Summary
Background and Objective
Tissue factor pathway inhibitor (TFPI) and thrombomodulin (TM) are endothelial-associated anticoagulant proteins thought to control hemostasis in specific vascular beds. Here, we have examined the consequences of TFPI deficiency in the presence of a compounding procoagulant state caused by reduced TM function.
Methods and results
TFPI+/−/TMpro/pro mice are born at less than expected frequency in either TFPI+/−/TMpro/+ or TMpro/pro mothers but are born at near the expected frequency in TMpro/+mothers. Adult TFPI+/−/TMpro/pro mice have elevated thrombin–antithrombin complex and increased thrombus volume in an electrical injury model of venous thrombosis. In striking contrast to mice with single deficiency of TFPI or TM, TFPI+/−/TMpro/pro mice exhibit augmented fibrin deposition not only in the liver, but also in the cerebral microvasculature.
Conclusions
TFPI+/−/TMpro/pro mice exhibit partial intrauterine lethality when carried by mothers with an underlying prothrombotic state, providing the first experimental evidence in an animal model that TFPI-dependent control of hemostasis in the vascular bed of the placenta fulfills a critical role for successful pregnancy outcome. In addition to the placenta, partial TFPI deficiency interacts with decreased TM function in an organ selective manner to produce fibrin deposition in other specific vascular beds, the liver and brain.
Keywords: fibrin, thrombomodulin, thrombosis, tissue factor pathway inhibitor
Introduction
Established genetic risk factors for thrombotic disease in humans that have been linked to genetic mutations, such as factor (F)V Leiden, or deficiency of antithrombin, protein C or protein S, have variable penetrance. Some individuals with one of these risk factors have thrombotic disease at an early age, while others, with the same genetic defect, will never have thrombotic disease. Clearly, multiple factors contribute to the ultimate severity of inherited thrombotic disorders. In addition, various hypercoagulable states are associated with local thrombus formation in specific vascular beds [1]. The goal of the work presented here is to characterize mice with combined tissue factor pathway inhibitor (TFPI) deficiency and thrombomodulin (TM) dysfunction in order to examine how partial TFPI deficiency alters the thrombotic predisposition of other thrombotic risk factors and to define vascular bed specific anticoagulant properties of these two endothelial-associated proteins.
TFPI is an anticoagulant protein found associated with the endothelial surface [2,3], within platelets [4,5] and circulating in plasma [6]. It exerts its anticoagulant activity through inhibition of factor Xa (FXa) and the tissue factor–FVIIa (TF–FVIIa) catalytic complex that initiates blood coagulation [7]. In humans, low plasma TFPI is not a well-established risk factor for thrombosis [8–11]. However, the development of recombinant FVIIa therapy has shown the tissue-factor pathway to be an important target for treatment in patients with acquired inhibitors to FVIII. This suggests that modulation of TFPI activity could be an attractive therapeutic target for treatment of hemophilia [12]. In mice, TFPI heterozygosity (TFPI+/−) in the absence of other genetic abnormalities has not been shown (previous to this study) to produce a hypercoagulable state despite the presence of only 50% normal TFPI activity [13]. However, when TFPI+/− mice are bred onto the homozygous FV Leiden (FVQ/Q) background, the TFPI+/−/FVQ/Q mice suffer from severe perinatal thrombosis [14], demonstrating that partial TFPI deficiency can synergize with other thrombotic risk factors to produce heightened thrombophilia.
TM, like TFPI, is an endothelium-associated anticoagulant protein. TM exerts its anticoagulant activity by binding thrombin and acting as a cofactor that allows thrombin to activate protein C. Activated protein C produces anticoagulant activity through degradation of FVa and FVIIIa. A mouse model of TM deficiency, called TMpro, has been developed [15]. These mice express a mutated form of TM containing a glutamine to proline mutation in the region between the fourth and fifth epidermal growth factor domains [16]. This mutation reduces TM expression and disrupts TM-dependent activation of protein C [15,16]. Mice homozygous for the TMpro mutation (TMpro/pro) mice are viable and fertile. They do not exhibit spontaneous thrombosis on the C57Bl/6 background but have evidence of tissue fibrin deposition when bred onto the 129SvPas background [17].
Studies using an in vitro model system of purified coagulation factors demonstrated that TFPI and TM act synergistically to quench TF-mediated thrombin generation via neutralization of prothrombinase activity [18]. However, it is unclear how TFPI and TM anticoagulant activities may cooperate within different vascular beds in vivo. For example, within the brain, which produces abundant TF, TM expression is very low [19,20] and TMpro/pro mice do not have fibrin deposition within the brain even after injection of lipopolysaccharide [17] suggesting that other anticoagulants, such as TFPI, may be important for prevention of cerebral thrombosis. In the present study, the TFPI+/− phenotype was bred with mice carrying the TMpro mutation to examine how these two key endothelium-associated anticoagulant proteins cooperate in vivo to prevent the development of intravascular thrombosis.
Methods
Transgenic animals
Mice containing a TFPI mutation such that the first Kunitz domain is not produced were a gift of Dr George Broze Jr (Washington University, St Louis). TMpro/pro mice have been described earlier [15,17]. The phenotypic penetrance of thrombosis in mice is strongly influenced by the genetic background. Therefore, all mouse strains were backcrossed to wild-type C57Bl/6 mice for at least 10 generations. All animal experiments were approved by the institutional animal care and use committee at the Medical College of Wisconsin.
Analysis of baseline coagulation and tissue fibrin deposition
Plasma thrombin–antithrombin complexes (TAT) were determined using a commercially available ELISA assay (Dade Behring, Newark, DE, USA). Plasma for these assays was obtained from mice injected 10 min prior to sample collection with 500 U of unfractionated heparin. Blood (400–1000 µL) was collected by venipuncture of the inferior vena cava and plasma samples frozen at −80 °C for batch analysis.
