Abstract
Introduction:
Accumulating evidence has linked depressive symptoms and sex hormones to risk for relapse; however, the specific mechanisms involved in these associations remain unknown. This randomized crossover study assessed physiological response to nicotine by menstrual phase in female smokers with and without depressive symptoms following acute smoking abstinence.
Methods:
Females, ages 18–40 years with regular menstrual cycles, not on exogenous hormones or psychotropic medications, who reported smoking ≥5 cigarettes/day were enrolled. Participants were stratified into 2 groups: no depressive symptoms (NDS; n = 23) and depressive symptoms (DS; n = 24). After 4 days of biochemically verified smoking abstinence, participants completed 2 laboratory sessions in the follicular (F) and luteal (L) phases. Participants used nicotine nasal spray at Time 0, and blood pressure, heart rate, and serum nicotine were measured at Time −1, 5, 10, 20, 30, 45, 60, and 90min.
Results:
Participants (n = 47) were 29.1±6.8 years old and smoked an average of 12.5±5.1 cigarettes/day. The NDS group had more pronounced menstrual phase differences (F > L) in diastolic blood pressure, heart rate, and maximum concentrations of nicotine compared with the DS group (p < .05).
Conclusions:
This study observed an interaction between sex hormones and depressive symptoms such that those without depressive symptoms had a greater menstrual phase difference in the physiological response to nicotine. These data offer additional support for the role of sex hormones in the physiological response to nicotine, which may play a role in menstrual phase effects on smoking cessation.
INTRODUCTION
In women who are attempting to quit smoking, menstrual phase, perhaps via sex hormones (specifically progesterone and estradiol), appears to be associated with risk for smoking relapse (Allen, Bade, Center, Finstad, & Hatsukami, 2008; Allen S.S, Allen A.M, Lunos, & Hatsukami, 2009b; Carpenter, Saladin, Leinbach, Larowe, & Upadhyaya, 2008; Franklin et al., 2008; Mazure, Toll, McKee, Wu, & O’Malley, 2011). Women who attempted to quit smoking during the luteal phase (the last 14 days of the menstrual cycle with relatively high levels of progesterone and low levels of estrogen) had improved smoking cessation outcomes compared with those who quit during the early follicular phase (begins after menses with relatively low levels of both progesterone and estrogen) in the absence of nicotine replacement therapy (NRT) (Allen et al., 2008; Allen et al., 2009b; Mazure et al., 2011). However, other studies that used NRT observed improved smoking cessation outcomes in the follicular phase compared with the luteal phase (Carpenter et al., 2008; Franklin et al., 2008). Aside from differences in study methodology, one theory to explain these seemingly discordant observations is that among women smokers who did not use NRT, estradiol (which peaks in the late follicular phase) may enhance the reinforcing effects of nicotine and, therefore, favor relapse. The pre-clinical model has provided strong evidence for this theory indicating that estradiol is associated with the facilitation of drug self-administration, whereas progesterone reduces drug self-administration (Carroll & Anker, 2010; Lynch & Sofuoglu, 2010).
There is evidence indicating that changes in sex hormones may impact the pharmacokinetics of nicotine. This is illustrated by studies showing that exogenous administration of hormones (i.e., oral contraceptives) and clinical situations in which the concentrations of hormones are altered (i.e., pregnancy and menopause) impact nicotine metabolism (Benowitz, Lessov-Schlaggar, Swan, & Jacob, 2006; Berlin, Gasior, & Moolchan, 2007; Dempsey, Jacob, & Benowitz, 2000; Selby, Hackman, Kapur, Klein, & Koren, 2001). Conversely, in a small clinical study, nicotine metabolism did not differ by menstrual phase in non-smoking women (n = 11) who received an infusion of nicotine and cotinine (Hukkanen, Gourlay, Kenkare, & Benowitz, 2005). These conflicting data indicate additional research is needed to more fully characterize the role of sex hormones on nicotine pharmacokinetics.
Risk for relapse is further exacerbated in women who have a comorbidity of depression (Husky, Mazure, Paliwal, & McKee, 2008). Considerable evidence has been amassed to suggest that depression (including both depressive symptomatology and major depressive disorder [MDD]) also plays a significant role in undermining quit attempts in smokers. Numerous clinical studies and population-based surveys have confirmed that the presence of MDD (past or present) is associated with a decreased likelihood of smoking cessation (e.g., Hitsman, Borrelli, McChargue, Spring, & Niaura, 2003; Killen, Fortmann, Schatzberg, Hayward, & Varady, 2003; Murphy et al., 2003). This association is of particular concern for women as depressive symptoms are more predictive of smoking behavior in women compared with men (Borrelli, Bock, King, Pinto, & Marcus, 1996; Husky et al., 2008; Pratt & Brody, 2010). The specific mechanisms involved in the increased risk for relapse among smokers with depressive symptoms, especially women, remain unknown.
