats were tested on the elevated plus maze (EMP) for anxiety and OR task for visual memory on day-8, the Y-maze for spatial memory on day-9, and the forced swim task (FST) for depression on day-10. Following the completion of the FST (30-min. post-swim) brain and blood samples were collected for analysis (Figure 1). The order of behavioral testing was deemed to provide optimal information regarding the interactive effects of stress and alcohol. Thus, the EPM was conducted on day-8 not only to test for anxiety, but also test for any possible withdrawal effects of alcohol. It was expected that conducting the OR task immediately after the EPM would have no effect on performance. To confirm, correlation analyses were run and no significant correlation was found between activity on the EPM and performance on the OP task (data not shown). The Y-maze was done alone on day-9, as it was the task that took the longest (~6-hours). To test for depression and limit any possible confounding effects of stress-induced changes in memory, the FST was run last. Additionally, the FST provided a novel stressor to assess hormone release.
Figure 1.
Time-line for Cohort 1 and Cohort 2. Each tick mark represents one day.
Cohort 2: Rats were allowed 1-week to acclimate before stress and/or alcohol treatment began. Blood samples were taken for BAC and CORT assays 30-minutes after gavage following restraint stress on day-1. Brain tissue and blood was collected on day-7 to assess effects of treatment on alcohol content, hormone adaptation, and neurotransmitter expression. Stress and alcohol exposure was identical to Cohort 1, but no behavioral testing was conducted (Figure 1). Behavioral testing has been shown to effect neuronal structures (Conrad et al., 2012; Eilam-Stock et al., 2012), thus Cohort 2 was designed to control for this possible change.
Stress and Alcohol Administration
Rats were restrained, not immobilized, for 6hr/day/7days (10:00-16:00) in a Plexiglas restrainer measuring 21.5cm long × 6.3cm internal diameter (Harvard Apparatus). Pure ethanol (200 proof, Sigma-Aldrich) was diluted in saline (0.9% NaCl, Fisher Scientific) to produce a concentration of 20% v/v ethanol. Gavage was performed using a feeding needle (18G × 2”) affixed to a 10 cc syringe (VWR). All rats were gavaged with a dose of 2 g/kg of alcohol or saline in a counterbalanced fashion at the same time every day (~16:15). Blood alcohol concentration (BAC) were determined 30-minutes after the first and final alcohol administration; blood samples were taken via tail-nick and BAC was analyzed using 5μl of sera in an Analox GM7 micro-stat machine.
Memory Tasks
The OR task is a pre-frontal cortex/hippocampal dependent working memory task comprised of a sample trial (T1) and a retention trial (T2). During T1, two identical objects were placed in corners of an open field (Plexiglas: 70 × 70 × 30 cm) and rats were allowed to explore for 3-minutes. Exploring was determined as touching, whisking, or sniffing the objects from no more than 2cm away. Following a 4-hour intertrial delay, one of the objects was replaced with a new object and rats were reintroduced and allowed to explore for 3-minutes. Increased exploration/preference for the new object is indicative of intact recognition memory (Ennaceur et al., 1997). Placement and new/old objects were presented in a counterbalanced fashion to reduce possible confounding effects of place or object preference.
The Y-maze is a hippocampal dependent spatial memory task that requires rats to use external maze cues to navigate the identical internal arms. The maze was placed in a room with high-contrast external cues (bullseyes, checkerboards). The arms were 56 × 19 × 36 cm and connected to create an equilateral triangle at the center with 60° angles (outer angels were 120°). The floor was covered with cage bedding that was thoroughly mixed before every trial to eliminate any odor cues (Conrad et al., 2003). During the training trial, one arm was blocked and rats were allowed to freely explore the start and other arm for 15-minutes. During the 4-hour intertrial delay, the Y-maze was rotated 120° clockwise, to reduce any possible marking effects (Conrad et al., 1996), and the previously blocked arm was open. Rotating the maze controls for marking confounds by presuming the rat is exploring the arm due to its novel location not any previously marked internal cues. Memory was assessed during a 5-minute probe trial as entries into the novel (new region) versus the other arm. An entry was designated as the rat crossing the threshold of the arm with both forepaws and half its torso. The novel, other, and start arms were counterbalanced to reduce any confounding place preference. All behavior was videotaped and analyzed off-line by an experimenter blind to the conditions.
Anxiety and Depression
The EPM was designed to test general anxiety levels in animal models (Pellow et al., 1985). The EPM consists of two open (50 × 12 cm) and two closed arms (50 × 12 × 40 cm). An entry was designated as the rat crossing the threshold of an arm with both forepaws and half its torso. Rats were allowed 5-minutes to explore the arms and behavior was videotaped and scored off-line.
The modified FST was designed to measure general depression in rat models. Originally developed by Prosolt et al. (1977), the FST measures swimming and immobile behaviors with increases in immobility associated with depression. Rats were placed in a Plexiglas cylinder (30 cm diameter) filled with 30 cm of water (~25°C). Rats were tested for 5-minutes and behavior was videotaped and scored off-line. Behaviors consist of swimming (horizontal movement), climbing (upward thrashing), and immobility (erect floating). Behaviors were counted at 5-second intervals over the 5-minutes and scores were converted into percentages and analyzed within groups.