Detection of tissue fibrin deposition
Synthesis of the fibrin-fibronectin binding peptide, CGLIIQKNEC [21], was performed according to standard FMOC (9-fluorenylmethoxycarbonyl) protocols. The peptide was purified by high-performance liquid chromatograph (HPLC), and N-terminally labeled with FAM succinimide ester (S, E). The peptide was cyclized with DMSO, the DMSO removed by HPLC, and the peptide was lyophilized. The peptide (400 µg) was diluted in 200 µl 0.05 M carbonate buffer, pH 9.6 for retro-orbital injection. Three hours later, mice were perfused with 4% paraformaldehyde, and tissues were harvested and incubated overnight in 30% sucrose. Tissues were mounted in OCT embedding media (Tissue-Tech, Torrance, CA, USA) and stored at −80 °C. Frozen tissue sections were fixed in 4% paraformaldehyde and mounted in fluorescence mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA). Sections were analyzed using the Zeiss Axioskop fluorescence microscope (Zeiss, Thornwood, NY, USA). Tissue sections were also analyzed using the Martius, Scarlet and Blue fibrin stain [22]. With this technique, fibrin stains red, fresh fibrin stains yellow and connective tissue stains blue. Sections were analyzed using a Nikon Eclipse E600 microscope (Nikon, Melville, NY, USA).
Venous thrombosis induced by electrical injury
Age- and sex-matched groups of mice were anesthetized with intraperitoneal pentobarbital (50 mg/kg body weight). An electric injury-based model of venous thrombosis was applied to the femoral vein, as described previously [23]. Briefly, a microsurgical needle was placed within the vein lumen and a direct 1.5-volt current was applied to the needle for 1 min. After current application and needle removal, a non-occlusive thrombus subsequently grew at the site. Vessels with indwelling thrombi were harvested 30 min after thrombus induction, fixed in formalin, paraffin-sectioned in their entirety, and histomorphometrically analyzed to determine thrombus volumes. The 30-min time point was chosen because it represents the time of stable, peak thrombus growth [23].
Statistical analysis of data
The significance of survival differences between groups was determined using the chi-square analysis. A double-tailed t-test was used for assigning significance to the TAT values. Venous thrombus volumes were compared among groups with anov, using posthoc Student–Newman–Kuels tests for between-group comparisons. P-values of <0.05 were considered significant.
Results
Decreased embryonic survival of mice with combined TFPI and TM deficiency
In mice, TFPI and TM are located 37 centimorgans apart on chromosome 2 resulting in allele segregation in about 40% of meioses. Because linkage between TFPI and TM is weak, breeding studies were performed without defining the haplotypes of individual mice.
TFPI+/−/TMpro/pro mice were generated in three different crosses in which the mother had a different prothrombotic phenotype. Embryonic lethality was observed in two of the three crosses. In the first cross, breeding of TFPI+/−/TMPro/+ females with TMPro/Pro males produced significantly reduced numbers of TFPI+/−/TMPro/Pro pups (total number analyzed, n = 151; 41 TMPro/Pro; 7 TFPI+/−/TMPro/Pro; 48 TMPro/+; 55 TFPI+/−/TMPro/+; P = 7.5E-09). In the second cross, the reverse mating of TMPro/Pro females with TFPI+/−/TMPro/+ males similarly produced reduced numbers of TFPI+/−/TMPro/Pro pups (n = 129; 45 TMPro/Pro; 11 TFPI+/−/TMPro/Pro; 22 TMPro/+; 51 TFPI+/−/TMPro/+; P = 1.6E-05). Combined data from these two matings is presented in Table 1. It is important to note that both of these matings would produce equal numbers of TMpro/+ and TFPI+/−/TMpro/pro offspring regardless of the rate of crossover; the dramatically reduced number of TFPI+/−/TMpro/pro offspring compared with that of the TMpro/+ offspring demonstrates unequivocally that the TFPI+/−/TMpro/pro embryos suffer from lethality prior to weaning.
Table 1.
Combined survival at wean of (M × F) TMpro/pro × TFPI+/−/TMpro/+ and TFPI+/−/TMpro/+ × TMpro/pro
| TMpro/pro | TFPI+/−/ TMpro/pro |
TMpro/+ | TFPI+/−/ TMpro/+ |
|
|---|---|---|---|---|
| Number of mice | 86 | 18 | 70 | 106 |
| Percent present | 30.7 | 6.4 | 25.0 | 37.9 |
To determine if the TFPI+/−/TMPro/Pro mice die during embryonic development or during the perinatal period, E18.5 embryos from TMPro/Pro females mated to TFPI+/−/TMPro/+ males were genotyped. Significantly reduced numbers of TFPI+/−/TMPro/Pro embryos were present at E18.5 (n = 28; 1 TFPI+/−/TMPro/Pro; P = 0.009) (Table 2). These data show that TFPI+/−/TMPro/Pro mice die in utero during embryonic development. Genotyping of embryos between E11.5 and E13.5 revealed normal numbers of TFPI+/−/TMPro/Pro embryos indicating that embryonic death occurs after E13.5 (n = 18; 5 TFPI+/−/TMPro/Pro; P = 0.785).
Table 2.