In women, the risk for developing depressive symptoms is highest during times of dramatic fluctuations in sex hormones such as during the postpartum period (when a substantial decline in progesterone and estrogen occurs) and the transition to menopause (when sex hormones fluctuate dramatically). Although the clinical literature is mixed, the evidence appears to indicate that lower levels of estradiol may be associated with an increased risk for depressive symptoms. For example, women who develop postpartum depressive symptoms have lower levels of estradiol compared with those without depressive symptoms (Klier et al., 2007). Similarly, the overall decline in estradiol levels during the menopause transition is associated with an increase risk in developing depressive symptoms (Ryan et al., 2009). Finally, in regularly menstruating women, those with a history of depression had lower levels of estradiol in the follicular phase compared with a control group (Deecher, Andree, Sloan, & Schechter, 2008).
It is possible that sex hormones and depressive symptoms may act synergistically to minimize quit attempts in women; however, to date, no data are available to evaluate this relationship. Therefore, the purpose of this study is to characterize the independent and combined effects of menstrual phase and depressive symptoms on physiological response to nicotine during acute smoking abstinence in a sample of premenopausal female smokers. Specifically, our three aims are to (a) investigate the difference in physiological response to nicotine in women with depressive symptoms (DS) compared with women without depressive symptoms (NDS), (b) explore differences in the physiological response to nicotine by menstrual phase, and (c) determine if depressive symptom status (DS vs. NDS) is an effect modifier between menstrual phase and physiological response to nicotine. We hypothesized that physiological response to nicotine will be greater in the DS group compared with the NDS group, and that the response will be differentially affected by menstrual phase. We also anticipated that depressive symptoms would be a significant effect modifier on the relationship between menstrual phase and physiological response to nicotine.
METHODS
Study Sample
Women between the ages of 18–40 years were recruited via mass-media advertising for this randomized crossover laboratory study. Eligibility was initially assessed over the phone followed by an in-person screening visit conducted during the early part of the menstrual cycle (days 2–7) when hormone levels were low. To be eligible, women had to smoke at least 5 cigarettes/day for at least the past year, have regular menstrual cycles for at least the past 3 months, and be in stable physical/mental health. Exclusionary items included the use of any exogenous hormones, psychotropic medications, or other forms of tobacco or nicotine, as well as pregnancy, nursing, or planning to become pregnant within the next 6 months. Potential study participants also had to score an 11 or higher on the Shortened Premenstrual Form (Allen, McBride, & Pirie, 1991), to ensure study participants experienced premenstrual symptoms. However, those who met criteria for Premenstrual Dysphoric Disorder (PMDD) or MDD within the past 6 months, as assessed using the DSM-IV criteria via the Composite International Diagnostic Interview (CIDI), a computer-assisted interview (Wittchen et al., 1991), were excluded and referred for treatment. Finally, given the crossover design of this study, potential participants who indicated they wanted to permanently abstain from smoking were excluded and referred to other smoking cessation studies or programs.
Study Protocol and Measures
Upon confirmation of study eligibility, participants were stratified into one of two groups—depressive symptoms (DS) and no depressive symptoms (NDS). The DS group was defined as meeting at least one of the following criteria: (a) lifetime presence (assessed by the CIDI) of depressed mood or loss of interest/pleasure for at least 14 consecutive days, (b) lifetime presence of four or more DSM-IV behavioral symptoms (assessed by the CIDI), and/or (c) a score of five or greater on the Patient Health Questionnaire-9 (Kroenke, Spitzer, & Williams, 2001). Although participants with current (i.e., within the past 6 months) PMDD or MDD were excluded, the DS group included participants who had a history of MDD and/or current depressive symptoms but did not meet criteria for current MDD (i.e., items 1 and 2 above, both within the past 6 months). The NDS group was defined as not meeting any of the criteria for the DS group.
After stratification, participants were randomized to test in the follicular phase first followed by the luteal (F–L) phase or vice versa (L–F). The 6-day testing week started on the day after the onset of menses for F phase, to time the nicotine laboratory session in early F phase (day 7) and started 2 days after ovulation (determined with urine luteinizing hormone tests as previously described; Allen et al., 2008) to time the nicotine laboratory session in L phase (8 days after ovulation). If schedule conflicts occurred, the entire testing week was shifted 1 day earlier or 1 day later.
Each testing week included daily clinic visits for six consecutive days. On testing Day 1, participants were smoking ad libitum and attended a 1-hr clinic visit to be trained on study procedures and learn how to use the nicotine nasal spray. On testing Day 2, participants continued to smoke ad libitum and completed a 2.5-hr nicotine nasal spray exposure session (results not presented). At midnight on testing Day 2, participants quit smoking and remained abstinent for the rest of the testing week. On testing Days 3–5, participants attended 30-min clinic visits to biologically confirm smoking status and provide blood samples for measurement of sex hormones. Smoking status was confirmed on Days 3–6 via the following values indicating abstinence: carbon monoxide breathalyzer (done on Days 3–6): <5 parts per million; salivary cotinine samples (done on Day 6): <15ng/ml; and serum nicotine samples (done on Day 6): <2ng/ml (Benowitz, Bernert, Caraballo, Holiday, & Wang, 2009; Jarvis, Tunstall-Pedoe, Feyerabend, Vesey, & Saloojee, 1987). On testing Day 6, participants attended a 4-hr nicotine nasal spray laboratory session. During this session, participants used the nicotine nasal spray (2-mg dose at Time 0), and a series of eight-timed blood samples were collected for serum nicotine analysis (Time: −1, 5, 10, 20, 30, 45, 60, and 90min). Nicotine serum samples were later analyzed in batches at Hennepin County Medical Center Laboratories using gas chromatography with nitrogen phosphorus detection (Jacob, Wilson, & Benowitz, 1981). Intraassay and interassay coefficient of variations ranged between 2.7% and 5.6%, respectively. At each timepoint, participants completed the Subjective State Scale (al’Absi, Lovallo, McKey, & Pincomb, 1994; al’Absi et al., 1998) for measurement of positive (i.e., cheerful) and negative affect (i.e., angry) using an 8-point scale. Blood pressure and heart rate were also measured via an automated blood pressure machine at each timepoint.