Western Blots
After the final gavage (day-7) or FST (day-10), rats were rapidly decapitated. Brains were removed, split down the mid-sagittal line, and one hemisphere was fast frozen on dry ice. Hippocampal tissue was later dissected, homogenized, and separated into membrane and post-synaptic density fractions (as detailed in Sacktor et al., 1993). Total protein for each sample was determined by BCA assay (Pierce Rockford, IL) and protein concentrations were standardized to load 10μg total protein for each sample prior to SDS-PAGE. Gels were run with an Invitrogen XCell Mini Electrophoresis system using 4-20% Tris-Glycine gels (Life Technologies, Grand Island, NY). Samples were then transferred to nitrocellulose membranes, incubated in hemoglobin-based blocker for 30-minutes and then incubated overnight at 4°C in primary anti-bodies to detect GABAα4 (Anti-GABA A Receptor alpha 4 antibody, 66 kD – no. ab4120, Abcam Inc., Cambridge, MA), GluN2B (Anti-NMDAR2B antibody, 148 kD – no. ab81271, Abcam Inc., Cambridge, MA), and GAPDH (Anti-Glyceraldehyde-3-Phosphate Dehydrogenase Antibody, 38 kD – no. MAB374, Millipore Co., Temecula, CA). GABAα4 and GluN2B primaries were diluted to 1:500 and GAPDH was diluted to 1:2000 using a hemoglobin-based blocker. Membranes were incubated in alkaline phosphatase-conjugated secondary anti-bodies for 2-hours at room temperature (Rabbit for GABA and NMDA – no. A3687 and Mouse for GAPDH – no. A9316; Sigma-Aldrich Co., St. Louis, MO). After secondary incubation, membranes where developed using BCIP/NBT phosphatase substrate for colorimetric detection (KPL Inc., Gaithersburg, MD). Developed blots were scanned and analyzed using Image-J software provided by the NIH.
Corticosterone
For Cohort 1, blood samples were collected from truck blood 30-minutes after the FST on day-10. For Cohort 2, samples were collected from the tail vein 30-minutes after gavage on day-1 and day-7. Samples were centrifuged at 3000g in 4°C for 5-minutes and sera was collected. Using a corticosterone ELISA kit (Neogen Corp., Lexington, KY), 100μL of plasma was dissolved in ethyl ether and allowed to evaporate for 48-hours. Extraction with ethyl ether allows for measurement of bound corticosterone. The ELIZA kit used polyclonal rabbit antibodies, had a sensitivity range from 0.05-5.0 ng/ml, and had an inter-assay and intra-assay validation of ≤10%. Samples went through a series of washes and incubations as directed by the kit instructions. Samples and standards (50μL) were assayed in a kit-provided 96-well plate and read in a microplate reader at 650nm. Output was converted into corticosterone levels at ng/ml via equations provided by Neogen Corp.
Statistics
An independent samples t-test was used to test differences of BAC in Cohort 1. Repeated measures ANOVAs were used to analyze BAC and corticosterone in Cohort 2, body weights, object recognition, Y-maze, and the forced swim task. Univariate 2×2 ANOVAs were used to analyze corticosterone in Cohort 1, the elevated plus maze, and neurotransmitter expression. All statistical analyses were run on PASWStatistics v.18 (SPSS IBM, USA).
Results
Physiology Effects
In Cohort 1, BAC was measured from blood sampled 30-minutes after the last alcohol administration on day-7. No difference was found between the ALC and STR+ALC groups [t(13)=0.82, p=0.43] (Figure 2A). In Cohort 2, BAC was measured on day-1 and day-7 and was not altered by treatment at either time. A 2-way repeated-measures ANOVA found no significant main effects of group [F(1,26)=1.10, p=0.30], day [F(1,26)=0.26, p=0.61], or group by day interaction [F(1,26)=0.18, p=0.68], suggesting no stress-induced change in metabolism (Figure 2B).
Figure 2.
Physiological Effects: (A,B) Comparison of blood alcohol concentration, presented as means±SEM. (C) Corticosterone levels presented as means±SEM. Asterisk |*| p<0.05. (D) |a| indicates the STR+ALC group had the highest level of CORT on day-1 compared to all other groups (p<0.05). |b| indicates the CON group had a greater release of CORT on day-7 compared to the STR and STR+ALC groups. Asterisk |*| shows only the STR+ALC group had a significant within group difference in CORT levels from day-1 to day-7. (E) Body weights over days of stress treatment displayed in means±SEM, asterisk |*| p<0.05 over day range. (F) Asterisk |*| significant difference between the CON/ALC groups and the STR/STR+ALC groups over day range. Double Asterisk |**| significant difference between CON andALC and STR/STR+ALC groups over day range (p<0.05).
In Cohort 1, corticosterone (CORT) levels were analyzed 30-minutes post-forced swim. All rats mounted a CORT response, but a 2×2 ANOVA found effects of alcohol [F(1,28)=4.69, p=0.04] and stress [F(1,28)=10.28, p=0.003], whereby the STR+ALC group mounted the greatest CORT release [LSD, p<0.02] (Figure 2C). In Cohort 2, the stress response to restraint and alcohol exposure was measured 30-minutes after gavage on day-1 and day-7. Repeated-measures ANOVA (2×2×2, day × drug × stress) showed significant effects of day [F(1,27)=12.86, p=0.001], day by alcohol [F(1,27)=25.72, p=0.0001], day by stress [F(1,27)=12.33, p=0.002], and day by alcohol by stress [F(1,27)=7.06, p=0.013] interactions (Figure 2D). On day-1, the STR+ALC group had a higher CORT level compared to the other groups and the ALC group was greater than the CON and STR groups [F(3,27)=15.79, p=0.0001]. On day-7, the STR and STR+ALC group had lower CORT levels than the CON group (p<0.02). Within groups, only the STR+ALC group showed a difference in CORT levels between day-1 and day-7 (p<0.002), all other groups had similar CORT levels between days (Figure 2D).