E18.5 survival (M × F) TFPI+/−/TMpro/+ × TMpro/pro
| TMpro/pro | TFPI+/−/ TMpro/pro |
TMpro/+ | TFPI+/−/ TMpro/+ |
|
|---|---|---|---|---|
| Number of mice | 8 | 1 | 6 | 13 |
| Percent present | 28.6 | 3.6 | 21.4 | 46.4 |
To determine if the degree of the prothrombotic state of the mother contributes to the death of TFPI+/−/TMPro/Pro embryos, a third cross in which TFPI+/−/TMPro/Pro males were mated to TFPI+/+/TMPro/+ females (the least prothrombotic mothers that can produce TFPI+/−/TMPro/Pro offspring) was performed. In these breeding experiments, the TFPI+/−/TMPro/Pro embryos were produced at near-expected frequency (n = 75; 15 TFPI+/−/TMPro/Pro; P = 0.317) (Table 3). These data demonstrate that the maternal phenotype is an important determinant of intrauterine survival of TFPI+/−/TMPro/Pro mice.
Table 3.
Survival at wean of (M × F) TFPI+/−/TMpro/pro × TMpro/+
| TMpro/pro | TFPI+/−/ TMpro/pro |
TMpro/+ | TFPI+/−/ TMpro/+ |
|
|---|---|---|---|---|
| Number of mice | 19 | 15 | 13 | 28 |
| Percent present | 25.3 | 20.0 | 17.3 | 37.3 |
TFPI+/−/TMpro/pro mice have elevated plasma TAT levels
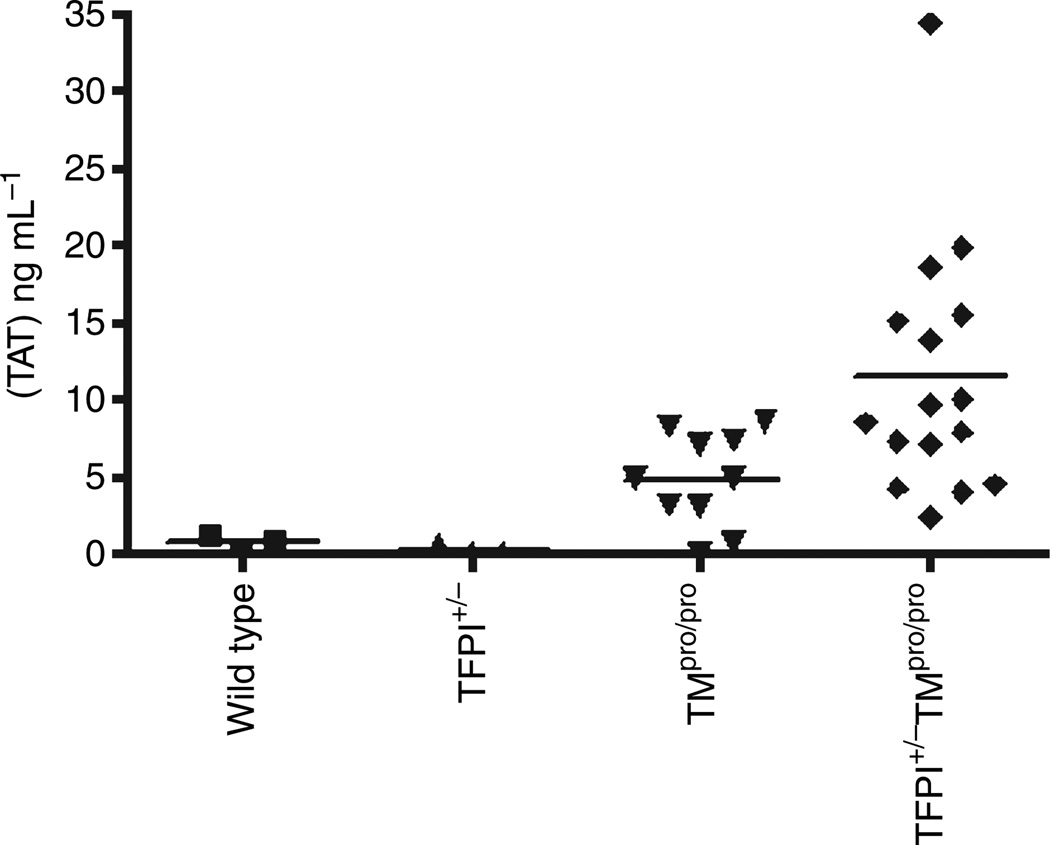
TFPI+/− mice do not exhibit a prothrombotic state when maintained under standard husbandry conditions and their plasma TAT levels are indistinguishable from wild-type mice (Fig. 1). TMpro/pro mice on the C57Bl/6 background exhibit a mild hypercoagulable state associated with increased plasma TAT levels (Fig. 1). Combination of these two prothrombotic phenotypes in the TFPI+/−/TMpro/pro mice produces significantly elevated plasma TAT levels when compared with TMpro/pro mice, demonstrating a synergistic effect of TFPI and TM deficiencies toward creating a systemic procoagulant state (Fig. 1).
Fig. 1.
Plasma thrombin–antithrombin complexes (TAT) concentration in wild-type, TFPI+/−, TMpro/pro and TFPI+/+/TMpro/pro mice. Values for wild-type (n = 3) and TFPI+/− mice (n = 3) are indistinguishable. The average ± SD values are 4.82 ± 3.05 for the TFPI+/+/TMpro/pro mice (n = 10) and 11.4 ± 8.12 for the TFPI+/+/TMpro/pro mice (n = 16), P < 0.03.