Upon completion of the testing week, participants resumed ad libitum smoking until they arrived at the alternate menstrual phase (approximately 6 weeks later depending on cycle length). Identical data collection procedures were then completed. Participants were compensated for their time. All procedures were approved by the human subjects committee at the University of Minnesota.
Analysis
Maximal nicotine concentration (Cmax) was the highest observed nicotine concentration, and time to maximal nicotine concentration (Tmax) was the first timepoint at which this concentration occurred. In instances when all of the concentrations for a given laboratory period were below the lower limit of quantification (which was 2ng/ml), a value of 1ng/ml was assigned as the Cmax and the 5-min timepoint was assigned as the Tmax.
Descriptive statistics were calculated for demographics and baseline characteristics (mean and standard deviation for continuous variables, and count and percent for categorical variables). Change scores for heart rate, systolic blood pressure, and diastolic blood pressure were calculated by subtracting baseline values (Time −1min) from the testing values (Time 5, 10, 20, 30, 45, 60, and 90min). Random-intercept models with variables for sequence (carry-over) and time effects were used to investigate the association of menstrual phase and depressive symptoms group with serum nicotine, heart rate, systolic blood pressure, and diastolic blood pressure. In subsequent analyses, the depressive symptoms group-menstrual phase interactions were included in the models. To examine the effect of sex hormone values (estradiol, progesterone, and progesterone/estradiol ratio) on serum nicotine, heart rate, systolic blood pressure, and diastolic blood pressure, the models were rerun replacing menstrual phase with the sex hormone value. The same analysis was done to assess the positive and negative affect outcomes. A total of 52 participants (13 in each phase order/depressive symptoms group) were enrolled to have a power of 0.79 to find a large effect size, f = 0.40, for the main effects and the interaction between menstrual phase and depressive symptoms. The p values less than .05 were deemed statistically significant. No adjustments for multiple comparisons were made. SAS V9.1.3 (SAS Institute, Cary, NC) was used for the statistical analyses.
RESULTS
Study Sample
A total of 52 participants completed the full protocol; however, three participants were excluded from the analysis due to serum hormone levels inconsistent with assigned menstrual phase and two were excluded for not remaining abstinent from nicotine based on serum nicotine samples. Therefore, the final sample includes 47 participants. There were no significant differences in demographics, smoking behavior, cardiovascular variables, or hormone levels by stratification or randomization (Table 1). Overall, correlations between serum nicotine, heart rate, and diastolic blood pressure indicate a dose-related physiological response to nicotine was achieved (Table 2). No significant differences were found for sequence or time effects (data not shown).
Table 1.
Demographics and Baseline Characteristics by Stratification and Randomization (n = 47)a
| Total (n = 47) | No depressive symptoms group | Depressive symptoms group | |||
|---|---|---|---|---|---|
| Follicular phase (n = 13) | Luteal phase (n = 10) | Follicular phase (n = 12) | Luteal phase (n = 12) | ||
| Demographics | |||||
| Age (years) | 29.1±6.8 | 29.5±6.2 | 28.3±7.8 | 29.6±7.1 | 28.8±7.1 |
| Race | |||||
| White (%) | 53 | 46 | 50 | 50 | 67 |
| Black (%) | 34 | 46 | 20 | 33 | 33 |
| Education (≥ high school [%]) | 96 | 100 | 80 | 100 | 100 |
| Smoking behavior | |||||
| Cigarettes/day | 12.5±5.1 | 13.4±6.8 | 11.6±4.9 | 12.7±4.0 | 12.3±4.7 |
| Fagerström scoreb | 4.1±1.8 | 4.4±1.2 | 3.1±2.0 | 4.3±1.9 | 4.4±1.8 |
| Cardiovascular | |||||
| Heart rate (beats/min) | – | 67.5±11.7 | 69.4±8.1 | 66.3±9.2 | 68.5±8.2 |
| Systolic blood pressure (mmHg) | – | 109.6±11.7 | 110.6±11.9 | 106.6±10.6 | 113.0±10.4 |
| Diastolic blood pressure (mmHg) | – | 67.0±10.1 | 65.3±6.6 | 65.2±7.3 | 68.2±9.4 |
| Hormones | |||||
| Progesterone (ng/ml) | – | 1.2±1.3 | 12.7±6.1 | 0.7±0.2 | 13.2±5.2 |
| Estradiol (pg/ml) | – | 62.0±32.2 | 113.1±52.5 | 64.4±42.9 | 139.0±55.4 |
| Estradiol/progesterone ratio | – | 95.7±94.8 | 9.9±4.4 | 107.4±109.6 | 13.6±15.5 |
| Affect | |||||
| Positivec | – | 21.8±9.8 | 21.4±3.5 | 16.3±8.2 | 21.9±10.8 |
| Negatived | – | 5.0±6.2 | 7.7±5.9 | 8.8±7.3 | 8.1±6.8 |
aMean ± standard deviation, unless noted.
bRange of sample 0–8 (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991).
cRange of sample 2–42 (al’Absi et al., 1994 ; al’Absi et al., 1998).
dRange of sample 0–24; (al’Absi et al., 1994 ; al’Absi et al., 1998).