Rats in Cohort 1 that were exposed to stress showed a reduction in weight gain over the 7-day stress period. Repeated measures ANOVA (7×2×2, day × drug × stress) showed effects of day [F(6,168)=37.91, p=0.0001], day by stress [F(6,168)=23.90, p=0.0001], and day by alcohol by stress [F(6,168)=6.17, p=0.0001] interactions. Post-hoc analysis revealed that by day-4, rats in the CON and ALC groups weighed more than rats in the STR and STR+ALC groups [F(3,28)=4.28, p=0.01]. The difference in weight continued until the final day of stress (Figure 2E). In Cohort 2, repeated-measures ANOVA (7×2×2, day × drug × stress) showed effects of day [F(6,168)=32.51, p=0.0001], day by alcohol [F(6,168)=3.64, p=0.02], and day by stress [F(6,168)=8.40, p=0.0001] interactions. There was no difference between groups on day-1, but from day-2 to day-4 the CON and ALC groups outweighed the STR and STR+ALC groups [F(3,28)=15.97, p=0.0001]. From day-5 to day-7 the CON group weighed more than all other groups, but the ALC group was still greater than the STR and STR+ALC groups [F(3,28)=25.80, p=0.0001 (Figure 2F).
Working Memory Using Object Recognition
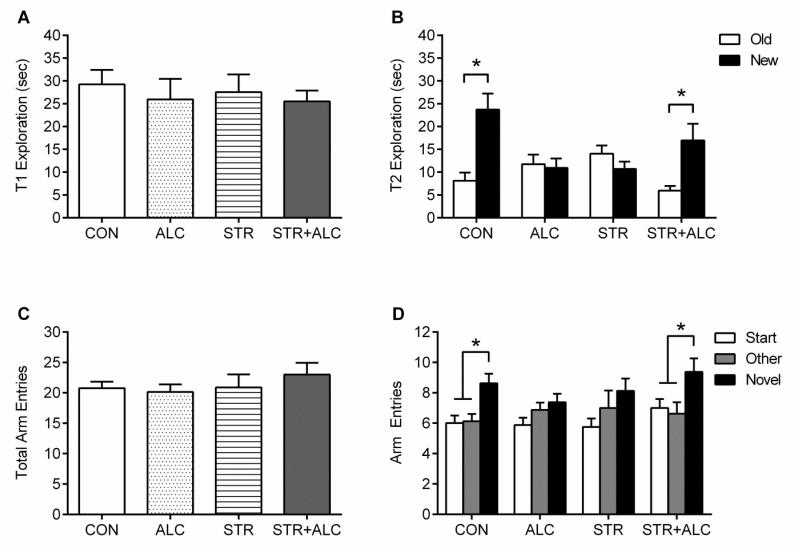
During the initial sample trial (T1) of the object recognition memory test, there was no difference in exploration times between groups [F(3,28)=0.23, p=0.88] (Figure 3A). For the retention trial (T2), exploration time with each object was analyzed with a repeated measures ANOVA (2×2×2, object × drug × stress). There was an effect of object [F(1,28)=14.32, p=0.001] and an object by drug by stress [F(1,28)=26.82, p=0.0001] (Figure 3B). Post hoc paired t-test showed that the CON and STR+ALC groups spent more time exploring the new object compared to the old [t(7)=3.97, p=0.005 and t(7)=3.47, p=0.01, respectively]. The ALC and STR groups spent equal time exploring each object [t(7)=0.30, p=0.76 and t(7)=1.90, p=0.09, respectively]. Thus, in comparison to controls, exposure to alcohol after a stressor blocks recognition memory impairments attributed to alcohol or stress alone.
Figure 3.
Memory Effects: Object Recognition - (A) Exploration time (means±SEM) of identical objects during the sample trial (T1). (B) Exploration time (means±SEM) of new and old objects during retention trail (T2). Asterisk |*| p<0.05, CON/STR+ALC spent more time exploring new object, while ALC/STR showed no difference. Y-Maze - (C) Total entries into the Start, Other, and Novel arms (means±SEM). (D) Entries into each arm during the probe trial. CON and STR+ALC groups explored the novel arm at a greater rate, while ALC/STR groups showed no difference. Asterisk |*| p<0.01.