TFPI+/−/TMpro/pro mice have fibrin deposition in liver and brain
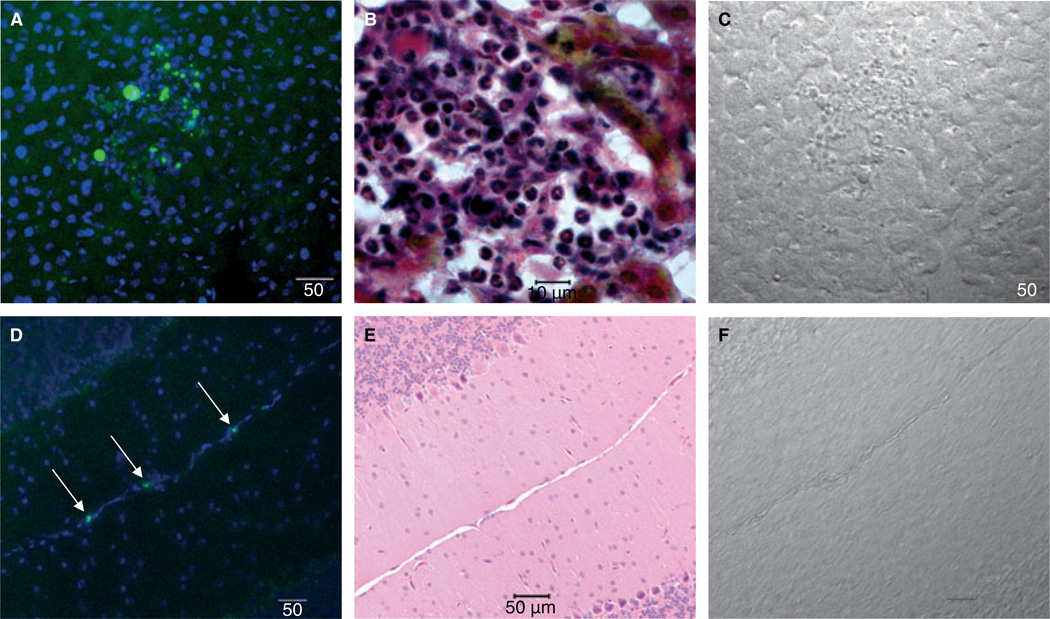
Pilch and co-workers developed and characterized a cyclic peptide that specifically binds to fibrin–fibronectin complexes in mice [21]. This peptide was injected into TFPI+/−/TMpro/pro, TFPI+/−, TMpro/+ and TMpro/pro mice to evaluate the development of tissue fibrin deposition. The TFPI+/−/TMpro/pro mice have distinct, multifocal areas of hepatic fluorescence demonstrating fibrin deposition within the liver. These fluorescent areas are not found in the livers of mice with other genotypes (Fig. 2A). Peptide deposition is often observed in areas where the normal hepatic architecture appears to be disrupted from thrombotic disease. When observed with the Martius, Scarlet and Blue fibrin stain, multifocal areas of fibrin deposition are present in association with macrophages and neutrophils consistent with a sterile inflammatory response to fibrin deposition with associated disruption of the hepatic architecture (Fig. 2B). In addition to the liver, fibrin deposition is evident within the brain of TFPI+/−/TMpro/pro mice as distinct punctuate fluorescence within vessels of the pia matter surrounding the cerebellum (Fig. 2D). This pattern of fluorescence was relatively rare but consistently present in the TFPI+/−/TMpro/pro mice; identified in two to four vessels of all four mice examined. In contrast, this pattern of fluorescence was found in only one vessel of one control mouse (TFPI+/−) (data not shown). Examination of the vascular beds from the other major organ systems did not reveal differences in peptide deposition in any of the mouse genotypes. All mice examined had significant amounts of peptide within the kidneys consistent with its renal clearance from the circulation (data not shown).
Fig. 2.
Fibrin deposition in liver and brain of TFPI+/−/TMpro/pro mice. Panels A, B and C are liver; panels D, E and F are brain. All panels are at 20× magnification with 50 micron scale bars except panel B that is 100 × magnification with 10 micron scale bar. (A) Fibrin deposits (green) within the interstitia of the liver from a TFPI+/−/TMpro/pro mouse detected using a fluorescently labeled peptide (green) that specifically binds to fibrin–fibronectin complexes [21]. Cell nuclei are stained blue with DAPI. (B) Martius, Scarlet and Blue fibrin stain of a representative liver lesion. This stain colors fibrin red, fresh fibrin yellow and connective tissue blue. Multiple neutrophils are present within the lesion. (C) Brightfield image of the liver lesion presented in panel A. (D) Fibrin deposition (green) within vessels of the pia matter surrounding the cerebellum of TFPI+/−/TMpro/pro mice detected using the fibrin binding peptide (arrows). Cell nuclei are stained blue with DAPI. (E) Hematoxylin and eosin stain of section similar to that shown in panel D showing the pia matter of the cerebellum where the fibrin deposition was most commonly observed. (F) Brightfield image of brain presented in panel D.