Table 2.
Serum Nicotine Concentrations and Cardiovascular Response by Depressive Symptoms Group and Menstrual Phase (n = 47)
| All participants (n = 47) | No depressive symptoms group (n = 23) | Depressive symptoms group (n = 24) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Follicular phasea | Luteal phasea | Phase differencea | Overall averagea | Follicular phasea | Luteal phasea | Overall averagea | Follicular phasea | Luteal phasea | |
| Serum nicotine | |||||||||
| Tmax | 5.1 (0.3) | 5.5 (0.3) | −0.4±0.4 | 5.3 (0.3) | 5.2 (0.4) | 5.4 (0.4) | 5.3 (0.3) | 5.0 (0.4) | 5.7 (0.4) |
| Cmax | 4.2 (0.4) | 3.4 (0.4) | 0.8±0.4* | 3.1 (0.5) | 3.9 (0.6)*** | 2.3 (0.6)*** | 4.4 (0.5) | 4.4 (0.6)*** | 4.5 (0.6)*** |
| Cardiovascular response | |||||||||
| HRb | 11.2 (1.4) | 8.8 (1.4) | 2.4±1.3** | 9.8 (1.8) | 12.2 (2.0)**** | 7.3 (2.0)**** | 10.4 (1.7) | 10.2 (1.9)**** | 10.5 (2.0)**** |
| SBPb | 5.6 (1.3) | 3.3 (1.3) | 2.4±1.7 | 4.6 (1.4) | 6.5 (1.8) | 2.6 (1.9) | 4.4 (1.4) | 4.8 (1.8) | 4.0 (1.9) |
| DBPb | 5.4 (1.2) | 5.1 (1.2) | 0.2±1.7 | 4.5 (1.2) | 6.2 (1.7)***** | 2.6 (1.7)***** | 6.1 (1.2) | 4.5 (1.7)***** | 7.8 (1.7)***** |
| Correlations | |||||||||
| Cmax − HR | 0.46 | 0.50 | – | – | 0.42 | 0.41 | – | 0.49 | 0.51 |
| Cmax − SBP | 0.12 | 0.16 | – | – | 0.08 | 0.03 | – | 0.17 | 0.19 |
| Cmax − DBP | 0.31 | 0.23 | – | – | 0.31 | −0.21 | – | 0.35 | 0.41 |
| Affect | |||||||||
| Positivec | 1.0 (0.7) | 0.6 (0.7) | 0.3±1.0 | 1.1 (0.7) | 0.8 (1.0) | 1.5 (1.0) | 0.5 (0.7) | 1.2 (1.0) | −0.2 (1.0) |
| Negativec | −1.7 (0.6) | −1.9 (0.6) | 0.2±0.7 | 1.6 (0.6) | −0.8 (0.8)****** | −2.4 (0.8)****** | −2.0 (0.6) | −2.5 (0.8)****** | −1.4 (0.8)****** |
Note. Tmax = time at maximum nicotine concentration (min); Cmax = maximum nicotine concentrations (ng/ml); HR = heart rate (beats/min); SBP = systolic blood pressure (mmHg); DBP = diastolic blood pressure (mmHg).
aMean ± standard error.
bChange from baseline (Time −1) at Cmax.
cChange from baseline (Time −30) at 5min as measured by the Subjective State Scale (al’Absi et al., 1994 ; al’Absi et al., 1998).
*p = .0553; **p = .0749.
Group-phase interactions: ***p = .0216; ****p = .0480; *****p = .0472; ******p = .0665.
Physiological Response to Nicotine by Menstrual Phase
There were no statistically significant differences in serum nicotine values, blood pressure, heart rate, or affect by menstrual phase. However, two trends were noted suggesting that F phase may be associated with higher Cmax nicotine (p = .0553) and heart rate at Cmax (p = .0749; Table 2).
Physiological Response to Nicotine by Depressive Symptoms Group
There were no significant differences in serum nicotine values, heart rate, blood pressure, or affect by depressive symptoms group (Table 2).