Spatial Memory Using the Y-maze
The Y-maze showed the same pattern of results as the OR task. Total entries were analyzed and no significant differences were found [F(3,28)=0.55, p=0.65] (Figure 3C). During the probe trial, entries into each individual arm were analyzed with a multifactorial ANOVA (3×2×2, arm × drug × stress). The analysis found an effect of arm [F(2,56)=23.02, p=0.0001] and an arm by drug by stress [F(2,56)=3.65, p=0.03] interaction. The difference in exploration of each arm was determined within groups, showing the CON and STR+ALC explored the novel arm more than the others [F(2,21)=7.55, p=0.003 and F(2,21)=3.90, p=0.03, respectively] (Figure 3D). There was no difference between arm exploration within the ALC or STR groups [F(2,21)=2.24, p=0.13 and F(2,21)=1.84, p=0.18, respectively]. Therefore showing an interactive effect between stress and alcohol that spares spatial memory impairments caused by each treatment alone.
Anxiety and Depression Measures
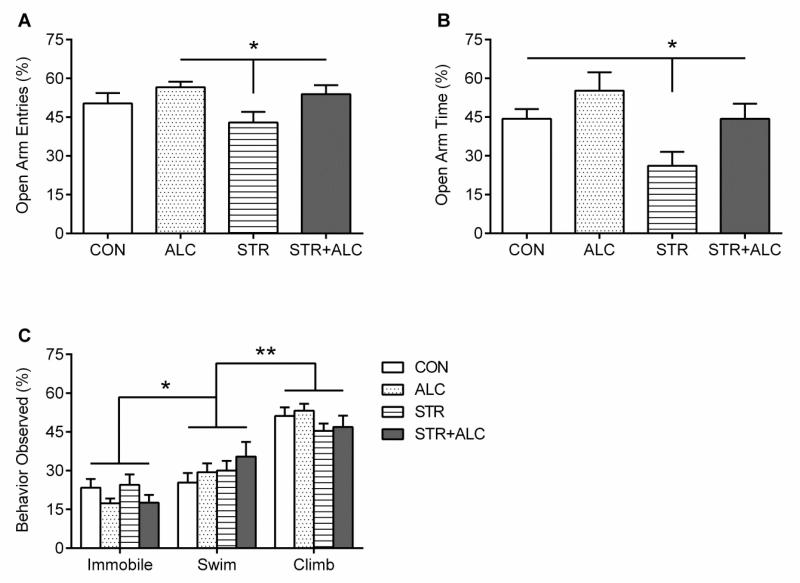
On the elevated plus maze, the number of entries and amount of time spent in the open arms were converted into open arm percentages and analyzed. A 2×2 ANOVA found a between groups interaction effect of alcohol by stress on percentage of open arm entries [F(1,27)=5.75, p=0.02] (Figure 4A). Additionally, there was an effect of stress [F(1,27)=6.82, p=0.015] and an alcohol by stress interaction [F(1,27)=6.85, p=0.014] on percentage of time spent in the open arms. Post-hoc LSDs found the STR group made less entries into the open arms compared to the ALC and STR+ALC groups and spent less time in the open arms compared to all other groups (p<0.03), an indication of increased anxiety (Figure 4B).
Figure 4.
Mood Effects: (A) Elevated Plus Maze – Percent of entries into the open arms (means±SEM). The STR group made fewer entries into the open arms compared to the ALC and STR+ALC groups, asterisk |*| p<0.05. (B) Percent of time spent in the open arms (means±SEM). The STR group spent significantly less time in the open arms, asterisk |*| p<0.05. (C) Forced Swim Task – Swimming, immobile, and climbing behavior as mean±SEM. No difference between groups for each behavior was found. Climbing behavior was greater than both Swim and Immobile, asterisk |*| p<0.0001. Swim behavior was greater than Immobile behavior, double asterisk |**| p<0.0001.
The forced swim task assessed swimming, climbing, and immobile behaviors at 5-second intervals for 5-minutes. The behavioral counts collected were analyzed with a repeated measures ANOVA (3×2×2, behavior × drug × stress). A significant effect of behavior was found showing climbing behavior was greater than immobile and swim behaviors and swimming behavior was greater than immobility [F(2,93)=64.23, p=0.0001, LSD p<0.0001] (Figure 4C). However, no difference in immobility, swimming, or climbing behavior was found between groups [F(3,28)=1.41, p=0.25, F(3,28)=0.95, p=0.42, and F(3,28)=1.19, p=0.33, respectively]. Treatment with alcohol, stress, or both had no effect on depressive-like behaviors as compared to controls.
Neuronal Effects
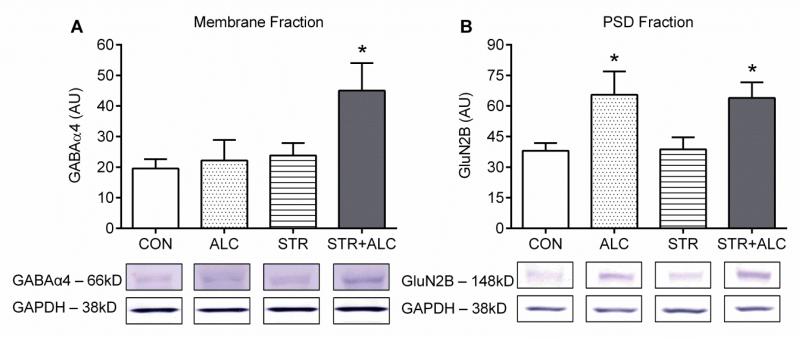
In Cohort 1, samples collected post-FST (day-10) showed no significant between group differences in expression of GABAα4 (M = 24.98 ± 2.52 |AU|) or GluN2B (M = 111.40 ± 12.87 |AU|) [F3,27=0.50, p=0.68; F3,28=0.49, p=0.69, respectively] (data not shown). In Cohort 2, on the final day of treatment (day-7), a significant stress effect on receptor expression of GABAα4 was found between groups [F(1,22)=4.74, p=0.04] and post-hoc LSD showed the STR+ALC group had an upregulation of GABAα4 compared to the other groups (p<0.02) (Figure 5A). For GluN2B, a significant alcohol effect was found between groups [F(1,24)=10.85, p=0.003]. The two groups treated with alcohol (ALC and STR+ALC) had upregulation of the glutamate receptor protein compared to the CON and STR groups (p<0.03) (Figure 5B).