Measurement of thrombus volume after venous injury
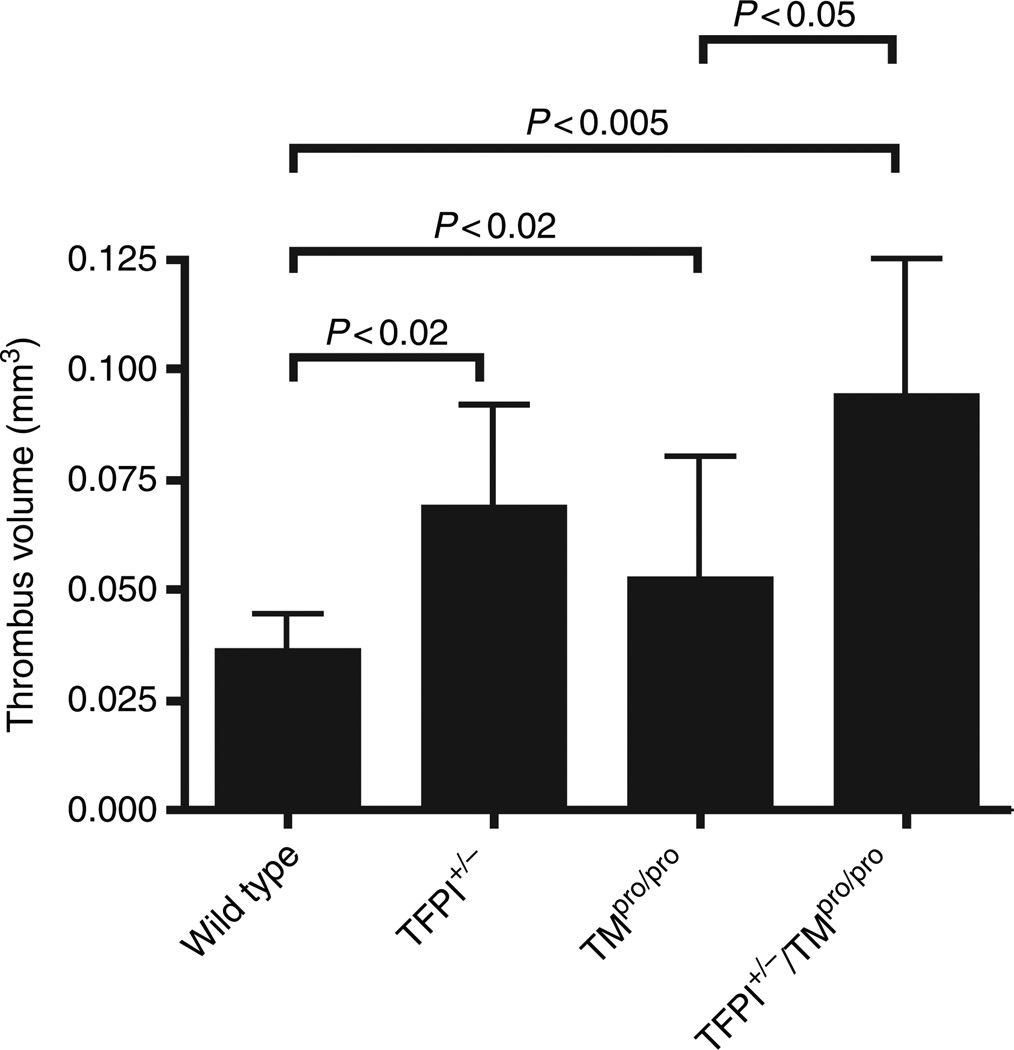
The extent of venous thrombus formation in wild-type, TFPI+/−, TMpro/pro and TFPI+/−/TMpro/pro mice was examined using an electrical injury model [23]. In this model, both TFPI+/− and TMpro/pro mice have significantly increased thrombus volume compared with wild-type mice (Fig. 3). These data are the first to demonstrate that partial TFPI deficiency in the absence of other genetic modifiers contributes to thrombus growth in a vascular injury model. Experiments performed with TFPI+/−/TMpro/pro mice demonstrate that addition of TFPI heterozygosity significantly increases injury-induced thrombus volume when compared with the TMpro/pro mice (Fig. 3).
Fig. 3.
Thrombus volume in a venous electrical injury model. Mice (n = 10 for all groups) were subjected to venous injury and thrombus volume measured by histomorphometry. P-values comparing different mice are as indicated.
Discussion
Totally TFPI-deficient humans have not been identified and the absence of TFPI results in embryonic death of mice. Many TFPI null mice die between days E9.5 and E11.5. Mice surviving beyond E11.5 have normal organ development but have short tails and other signs of coagulopathy including diffuse fibrin(ogen) deposition in the liver and rare intravascular thrombi in the brain [13]. These findings are consistent with unregulated TF–FVIIa activity producing a consumptive coagulopathy in the TFPI null embryos.
In contrast to the severe phenotype of the TFPI null mice, a propensity for thrombosis in TFPI+/− mice has not been described previously. Partial TFPI deficiency produces severe thrombosis when bred into FVQ/Q mice [14]. TFPI+/−/FVQ/Q mice have nearly complete perinatal mortality with fibrin deposition or tissue infarction present in the liver, lung and kidney, suggesting disseminated thrombosis. This is strikingly different from the FVQ/Q mice that develop normally and rarely exhibit spontaneous thrombosis depending on the genetic background of the mice [24]. The TFPI+/− phenotype has also been bred into ApoE null mice [25]. The TFPI+/−/ApoE null mice developed increased atherosclerotic burden in carotid and common iliac arteries providing further evidence that partial TFPI deficiency contributes to the development of vascular disease in the presence of other genetic risk factors.
In vitro experiments have demonstrated that TFPI and TM act synergistically to quench thrombin generation in TF-initiated assays [26]. Here, we have demonstrated that combined partial deficiencies of these two endothelium-associated anticoagulant proteins produce a severe thrombotic state in vivo. TFPI+/−/TMpro/pro mice have increased intrauterine lethality with death occurring between E13.5 and E18.5. Animals surviving to adulthood have an enhanced prothrombotic state when compared with either TFPI+/− or TMpro/pro mice.
The TFPI+/−/TMpro/pro mice have greatly reduced embryonic survival in either TFPI+/−/TMpro/+ or TMpro/pro mothers. As mice with the other genotypes are born at the expected frequencies in these mothers, the fetal genotype contributes to the death of the TFPI+/−/TMpro/pro embryos. This finding is consistent with our recent demonstration of the critical role of TFPI expressed on differentiated fetal trophoblast cells in the down-regulation of constitutive TF activity on these cells [27]. In contrast, TFPI+/−/TMpro/pro mice are born at near the expected frequency from TMpro/+ mothers, the least prothrombotic maternal phenotype that can produce TFPI+/−/TMpro/pro offspring. These data demonstrate that survival of the TFPI+/−/TMpro/pro embryos is also dependent on the maternal genotype. We have recently documented reduced intrauterine survival of TMpro/pro mice in mothers carrying the FV Leiden allele [28]. The current data provides experimental evidence that both maternal and fetal TFPI status also affects intrauterine survival of TMpro/pro mice. It is likely that embryonic death of the TFPI+/−/TMpro/pro mice is as a result of synergistic effects of fetal and maternal prothrombotic mutations producing thrombosis at the fetal–maternal interface within the placental vascular bed.