Physiological Response to Nicotine by Menstrual Phase and Depressive Symptoms
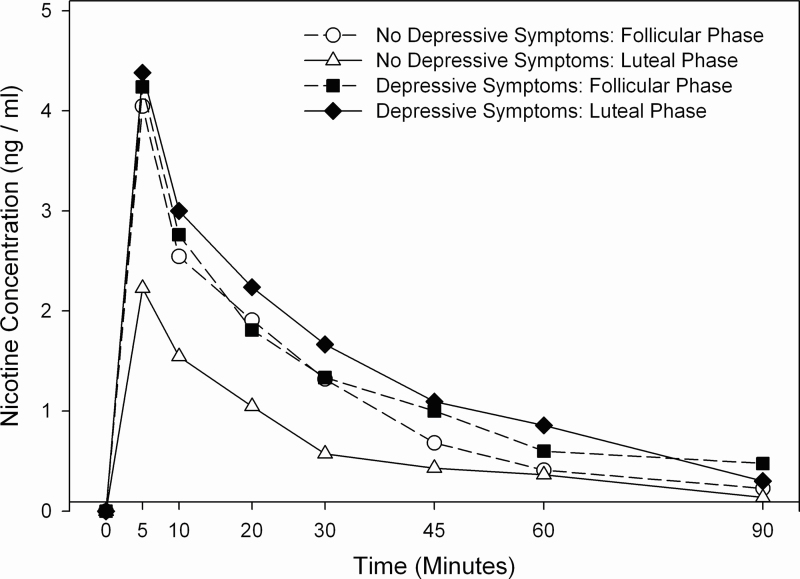
The women in the NDS group had significant menstrual phase differences in their physiological response to nicotine, such that Cmax, heart rate, and diastolic blood pressure were all significantly higher in the F phase compared with the L phase; whereas those in the DS group did not have a significant menstrual phase difference in their physiological response to nicotine (Table 2; Figure 1). A similar relationship was seen in the negative association between nicotine Cmax and progesterone, which was significant in the NDS group (b = −0.13, p < .001), but not in the DS group (b = −0.00, p = .929). Significant associations were also noted between progesterone and heart rate in the NDS group (b = −0.33, p = .014) and between progesterone and diastolic blood pressure in the DS group (b = 0.39, p = .010). Finally, two nearly statistically significant items were noted in affect: (a) the NDS group had a greater decrease in negative affect in the luteal phase than the follicular phase, whereas those in the DS group had a smaller menstrual phase difference (p = .067), and (b) the change in positive affect had a negative association with estradiol in the DS group but not the NDS group (b = −0.02, p = .063; data not shown). No other significant associations were noted between sex hormone levels (estradiol, progesterone, and progesterone/estradiol ratio) and study outcomes (data not shown).
Figure 1.
Mean serum nicotine concentrations (ng/ml) following nasal spray use. Measurements below the limit of quantitation (BLQ) were substituted as follows: 1) first of consecutive BLQ measurements was assigned a value of 1ng/ml with subsequent BLQ measurements assigned a value of 0ng/ml; 2) single BLQ measurements and BLQ measurements at baseline assigned a value of 0ng/ml.
DISCUSSION
This study is the first to systematically investigate the role of menstrual phase and depressive symptoms on the physiological response to nicotine. Contrary to our hypothesis, the physiological response to nicotine did not vary significantly by menstrual phase or depressive symptoms status. However, depressive symptoms status appeared to be a significant effect modifier on the association between menstrual phase and physiological response to nicotine. Specifically, compared with those with depressive symptoms, those without depressive symptoms experienced greater menstrual phase differences in change of heart rate, diastolic blood pressure, maximum concentrations of nicotine and, perhaps, negative affect after using nicotine nasal spray. These data suggest that those without depressive symptoms may be more sensitive to menstrual phase effects on physiological response to nicotine.
The literature is mixed on the effects of menstrual phase on physiological response to nicotine. Two previous studies observed no significant menstrual phase differences in physiological response to nicotine (Hukkanen et al., 2005; Marks, Pomerleau, & Pomerleau, 1999) . However, neither of these studies considered depressive symptoms as a factor. Also, one study assessed the physiological response to nicotine after overnight abstinence in a sample of heavily dependent female smokers (Marks et al., 1999), whereas the other study assessed the physiological response to nicotine in a sample of non-smoking females (Hukkanen et al., 2005). This study expands the literature by assessing physiological response to nicotine after a longer period of abstinence (4 days) in a larger sample of less dependent female smokers with and without depressive symptoms. Although statistical significance was not reached, this study suggests that follicular phase may be associated with higher maximum nicotine concentrations as compared with the luteal phase. Prior research has observed similar findings indicating maximum concentrations of intranasally administered cocaine are higher in the follicular phase compared with the luteal phase (Lukas et al., 1996). The specific explanations for these observations remain unknown. Altered nasal absorption is one possible explanation as changes are known to occur in the nasal mucosa over the course of the menstrual cycle (Taylor, 1961). However, the increases in vascular engorgement of the nasal turbinates that occur in the luteal phase would be expected to result in higher maximum nicotine concentrations during the luteal phase, rather that our observation of higher concentrations in the follicular phase. Further, if this were the explanation, we would also expect to see a similar menstrual phase variation in women with depressive symptoms. Additional research is needed to characterize the mechanisms by which nicotine concentrations differ based on menstrual phase. Studies assessing alternative (i.e., non-nasal) nicotine delivery methods would help determine if these effects are due to alterations in nasal absorption or are indicative of other changes in nicotine pharmacokinetics.