Figure 5.
Neurotransmitter receptor protein expression in hippocampus (Arbitrary Units, mean±SEM). All bands were not analyzable, thus, group sizes differ (GABAα4: CON, STR, STR+ALC – n=7, ALC – n=5; GluN2B: CON – n=6, ALC and STR – n=7, STR+ALC – n=8). (A) GABAα4 analysis shows a significant difference STR+ALC > CON/ALC/STR groups, asterisk |*| p<0.05. (B) GluN2B analysis shows a significant difference, ALC/STR+ALC > CON/STR, asterisk |*| p<0.05.
Discussion
The results show that alcohol administration after stress exposure altered behavioral responses compared to each treatment alone. Memory function was impaired by both stress and alcohol alone; however, performance of rats receiving both treatments was at control levels. This finding supports our previous work showing that alcohol consumption after a stressor alleviates spatial memory impairments (Gomez et al., 2012). Alcohol also reversed the stress-induced increase in anxiety shown on the plus maze. In the forced swim task, depressive-like behavior was unaltered by the treatments, either alone or in combination. Thus, as is predicted by the self-medication hypothesis, alcohol consumption may increase during times of anxiety as a means of reducing effects of stress during this state. Physiologically, corticosterone levels and body weights were altered by treatment. Western blot analysis revealed, on day-7, a significant upregulation of GABAα4 and GluN2B in rats exposed to the combination treatment.
Previous work by our lab has shown that chronic stress impairs non-spatial working memory on the object recognition task in male rats (Bowman et al., 2003). Other forms of stress (foot shock/novel cage exposure) and stress duration (7-days vs. 21-days) also produce memory impairments on object recognition tasks (Baker and Kim, 2002; Bowman et al., 2009; Scullion et al., 2009). Chronic ethanol administration shows similar impairments on non-spatial memory as chronic stress (Ryabinin et al., 2002). The Y-maze is a low-stress task measuring memory without stressors such as, food deprivation or forced swim (Conrad el al., 1996). Chronic stress impairs spatial memory on the Y-maze by reducing exploration of the novel arm (Conrad et al., 1996). Chronic alcohol (injection, voluntary, chronic intermittent exposure, liquid diet) studies have also shown a significant impairment of spatial memory (review, Matthews and Morrow, 2000). To our knowledge, few have evaluated cognitive effects when stress and alcohol are combined. Indirect evidence supports our data and may elucidate our findings. Ryabinin et al. (1995) found a significant decrease of hippocampal c-fos expression when rats were given alcohol after a stressor, suggesting an alcohol-induced amelioration in hippocampal activity. Moreover, knockout models of rats with defective CRH receptor systems show increases in alcohol sensitivity and reinstatement of alcohol seeking behavior caused by stress (Hansson et al., 2006). Our data show that alcohol administration after a stressor alleviated impairments caused by alcohol or stress alone. Others have found similar effects with other drugs of abuse. Nicotine and opioid antagonists (naloxone) administered during periods of stress, block impairments on memory tasks and have been shown to reestablish normal LTP function (Alkadhi et al., 2011; Schneider et al., 2009). We believe the results on memory function may be due to an altered stress response attributed to alcohol consumption, which was also seen in our physiological and neurochemical measures.
Alcohol intake is generally associated with decreases in anxiety (review Lewis, 1996). We found fewer entries and less time spent in the open arms of the EPM only with the STR group, suggesting a stress-induced increase in anxiety. Alcohol treatment after a stressor increased the entries made and time spent in the open arms of the maze. The anxiolytic properties of alcohol are usually found when low to moderate doses are given (Lister, 1987). Long-term alcohol intake of moderate to high doses, however, shows increases in anxiety during alcohol withdrawal (Rasmussen et al., 2001). Stress hormone levels may reflect the increase in anxiety produced by chronic alcohol consumption, whereby, after chronic alcohol intake, increases in anxiety can be blocked by treatment with CRH antagonists (Valdez et al., 2003). Congruently, the NMDA antagonist AP7 administered during a stressor, reduced time spent in the closed arms of the EMP (Padovan et al., 2000). Alcohol is an NMDA antagonist, which may help explain the results obtained in the present study. Thus, our results show that stress-induced increases in anxiety can be mitigated by alcohol exposure following a stressor.