Adult TFPI+/−/TMpro/pro mice are fertile and do not exhibit grossly apparent signs of thrombosis. Compared with either TFPI+/− or TMpro/pro mice, plasma TAT levels in TFPI+/−/TMpro/pro mice are significantly elevated demonstrating enhanced thrombin production and suggesting the presence of systemic activation of coagulation. Plasma TAT levels in TMpro/pro mice are elevated over wild-type while TFPI+/− mice have no change in TAT level. This indicates an augmented systemic activation of coagulation in mice with the TMpro/pro genotype. However, when compared in the venous thrombosis model, the TFPI+/− and TMpro/pro mice both had increased thrombus volume with no significant difference between the two phenotypes. This demonstrates that in the presence of an intravascular coagulation challenge, the hypercoagulable status of the TFPI+/− and TMpro/pro mice produces a similar effect on the developing venous thrombus regardless of the systemic activation of coagulation in the absence of thrombotic challenge.
Further evidence for the presence of systemic activation of coagulation in the TFPI+/−/TMpro/pro mice is provided by studies using a fluorescent peptide that specifically binds to clot-bound fibrin [21], a simple and sensitive means for detection of fibrin deposition in vivo. Experiments using this peptide demonstrated distinct areas of fibrin deposition within the liver and brain of TFPI+/−/TMpro/pro mice. It is notable that this same pattern of fibrin deposition in the liver and brain is observed in TFPI null embryos [13], but is distinctly different from the pattern of fibrin deposition in the lung and heart observed in TMpro/pro mice on the 129SvPas background [15,17] [the TMpro/pro mice on the C57Bl/6 background used in this study do not exhibit fibrin deposition in any organ system [17]] as well as in mice with severe protein C deficiency [29]. Thus, superimposing partial TFPI deficiency on the TMpro/pro mutation produces an organ-specific pattern of fibrin deposition similar to that produced by TFPI deficiency and not that of deficiency in the TM-protein C system. The lack of fibrin deposition within the heart and lung of TFPI+/−/TMpro/pro mice may be a result of the high amounts of TFPI produced by these tissues [2], such that sufficient amounts of TFPI remain to regulate TF-FVIIa mediated fibrin production even in animals with heterozygous deficiency. It is also interesting to note that TMpro/pro mice, regardless of genetic background, have not previously been shown to have fibrin deposition in the brain. This is true even after lipopolysaccharide challenge that induces fibrin deposition in multiple other vascular beds [17]. Here, it is demonstrated that partial TFPI deficiency induces intravascular fibrin deposition in the brain of TMpro/pro mice. These data suggest that within the brain vasculature, TFPI may be the primary endothelium-associated anticoagulant, with the TM-protein C system playing a secondary role.
It has been previously demonstrated that TFPI+/− mice have the same average time to occlusive arterial thrombosis as wild-type mice in a Rose bengal photo-induced model of carotid artery injury [14]. Here, we examined thrombus development in TFPI+/− mice using a venous electrical injury model [23]. This model has the unique advantage of generating a measurable non-occlusive venous thrombus under maintained conditions of flow, thus simulating early events in clinical deep vein thrombosis [23]. The developing thrombus is composed primarily of a fibrin clot, but has a dependence on platelet adhesion/aggregation. The finding of increased thrombus volume in TFPI+/− mice represents the first demonstration of an increased thrombotic response to vascular injury in these mice. Study of TFPI+/−/TMpro/pro mice with the venous injury model demonstrates that the addition of partial TFPI deficiency to the prothrombotic condition produced by reduced TM function further increases the risk for thrombosis associated with venous injury. These data indicate that TFPI+/− is an independent risk factor for development of venous thrombosis in this model even in the presence of other thrombotic risk factors.
The use of mouse models to investigate the role of TFPI in thrombotic disease is important because mice with well-defined deficiency of both cell-associated and plasma TFPI can be produced and examined. In contrast, identification of partial TFPI deficiency in humans is limited to measurement of the plasma TFPI concentration that does not necessarily represent the amount of endothelial and platelet associated TFPI [4–6] These differences in our ability to definitively define TFPI deficiency in mice and humans perhaps accounts for the differences in the pro-thrombotic effects of TFPI deficiency observed in mouse models and clinical studies. Mouse models have demonstrated a significant contribution of partial TFPI deficiency to thrombotic disease [14], while clinical studies have only weakly linked low plasma TFPI concentration to disease in humans. Several clinical studies have suggested the presence of a ‘threshold effect’ where patients with free (non-lipoprotein bound) plasma TFPI less than 10% of the normal mean value are at increased risk (~2-fold) of both deep venous thrombosis and myocardial infarction [8–11]. In these studies there was no difference in the mean free plasma TFPI level between the disease and control groups. In other clinical studies there is a considerable amount of conflicting data about the contribution of plasma TFPI levels and polymorphisms to the development of thrombosis [30–33]. This is likely a result of the wide normal range for plasma TFPI [6], and the various methods for measurement of plasma TFPI [34]. Thus, studies in mice are substantially more informative than clinical studies in humans because the TFPI+/− mice have a well-defined deficiency of endothelial, platelet and plasma TFPI. Extrapolation of the data obtained from studies in mice to human disease suggests that partial TFPI deficiency significantly contributes to the variable phenotypes observed in humans with established genetic risk factors for thrombosis. The results emphasize the need for development of new clinical assays to measure endothelial-associated and platelet TFPI.