Participants without depressive symptoms experienced a blunted physiological response to nicotine while in the luteal phase. This observation supports some of our earlier work. We previously observed that women experienced a blunted “response bias” (i.e., became less impulsive) while in the luteal phase versus the follicular phase after exposure to a nicotine challenge following 4 days of smoking abstinence (Allen A.M, Allen S.S, al’Absi, & Hatsukami, 2009a). Similarly, women without depressive symptoms had significantly greater smoking satisfaction in the follicular phase than the luteal phase versus women with depressive symptoms. Further, in women with depressive symptoms, no menstrual phase difference in smoking satisfaction was observed and, regardless of menstrual phase, smoking satisfaction was similar to heightened follicular phase levels observed in women without depressive symptoms (Allen, Lunos, & Allen, 2010).
The higher levels of nicotine absorption during follicular phase in women without depressive symptoms may offer additional evidence to explain the seemingly conflicting results of the studies that have assessed smoking cessation outcomes by menstrual phase (Allen et al., 2008; Allen et al., 2009b; Carpenter et al., 2008; Franklin et al., 2008; Mazure et al., 2011). The effectiveness of the nicotine patch may vary by menstrual phase given the differences in nicotine absorption, with higher nicotine levels attained in the follicular phase. Greater absorption of nicotine via the patch during follicular phase may lead to greater efficacy of the patch and less relapse, therefore accounting for lower relapse rates observed during the follicular phase in studies using NRT; whereas in studies without nicotine patch, no protective effects from the NRT are observed leading to higher relapse observed in the follicular phase (Franklin & Allen, 2009). Additional research is needed to test this theory.
This study has several limitations. First, approximately half of our participants had at least one undetectable nicotine concentrations after using the nicotine nasal spray during one of the two laboratory sessions. Although this rate of undetectable levels is similar to previous literature (Gourlay & Benowitz, 1997), it is not clear what effect this may have had on our analyses. One possibility is that there were differences in nicotine concentrations by menstrual phase or depressive status that were not able to be quantified and, therefore, the results underestimate the differences between groups. However, the frequency of undetectable levels did not appear to vary by menstrual phase (F: n = 13; L: n = 16) nor depressive symptoms status (NDS: n = 15; DS: n = 16) . In post-hoc analyses, excluding participants with any undetectable levels of serum nicotine, the overall results were largely unchanged (data not shown). Second, it is unknown how these observations may apply to smoking cessation efforts. It is well known that women receive less benefit from NRT (e.g., Bjornson et al., 1995; Perkins & Scott, 2008). Our results suggest that women may receive less beneficial effects from nicotine replacement during the luteal phase because lower levels of nicotine are achieved, accounting for higher relapse in this phase in studies that have used the nicotine patch. Finally, our results are limited by our relatively small sample size, which limits our power to detect smaller differences in the physiological response to nicotine by menstrual phase and/or depressive symptoms status. Despite these limitations, this study has several strengths including its detailed measurement of menstrual phase, sex hormones, depressive symptoms status, and smoking status. Further, the randomized crossover study design is a strength of this study as this type of design limits the bias and confounding typically occurring in other study designs.
In conclusion, this study provides further evidence for the role of sex hormones and depressive symptoms on physiological response to nicotine in women. Although additional research is needed to further characterize the role of menstrual phase and depressive symptoms on risk for smoking relapse in women, these observations should be taken into consideration in the development of new smoking cessation interventions.
FUNDING
This project was funded by National Institute on Drug Abuse (NIDA) grant R01 DA08075. This publication was made possible by support from the National Center for Research Resources (NCRR) grant M01 RR00400, a component of the National Institutes of Health (NIH). Additional support comes from the National Center for Research Resources (NCRR) grant 1UL1RR033183 and National Center for Advancing Translational Sciences (NCATS) grant 8UL1TR000114-02 of the NIH to the University of Minnesota Clinical and Translational Science Institute (CTSI). The University of Minnesota CTSI is part of a national Clinical and Translational Science Award (CTSA) consortium created to accelerate laboratory discoveries into treatments for patients. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CTSI, NIDA, NCRR, NCATS, or NIH.
DECLARATION OF INTERESTS
None declared.
ACKNOWLEDGMENTS
We would like to thank our research staff—Lindsay Farnsworth, Kathryn Resner, Sara Paradise, Nicole Tosun, Jennifer Widenmier, and Danielle Young—for their dedication to participant recruitment and follow-up, as well as data collection, entry, and management. We would also like to acknowledge the staff at Hennepin County Medical Center Laboratories for analyzing the serum nicotine samples.