The FST is a rodent model of depressive-like behaviors and measures the escape directed behaviors such as swimming and climbing in comparison to helplessness behaviors such as immobility (Porsolt et al, 1977). The behaviors observed in the FST were analyzed similarly to that seen in Cryan and Lucki (2000). Swimming, climbing, and immobility behaviors were compared between groups to determine if treatment altered depressive-like symptoms. Between groups, there was no difference for each behavior observed, however, all rats did show greater climbing behavior compared to swimming and immobility and more swimming than immobility. Others have found different compounds affect the frequency of behaviors observed and show increased climbing is associated with changes in to norepinephrine, while swimming is moderated by serotonin (Page et al., 1999). Given that we did not find any changes between groups, it is difficult to determine the cause of increased climbing and swimming behavior. Further research is needed to determine if the delay between the final alcohol/stress treatment and testing for depressive-like behaviors may have influenced the current results.
In Cohort 1, corticosterone was measured only after the forced swim task. Based on other work showing that non-stressed male rats have a mean daily CORT level of 60 ng/ml (Girotti et al., 2007), the results showed a sensitized response in the STR+ALC group, suggesting an interactive effect in the combination group. This result supports others who have also shown sensitized stress responses to a novel stressor (Nisenbaum et al., 1991; Weinberg et al., 2009). In Cohort 2, rats treated with alcohol showed an increase in plasma CORT 30-minutes after intake. CORT levels were further elevated if rats were exposed to 6-hours of restraint stress prior to alcohol intake. On day-7, there were no differences in CORT levels between groups and only the STR+ALC group showed an HPA-adaptation between the first and final day of treatment. Studies have shown HPA adaptation in response to chronic stress or alcohol, which supports the finding for the STR+ALC group, however these studies used 21-days of treatment, which may explain the lack of effect in the current ALC and STR groups (Spencer and McEwen, 1990; Dhabhar et al., 1997). Galea et al. (1997) has shown that CORT levels peak around 30-minutes of restraint, but return to normal after 6-hours of restraint. This finding supports our current results, which show that STR rats have low levels of CORT after 6-hours of restraint. Thus, alcohol consumption after a stressor may lead to a switch from drinking for its positive reinforcing effects to drinking for negative reinforcing effects (Koob and LeMoal, 2008) and may be a process linked to stress sensitization.
Rats exposed to a stressor after alcohol injection display lower peak blood alcohol concentrations, suggesting that stress increases alcohol metabolism (Mezey et al., 1979; Ryabinin et al., 1995). We did not find any change in blood alcohol between the stress and non-stress groups. However, unlike studies that have found a difference, we administered alcohol after the stressor. Perhaps the increase in alcohol metabolism was present only when animals were exposed to a stressor after consumption, reported in humans as a “sobering” effect (Breslin et al., 1994). The stress-induced increase in alcohol metabolism may explain the stress-induced increase in alcohol consumption, as a lower BAC may drive an individual to drink more to attain a regular BAC obtained when not stressed (Sommer et al. 2008).
Neurotransmitter receptor levels may have normalized in Cohort 1 following 3-days of behavioral testing without stress or alcohol treatment, thus, in Cohort 2, tissue was collected on the final day of treatment to determine GABAα4 and GluN2B levels before the rats experienced behavioral testing or possible recovery. We found that GABAα4 was elevated in the STR+ALC group. The alpha-4 subunit was chosen due to its association with the anxiolytic effects produced by alcohol or benzodiazepines (Gulinello et al. 2001). The upregulation of alpha-4 may have contributed to the difference in anxiety found between the STR and STR+ALC groups. Upregulation of GABAα4 may indicate that rats in the STR+ALC group had greater anxiolytic effects than those in the ALC alone group. Further testing is necessary to explore this possibility. GluN2B was elevated in the groups exposed to alcohol, perhaps indicating a non-specific action of alcohol on receptor expression. The GluN2B receptor is associated with tolerance, and perhaps in compensation for constant NMDA antagonism by alcohol, the GluN2B receptor is upregulated (Follesa and Ticku, 1995; Hu et al., 1996). The increase in receptor expression appears transient and others have found an upregulation of GluN2B immediately after 5- or 6-days of alcohol treatment and a return to control levels 48-hours post-treatment (Kalluri et al., 1998; Narita et al., 2000). Thus, the differences in receptor expression found in Cohort 2 may account for the behavioral effects seen in Cohort 1.
In conclusion, although alcohol exposure generally adversely affects memory, anxiety, and depression, we found that it also mitigates some behavioral effects of stress. These data may have implications for alcohol use and abuse during times of stress, and may provide an intermediate time point and behavioral component of focus that may be of value in understanding the interactive effect of alcohol consumption after a stressor. Additionally, the use of restraint stress provides a different form of stress (psychological) which is not often used in alcohol research. Restraint stress along with other psychological stressors (social defeat/isolation), may provide a more direct comparison to stressors experienced by humans than physical stressors such as foot/tail shock and cold water swims. Further research is necessary to determine the relationship between current and other possible neural changes that may underlie the effects of alcohol or stress and their combination on behavior.
Highlights.
Memory was impaired by stress or alcohol, but alleviated when combined.
Stress-induced anxiety was blocked by alcohol.
Corticosterone response to stress was altered by alcohol.
Neurotransmitter receptor expression was upregulated by stress and alcohol.