Acknowledgement
The authors thank M. Syring, T. Holyst, C.-Y. Chen and J. Ferrel for expert technical assistance.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Aird WC. Vascular bed-specific thrombosis. J Thromb Haemost. 2007;5:283–291. doi: 10.1111/j.1538-7836.2007.02515.x. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj MS, Kuppuswamy MN, Saito H, Spitzer SG, Bajaj SP. Cultured normal human hepatocytes do not synthesize lipoprotein-associated coagulation inhibitor: evidence that endothelium is the principal site of its synthesis. PNAS. 1990;87:8869–8873. doi: 10.1073/pnas.87.22.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maroney SA, Cunningham AC, Ferrel J, Hu R, Haberichter S, Mansbach CM, Brodsky RA, Dietzen DJ, Mast AE. A GPI-anchored co-receptor for tissue factor pathway inhibitor controls its intracellular trafficking and cell surface expression. J Thromb Haemost. 2006;4:1114–1124. doi: 10.1111/j.1538-7836.2006.01873.x. [DOI] [PubMed] [Google Scholar]
- 4.Novotny WF, Girard TJ, Miletich JP, Broze GJ., Jr Platelets secrete a coagulation inhibitor functionally and antigenically similar to the lipoprotein associated coagulation inhibitor. Blood. 1988;72:2020–2025. [PubMed] [Google Scholar]
- 5.Maroney SA, Haberichter SL, Friese P, Collins ML, Ferrel JP, Dale GL, Mast AE. Active tissue factor pathway inhibitor is expressed on the surface of coated platelets. Blood. 2007;109:1931–1937. doi: 10.1182/blood-2006-07-037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novotny WF, Brown SG, Miletich JP, Rader DJ, Broze GJ., Jr Plasma antigen levels of the lipoprotein-associated coagulation inhibitor in patient samples. Blood. 1991;78:387–393. [PubMed] [Google Scholar]
- 7.Girard TJ, Warren LA, Novotny WF, Liker KM, Brown SG, Miletich JP, Broze GJ., Jr Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature. 1989;338:518–520. doi: 10.1038/338518a0. [DOI] [PubMed] [Google Scholar]
- 8.Dahm A, van Hylckama VA, Bendz B, Rosendaal F, Bertina RM, Sandset PM. Low levels of tissue factor pathway inhibitor (TFPI) increase the risk of venous thrombosis. Blood. 2003;101:4387–4392. doi: 10.1182/blood-2002-10-3188. [DOI] [PubMed] [Google Scholar]
- 9.Golino P, Ravera A, Ragni M, Cirillo P, Piro O, Chiariello M. Involvement of tissue factor pathway inhibitor in the coronary circulation of patients with acute coronary syndromes. Circulation. 2003;108:2864–2869. doi: 10.1161/01.CIR.0000105900.21445.3D. [DOI] [PubMed] [Google Scholar]
- 10.Morange PE, Simon C, Alessi MC, Luc G, Arveiler D, Ferrieres J, Amouyel P, Evans A, Ducimetiere P, Juhan-Vague I. Endothelial cell markers and the risk of coronary heart disease: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) study. Circulation. 2004;109:1343–1348. doi: 10.1161/01.CIR.0000120705.55512.EC. [DOI] [PubMed] [Google Scholar]
- 11.Duering C, Kosch A, Langer C, Thedieck S, Nowak-Gottl U. Total tissue factor pathway inhibitor is an independent risk factor for symptomatic paediatric venous thromboembolism and stroke. Thromb Haemost. 2004;92:707–712. doi: 10.1160/TH04-05-0293. [DOI] [PubMed] [Google Scholar]
- 12.Erhardtsen E, Ezban M, Madsen MT, Diness V, Glazer S, Hedner U, Nordfang O. Blocking of tissue factor pathway inhibitor (TFPI) shortens the bleeding time in rabbits with antibody induced haemophilia A. Blood Coagul Fibrinolysis. 1995;6:388–394. doi: 10.1097/00001721-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Huang ZF, Higuchi D, Lasky N, Broze GJ., Jr Tissue factor pathway inhibitor gene disruption produces intrauterine lethality in mice. Blood. 1997;90:944–951. [PubMed] [Google Scholar]
- 14.Eitzman DT, Westrick RJ, Bi X, Manning SL, Wilkinson JE, Broze GJ, Ginsburg D. Lethal perinatal thrombosis in mice resulting from the interaction of tissue factor pathway inhibitor deficiency and factor V Leiden. Circulation. 2002;105:2139–2142. doi: 10.1161/01.cir.0000017361.39256.82. [DOI] [PubMed] [Google Scholar]
- 15.Weiler-Guettler H, Christie PD, Beeler DL, Healy AM, Hancock WW, Rayburn H, Edelberg JM, Rosenberg RD. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J Clin Invest. 1998;101:1983–1991. doi: 10.1172/JCI2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke JH, Light DR, Blasko E, Parkinson JF, Nagashima M, McLean K, Vilander L, Andrews WH, Morser J, Glaser CB. The short loop between epidermal growth factor-like domains 4 and 5 is critical for human thrombomodulin function. J Biol Chem. 1993;268:6309–6315. [PubMed] [Google Scholar]
- 17.Weiler H, Lindner V, Kerlin B, Isermann BH, Hendrickson SB, Cooley BC, Meh DA, Mosesson MW, Shworak NW, Post MJ, Conway EM, Ulfman LH, von Andrian UH, Weitz JI. Characterization of a mouse model for thrombomodulin deficiency. Arterioscler Thromb Vasc Biol. 2001;21:1531–1537. doi: 10.1161/hq0901.094496. [DOI] [PubMed] [Google Scholar]