REFERENCES
- al’Absi M., Lovallo W. R., McKey B. S., Pincomb G. A. (1994). Borderline hypertensives produce exaggerated adrenocortical responses to mental stress. Psychosomatic Medicine, 56, 245–250 [DOI] [PubMed] [Google Scholar]
- al’Absi M., Lovallo W. R., McKey B., Sung B. H., Whitsett T. L., Wilson M. F. (1998). Hypothalamic-pituitary-adrenocortical responses to psychological stress and caffeine in men at high and low risk for hypertension. Psychosomatic Medicine, 60, 521–527 [DOI] [PubMed] [Google Scholar]
- Allen A. M., Allen S. S., al’Absi M., Hatsukami D. K. (2009a, April). Menstrual phase differences in nicotine response after acute smoking abstinence. Paper presented at the College of Problems on Drug Dependence Annual Meeting, Reno, Nevada Retrieved from http://www.srnt.org/conferences/past/2009/pdf/2009_Poster_Sessions.pdf [Google Scholar]
- Allen A. M., Lunos S., Allen S. S. (2010, February). Reinforcing effects of smoking by depressive symptoms and menstrual phase: Are depressive symptoms an effect modifier?Paper presented at the Society for Research on Nicotine and Tobacco Annual Meeting, Baltimore, Maryland Retrieved from http://www.srnt.org/conferences/past/2010/pdf/2010_SRNT_Proceedings.pdf [Google Scholar]
- Allen S. S., Allen A. M., Lunos S., Hatsukami D. K. (2009b). Patterns of self-selected smoking cessation attempts and relapse by menstrual phase. Addictive Behaviors, 34, 928–931 doi:10.1016/j.addbeh.2009.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S. S., Bade T., Center B., Finstad D., Hatsukami D. (2008). Menstrual phase effects on smoking relapse. Addiction, 103, 809––821 Retrieved from http://dx.doi.org/10.1111/j.1360-0443.2008.02146.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S. S., McBride C. M., Pirie P. L. (1991). The shortened premenstrual assessment form. Journal of Reproductive Medicine, 36, 769––772 Retrieved from http://www.reproductivemedicine.com/ [PubMed] [Google Scholar]
- Anker J. J., Carroll M. E. (2010). Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug and Alcohol Dependence, 107, 264–267 doi:10.1016/j.drugalcdep.2009.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N. L., Bernert J. T., Caraballo R. S., Holiday D. B., Wang J. (2009). Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. American Journal of Epidemiology, 169, 236–248 doi:10.1093/aje/kwn301 [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Lessov-Schlaggar C. N., Swan G. E., Jacob P., III (2006). Female sex and oral contraceptive use accelerate nicotine metabolism. Clinical Pharmacology and Therapeutics, 79, 480–488 doi:10.1016/j.clpt.2006.01.008 [DOI] [PubMed] [Google Scholar]
- Berlin I., Gasior M. J., Moolchan E. T. (2007). Sex-based and hormonal contraception effects on the metabolism of nicotine among adolescent tobacco-dependent smokers. Nicotine & Tobacco Research, 9, 493–498 doi:10.1080/ 14622200701243193 [DOI] [PubMed] [Google Scholar]
- Bjornson W., Rand C., Connett J. E., Lindgren P., Nides M., Pope F. … O’Hara P. (1995). Gender differences in smoking cessation after 3 years in the Lung Health Study. American Journal of Public Health, 85, 223–230 doi:10.2105/AJPH.85.2.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli B., Bock B., King T., Pinto B., Marcus B. H. (1996). The impact of depression on smoking cessation in women. American Journal of Preventive Medicine, 12, 378–387 Retrieved from http://www.ajpmonline.org/ [PubMed] [Google Scholar]
- Carroll M. E., Anker J. J. (2010). Sex differences and ovarian hormones in animal models of drug dependence. Hormones and Behavior, 58, 44–56 doi:10.1016/j.yhbeh.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Carpenter M. J., Saladin M. E., Leinbach A. S., Larowe S. D., Upadhyaya H. P. (2008). Menstrual phase effects on smoking cessation: a pilot feasibility study. Journal of Women’s Health (2002), 17, 293–301 doi:10.1089/jwh.2007.0415 [DOI] [PubMed] [Google Scholar]
- Deecher D., Andree T. H., Sloan D., Schechter L. E. (2008). From menarche to menopause: exploring the underlying biology of depression in women experiencing hormonal changes. Psychoneuroendocrinology, 33, 3–17 doi:10.1016/j.psyneuen.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Dempsey D., Jacob P., Benowitz N. L. (2000). Accelerated metabolism of nicotine and cotinine in pregnant smokers. The Journal of Pharmacology & Experimental Therapeutics, 301, 594–598 doi:10.1124/jpet.301.2.594 [DOI] [PubMed] [Google Scholar]
- Franklin T. R., Allen S. S. (2009). Influence of menstrual cycle phase on smoking cessation treatment outcome: A hypothesis regarding the discordant findings in the literature. Addiction, 104, 1941–1942 Retrieved from http://www.wiley.com/WileyCDA/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. R., Ehrman R., Lynch K. G., Harper D., Sciortino N., O’Brien C. P., Childress , A. R. (2008). Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: a retrospective analysis. Journal of Women’s Health (2002), 17, 287–292 doi:10.1089/jwh.2007.0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay S. G., Benowitz N. L. (1997). Arteriovenous differences in plasma concentration of nicotine and catecholamines and related cardiovascular effects after smoking, nicotine nasal spray, and intravenous nicotine. Clinical Pharmacology and Therapeutics, 62, 453–463 doi:10.1016/S0009-9236(97)90124–7 [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerstrom K. O. (1991). The fagerstrom test for nicotine dependence: A revision of the fagerstrom tolerance questionnaire. British Journal of Addiction, 86, 1119−–1127 doi:10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hitsman B., Borrelli B., McChargue D. E., Spring B., Niaura R. (2003). History of depression and smoking cessation outcome: a meta-analysis. Journal of Consulting and Clinical Psychology, 71, 657–663 doi:10.1037/0022-006X.71.4.657 [DOI] [PubMed] [Google Scholar]