Acknowledgements
This study was funded in part by the NIH MBRS-RISE grant GM060665; RCMI grant number RR03037 from the National Center for Research Resources (NCRR); NIH grant number AA019413; and the Department of Defense grant W81XWH-08-1-0243.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkadhi KA, Alzoubi KH, Srivareerat M, Tran TT. Chronic psychosocial stress exacerbates impairment of synaptic plasticity in beta-amyloid rat model of Alzheimer’s disease: prevention by nicotine. Curr Alzheimer Res. 2011;8:718–731. doi: 10.2174/156720511797633188. [DOI] [PubMed] [Google Scholar]
- Allan CA. Alcohol problems and anxiety disorders--a critical review. Alcohol Alcohol. 1995;30:145–151. [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learn Memory. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Gupte M, Sommer W, Sun H, Schwandt ML, Lindell SG, Kasckow JW, Suomi SJ, Goldman D, et al. Functional CRH variation increases stress-induced alcohol consumption in primates. Proc Natl Acad Sci U S A. 2009;106:14593–14598. doi: 10.1073/pnas.0902863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulouard M, Lelong V, Daoust M, Naassila M. Chronic ethanol consumption induces tolerance to the spatial memory impairing effects of acute ethanol administration in rats. Behav Brain Res. 2002;136:239–246. doi: 10.1016/s0166-4328(02)00134-1. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Micik R, Gautreaux C, Fernandez L, Luine VN. Sex-dependent changes in anxiety, memory, and monoamines following one week of stress. Physiol Behav. 2009;97:21–29. doi: 10.1016/j.physbeh.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Breslin FC, Hayward M, Baum A. Effect of stress on perceived intoxication and the blood alcohol curve in men and women. Health Psychol. 1994;13:479–487. doi: 10.1037//0278-6133.13.6.479. [DOI] [PubMed] [Google Scholar]
- Brick J, Pohorecky LA. Ethanol-stress interaction: biochemical findings. Psychopharmacology (Berl) 1982;77:81–84. doi: 10.1007/BF00436103. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol Learn Mem. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Huynh TN, El-Ashmawy M, Sparks M. Chronic stress and a cyclic regimen of estradiol administration separately facilitate spatial memory: relationship with hippocampal CA1 spine density and dendritic complexity. Behav Neurosci. 2012;126:142–156. doi: 10.1037/a0025770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci. 2011;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Lucki I. Antidepressant-like behavioral effects mediated by 5-Hydroxytryptamine(2C) receptors. J Pharmacol Exp Ther. 2000;295:1120–1126. [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress--comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65:360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- Eilam-Stock T, Serrano P, Frankfurt M, Luine V. Bisphenol-A impairs memory and reduces dendritic spine density in adult male rats. Behav Neurosci. 2012;126:175–185. doi: 10.1037/a0025959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Ticku MK. Chronic ethanol treatment differentially regulates NMDA receptor subunit mRNA expression in rat brain. Brain Res Mol Brain Res. 1995;29:99–106. doi: 10.1016/0169-328x(94)00235-7. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Girotti M, Weinberg MS, Spencer RL. Differential responses of hypothalamus-pituitary-adrenal axis immediate early genes to corticosterone and circadian drive. Endocrinology. 2007;148:2542–2552. doi: 10.1210/en.2006-1304. [DOI] [PubMed] [Google Scholar]
- Gomez JL, Lewis MJ, Luine VN. The interaction of chronic restraint stress and voluntary alcohol intake: Effects on spatial memory in male rats. Alcohol. 2012;46:499–504. doi: 10.1016/j.alcohol.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176:289–296. doi: 10.1016/0014-2999(90)90022-x. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases alpha4 GABA(A) receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XJ, Follesa P, Ticku MK. Chronic ethanol treatment produces a selective upregulation of the NMDA receptor subunit gene expression in mammalian cultured cortical neurons. Brain Res Mol Brain Res. 1996;36:211–218. doi: 10.1016/0169-328x(95)00223-f. [DOI] [PubMed] [Google Scholar]
- Kalluri HS, Mehta AK, Ticku MK. Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Brain Res Mol Brain Res. 1998;58:221–224. doi: 10.1016/s0169-328x(98)00112-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–171. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Lewis MJ. Alcohol reinforcement and neuropharmacological therapeutics. Alcohol Alcohol. 1996;1:17–25. [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Stimulus conditioned to foot-shock stress reinstates alcohol-seeking behavior in an animal model of relapse. Psychopharmacology (Berl) 2003;168:184–191. doi: 10.1007/s00213-002-1267-z. [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Kushner MG, Rawleigh JM, Fiszdon J, Carroll ME. The effects of restraint stress on voluntary ethanol consumption in rats. Exp Clin Psychopharmacol. 1999;7:318–323. doi: 10.1037//1064-1297.7.4.318. [DOI] [PubMed] [Google Scholar]
- Martin KP, Wellman CL. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex. 2011;21:2366–2373. doi: 10.1093/cercor/bhr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB, Morrow AL. Effects of acute and chronic ethanol exposure on spatial cognitive processing and hippocampal function in the rat. Hippocampus. 2000;10:122–130. doi: 10.1002/(SICI)1098-1063(2000)10:1<122::AID-HIPO13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Mezey E, Potter JJ, Kvetnansky R. Effect of stress by repeated immobilization on hepatic alcohol dehydrogenase activity and ethanol metabolism. Biochem Pharmacol. 1979;28:657–663. doi: 10.1016/0006-2952(79)90151-5. [DOI] [PubMed] [Google Scholar]