- 18.van’t Veer C, Golden NJ, Kalafatis M, Mann KG. Inhibitory mechanism of the protein C pathway on tissue factor-induced thrombin generation. Synergistic effect in combination with tissue factor pathway inhibitor. J Biol Chem. 1997;272:7983–7994. doi: 10.1074/jbc.272.12.7983. [DOI] [PubMed] [Google Scholar]
- 19.Ishii H, Salem HH, Bell CE, Laposata EA, Majerus PW. Thrombomodulin, an endothelial anticoagulant protein, is absent from the human brain. Blood. 1986;67:362–365. [PubMed] [Google Scholar]
- 20.Wang L, Tran ND, Kittaka M, Fisher Mark J, Schreiber SS, Zlokovic BV. Thrombomodulin expression in bovine brain capillaries: anticoagulant function of the blood-brain barrier, regional differences, and regulatory mechanisms. Arterioscler Thromb Vasc Biol. 1997;17:3139–3146. doi: 10.1161/01.atv.17.11.3139. [DOI] [PubMed] [Google Scholar]
- 21.Pilch J, Brown DM, Komatsu M, Jarvinen TAH, Yang M, Peters D, Hoffman RM, Ruoslahti E. Peptides selected for binding to clotted plasma accumulate in tumor stroma and wounds. PNAS. 2006;103:2800–2804. doi: 10.1073/pnas.0511219103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buk SJ. Simultaneous demonstration of connective tissue elastica and fibrin by a combined Verhoeff’s elastic-Martius-scarlet-blue trichrome stain. Stain Technol. 1984;59:1–5. doi: 10.3109/10520298409113822. [DOI] [PubMed] [Google Scholar]
- 23.Cooley BC, Szema L, Chen CY, Schwab JP, Schmeling G. A murine model for deep vein thrombosis: characterization and validation in transgenic mice. Thromb Haemost. 2005;94:498–503. doi: 10.1160/TH05-03-0170. [DOI] [PubMed] [Google Scholar]
- 24.Cui J, Eitzman DT, Westrick RJ, Christie PD, Xu ZJ, Yang AY, Purkayastha AA, Yang TL, Metz AL, Gallagher KP, Tyson JA, Rosenberg RD, Ginsburg D. Spontaneous thrombosis in mice carrying the factor V Leiden mutation. Blood. 2000;96:4222–4226. [PubMed] [Google Scholar]
- 25.Westrick RJ, Bodary PF, Xu Z, Shen YC, Broze GJ, Eitzman DT. Deficiency of tissue factor pathway inhibitor promotes atherosclerosis and thrombosis in mice. Circulation. 2001;103:3044–3046. doi: 10.1161/hc2501.092492. [DOI] [PubMed] [Google Scholar]
- 26.van’t Veer C, Golden NJ, Kalafatis M, Simioni P, Bertina RM, Mann KG. An in vitro analysis of the combination of hemophilia A and factor V(Leiden) Blood. 1997;90:3067–3072. [PubMed] [Google Scholar]
- 27.Sood R, Kalloway S, Mast AE, Hillard CJ, Weiler H. Fetomaternal cross talk in the placental vascular bed: control of coagulation by trophoblast cells. Blood. 2006;107:3173–3180. doi: 10.1182/blood-2005-10-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sood R, Zogg M, Westrick RJ, Guo Y, Kerschen EJ, Girardi G, Salmon JE, Coughlin SR, Weiler H. Fetal gene defects precipitate platelet-mediated pregnancy failure in factor V Leiden mothers. J Exp Med. 2007;204:1049–1056. doi: 10.1084/jem.20062566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lay AJ, Liang Z, Rosen ED, Castellino FJ. Mice with a severe deficiency in protein C display prothrombotic and proinflammatory phenotypes and compromised maternal reproductive capabilities. J Clin Invest. 2005;115:1552–1561. doi: 10.1172/JCI24030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ariens RA, Alberio G, Moia M, Mannucci PM. Low levels of heparin-releasable tissue factor pathway inhibitor in young patients with thrombosis. Thromb Haemost. 1999;81:203–207. [PubMed] [Google Scholar]
- 31.Asakur H, Ontachi Y, Mizutani T, Kato M, Saito M, Morishita E, Yamazaki M, Suga Y, Takami A, Miyamoto K, Nakao S. Elevated levels of free tissue factor pathway inhibitor antigen in cases of disseminated intravascular coagulation caused by various underlying diseases. Blood Coagul Fibrinolysis. 2001;12:1–8. doi: 10.1097/00001721-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Velasco F, Lopez-Pedrera C, Borrell M, Fontcuberta J, Torres A. Elevated levels of tissue factor pathway inhibitor in acute non- lymphoblastic leukemia patients with disseminated intravascular coagulation. Blood Coagul Fibrinolysis. 1997;8:70–72. doi: 10.1097/00001721-199701000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Adams MJ, Cardigan RA, Marchant WA, Grocott MPW, Mythen MG, Mutch M, Purdy G, Mackie IJ, Machin SJ. Tissue factor pathway inhibitor antigen and activity in 96 patients receiving heparin for cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2002;16:59–63. doi: 10.1053/jcan.2002.29677. [DOI] [PubMed] [Google Scholar]
- 34.Ariens RAS. The quest for the Holy Grail of tissue factor pathway inhibitor deficiency has just begun. J Thromb Haemost. 2005;3:649–650. doi: 10.1111/j.1538-7836.2005.01263.x. [DOI] [PubMed] [Google Scholar]