- Hukkanen J., Gourlay S. G., Kenkare S., Benowitz N. L. (2005). Influence of menstrual cycle on cytochrome P450 2A6 activity and cardiovascular effects of nicotine. Clinical Pharmacology and Therapeutics, 77, 159–169 doi:10.1016/j.clpt.2004.10.012 [DOI] [PubMed] [Google Scholar]
- Husky M. M., Mazure C. M., Paliwal P., McKee S. A. (2008). Gender differences in the comorbidity of smoking behavior and major depression. Drug & Alcohol Dependence, 11, 176–179 doi:10.1016/j.drugalcdep.2007.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P., III, Wilson M., Benowitz N. L. (1981). Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. Journal of Chromatography, 222, 61–70 doi:10.1016/S0378-4347(00)81033–6 [DOI] [PubMed] [Google Scholar]
- Jarvis M. J., Tunstall-Pedoe H., Feyerabend C., Vesey C., Saloojee Y. (1987). Comparison of tests used to distinguish smokers from nonsmokers. American Journal of Public Health, 77, 1435–1438 doi:10.2105/AJPH.77.11.1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen J. D., Fortmann S. P., Schatzberg A., Hayward C., Varady A. (2003). Onset of major depression during treatment for nicotine dependence. Addictive Behaviors, 28, 461–470 doi:10.1016/S0306-4603(01)00266-0 [DOI] [PubMed] [Google Scholar]
- Klier C. M., Muzik M., Dervic K., Mossaheb N., Benesch T., Ulm B., Zeller M. (2007). The role of estrogen and progesterone in depression after birth. Journal of Psychiatric Research, 41, 273–279 doi:10.1016/j.jpsychires.2006.09.002 [DOI] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R. L., Williams J. B. (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16, 606–613 Retrieved from http://www.springer.com/medicine/internal/journal/11606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas S. E., Sholar M., Lundahl L. H., Lamas X., Kouri E., Wines J. D. … Mendelson J. H. (1996). Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology, 125, 346–354 doi:10.1007/BF02246017 [DOI] [PubMed] [Google Scholar]
- Lynch W. J., Sofuoglu M. (2010). Role of progesterone in nicotine addiction: evidence from initiation to relapse. Experimental and Clinical Psychopharmacology, 18, 451–461 doi:10.1037/a0021265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J. L., Pomerleau C. S., Pomerleau O. F. (1999). Effects of menstrual phase on reactivity to nicotine. Addictive Behaviors, 24, 127–134 doi:10.1016/S0306-4603(98)00033-1 [DOI] [PubMed] [Google Scholar]
- Mazure C. M., Toll B., McKee S. A., Wu R., O’Malley S. S. (2011). Menstrual cycle phase at quit date and smoking abstinence at 6 weeks in an open label trial of bupropion. Drug and Alcohol Dependence, 114, 68–72 doi:10.1016/j.drugalcdep.2010.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. M., Horton N. J., Monson R. R., Laird N. M., Sobol A. M., Leighton A. H. (2003). Cigarette smoking in relation to depression: historical trends from the Stirling County Study. The American Journal of Psychiatry, 160, 1663–1669 doi:10.1176/appi.ajp.160.9.1663 [DOI] [PubMed] [Google Scholar]
- Perkins K. A., Scott J. (2008). Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine & Tobacco Research, 10, 1245–1250 doi:10.1080/14622200802097506 [DOI] [PubMed] [Google Scholar]
- Pratt L.A., Brody D.J. (2010). Depression and smoking in the U.S. household population aged 20 and over, 2005–2008. NCHS Data Brief, 34, Hyattsville, MD: National Center for Health Statistics. Retrieved from http://www.cdc.gov/nchs/data/databriefs/db34.pdf [PubMed] [Google Scholar]
- Ryan J., Burger H. G., Szoeke C., Lehert P., Ancelin M. L., Henderson V. W., Dennerstein L. (2009). A prospective study of the association between endogenous hormones and depressive symptoms in postmenopausal women. Menopause, 16, 509–517 doi:10.1097/gme.0b013e31818d635f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby P., Hackman R., Kapur B., Klein J., Koren G. (2001). Heavily smoking women who cannot quit in pregnancy: evidence of pharmacokinetic predisposition. Therapeutic Drug Monitoring, 23, 189–191 doi:10.1097/00007691-200106000- 00001 [DOI] [PubMed] [Google Scholar]
- Taylor M. (1961). An experimental study of the influence of the endocrine system on nasal respiratory musoca. Journal of Larynology and Otology, 75, 972–977 Retrieved from http://journals.cambridge.org/action/displayJournal?jid=JLO [DOI] [PubMed] [Google Scholar]
- Wittchen H. U., Robins L. N., Cottler L. B., Sartorius N., Burke J. D., Regier D. (1991). Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The Multicentre WHO/ADAMHA Field Trials. The British Journal of Psychiatry, 159, 645–653 doi:10.1192/bjp.159.5.645 [DOI] [PubMed] [Google Scholar]