- Narita M, Soma M, Mizoguchi H, Tseng LF, Suzuki T. Implications of the NR2B subunit-containing NMDA receptor localized in mouse limbic forebrain in ethanol dependence. Eur J Pharmacol. 2000;401:191–195. doi: 10.1016/s0014-2999(00)00428-3. [DOI] [PubMed] [Google Scholar]
- Nisenbaum LK, Zigmond MJ, Sved AF, Abercrombie ED. Prior exposure to chronic stress results in enhanced synthesis and release of hippocampal norepinephrine in response to a novel stressor. J Neurosci. 1991;11:1478–1484. doi: 10.1523/JNEUROSCI.11-05-01478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchinik M, Weiland NG, McEwen BS. Chronic exposure to stress levels of corticosterone alters GABAA receptor subunit mRNA levels in rat hippocampus. Brain Res Mol Brain Res. 1995;34:29–37. doi: 10.1016/0169-328x(95)00118-c. [DOI] [PubMed] [Google Scholar]
- Padovan CM, Del Bel EA, Guimaraes FS. Behavioral effects in the elevated plus maze of an NMDA antagonist injected into the dorsal hippocampus: influence of restraint stress. Pharmacol Biochem Behav. 2000;67:325–330. doi: 10.1016/s0091-3057(00)00361-0. [DOI] [PubMed] [Google Scholar]
- Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology. 1999;147:162–167. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- Patterson-Buckendahl P, Kubovcakova L, Krizanova O, Pohorecky LA, Kvetnansky R. Ethanol consumption increases rat stress hormones and adrenomedullary gene expression. Alcohol. 2005;37:157–166. doi: 10.1016/j.alcohol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Mitton DR, Green J, Puchalski S. Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res. 2001;25:999–1005. [PubMed] [Google Scholar]
- Roman E, Ploj K, Nylander I. Maternal separation has no effect on voluntary ethanol intake in female Wistar rats. Alcohol. 2004;33:31–39. doi: 10.1016/j.alcohol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Melia KR, Cole M, Bloom FE, Wilson MC. Alcohol selectively attenuates stress-induced c-fos expression in rat hippocampus. J Neurosci. 1995;15:721–730. doi: 10.1523/JNEUROSCI.15-01-00721.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Miller MN, Durrant S. Effects of acute alcohol administration on object recognition learning in C57BL/6J mice. Pharmacol Biochem Behav. 2002;71:307–312. doi: 10.1016/s0091-3057(01)00661-x. [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci U S A. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider AM, Simson PE, Spiller K, Adelstein J, Vacharat A, Short KR, Kirby LG. Stress-dependent enhancement and impairment of retention by naloxone: evidence for an endogenous opioid-based modulatory system protective of memory. Behav Brain Res. 2009;205:290–293. doi: 10.1016/j.bbr.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullion GA, Kendall DA, Sunter D, Marsden CA, Pardon MC. Central noradrenergic depletion by DSP-4 prevents stress-induced memory impairments in the object recognition task. Neuroscience. 2009;164:415–423. doi: 10.1016/j.neuroscience.2009.08.046. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Spencer RL, McEwen BS. Adaptation of the hypothalamic-pituitary-adrenal axis to chronic ethanol stress. Neuroendocrinology. 1990;52:481–489. doi: 10.1159/000125632. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Maickel RP. Effects of stress and ebiratide (Hoe-427) on free-choice ethanol consumption: comparison of Lewis and Sprague-Dawley rats. Life Sci. 1994;55:873–878. doi: 10.1016/0024-3205(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Fiellin DA, O’Connor PG. The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med. 2005;118:330–341. doi: 10.1016/j.amjmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Thatcher-Britton K, Koob GF. Alcohol reverses the proconflict effect of corticotropin-releasing factor. Regul Pept. 1986;16:315–320. doi: 10.1016/0167-0115(86)90031-5. [DOI] [PubMed] [Google Scholar]
- Trevisan L, Fitzgerald LW, Brose N, Gasic GP, Heinemann SF, Duman RS, Nestler EJ. Chronic ingestion of ethanol up-regulates NMDAR1 receptor subunit immunoreactivity in rat hippocampus. J Neurochem. 1994;62:1635–1638. doi: 10.1046/j.1471-4159.1994.62041635.x. [DOI] [PubMed] [Google Scholar]
- Trudeau LE, Aragon CM, Amit Z. Effects of ethanol on locomotor depression and corticosterone release induced by restraint-stress: support for a stress-ethanol interaction. Pharmacol Biochem Behav. 1990;36:273–278. doi: 10.1016/0091-3057(90)90403-5. [DOI] [PubMed] [Google Scholar]
- Trudeau LE, Aragon CM, Amit Z. Involvement of endogenous opioid mechanisms in the interaction between stress and ethanol. Psychopharmacology (Berl) 1991;103:425–429. doi: 10.1007/BF02244299. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Maciejewski PK, McKee SA, Reutenauer EL, Mazure CM. Gender differences in associations between lifetime alcohol, depression, panic disorder, and posttraumatic stress disorder and tobacco withdrawal. Am J Addict. 2009;18:140–147. doi: 10.1080/10550490802544888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Enriched environment prevents chronic stress-induced spatial learning and memory deficits. Behav Brain Res. 2008;187:41–47. doi: 10.1016/j.bbr.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]