Abstract
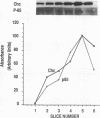
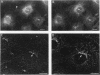
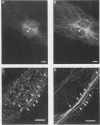
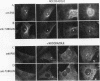
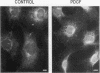
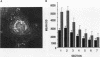
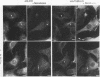
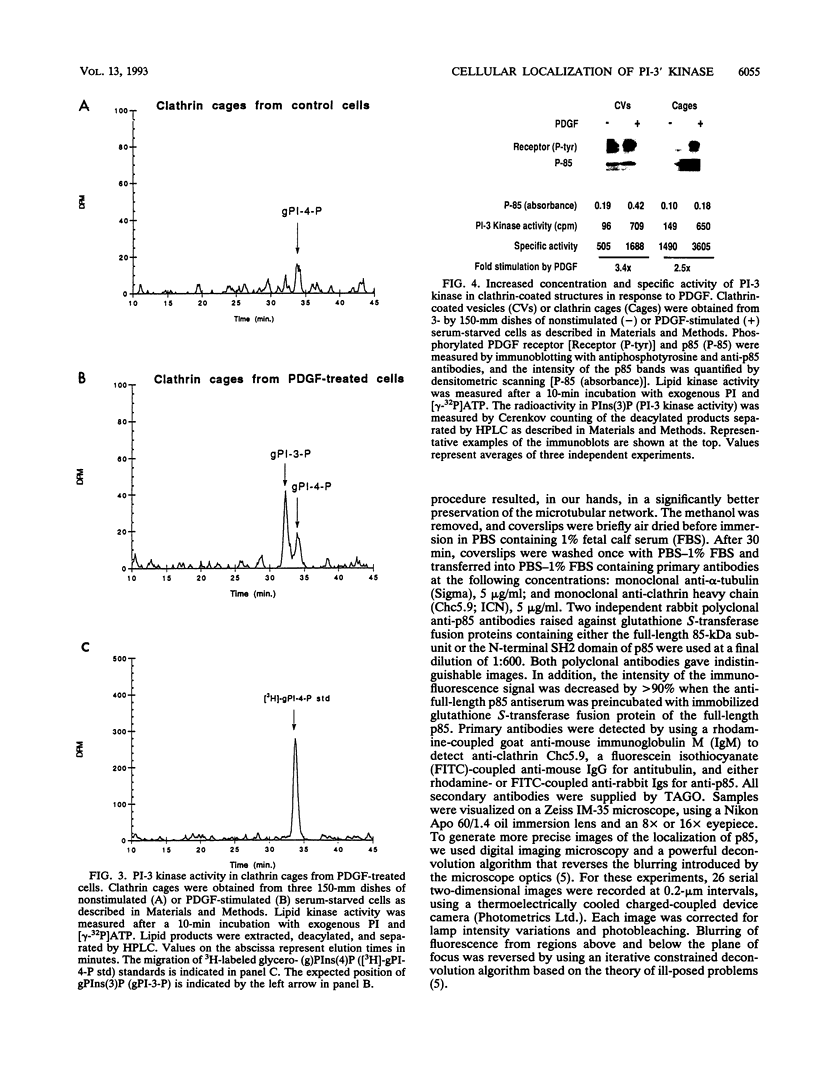
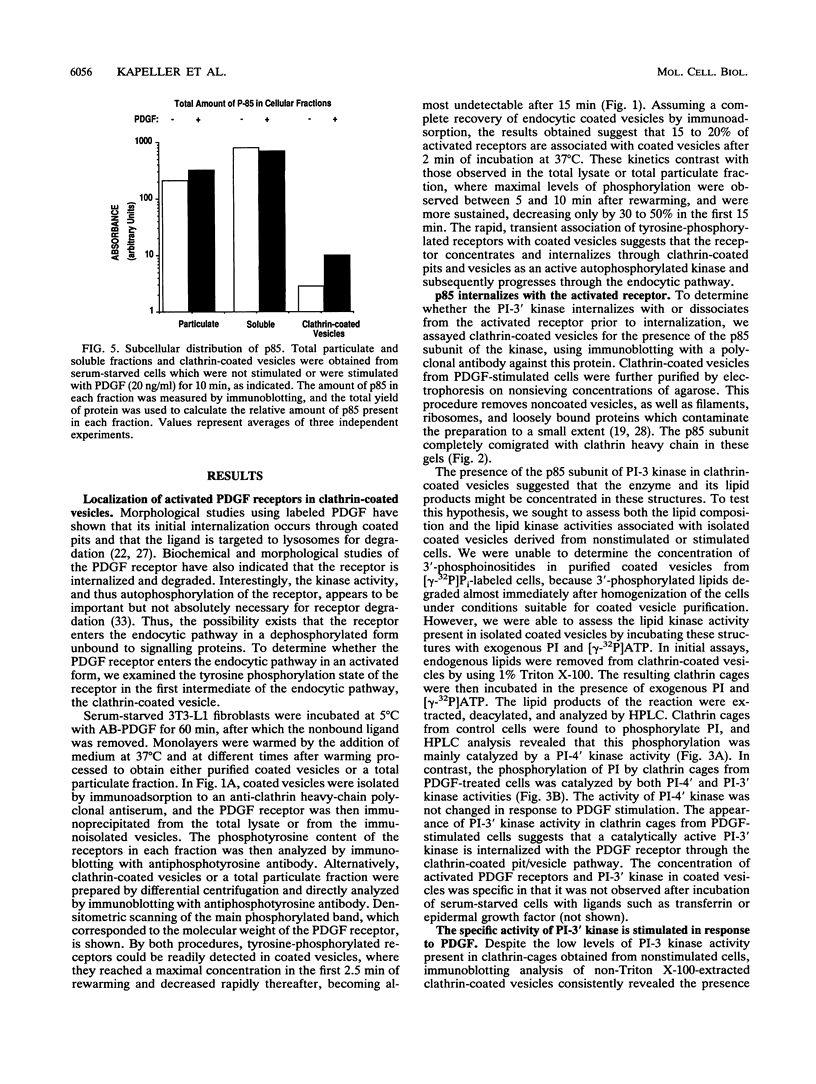
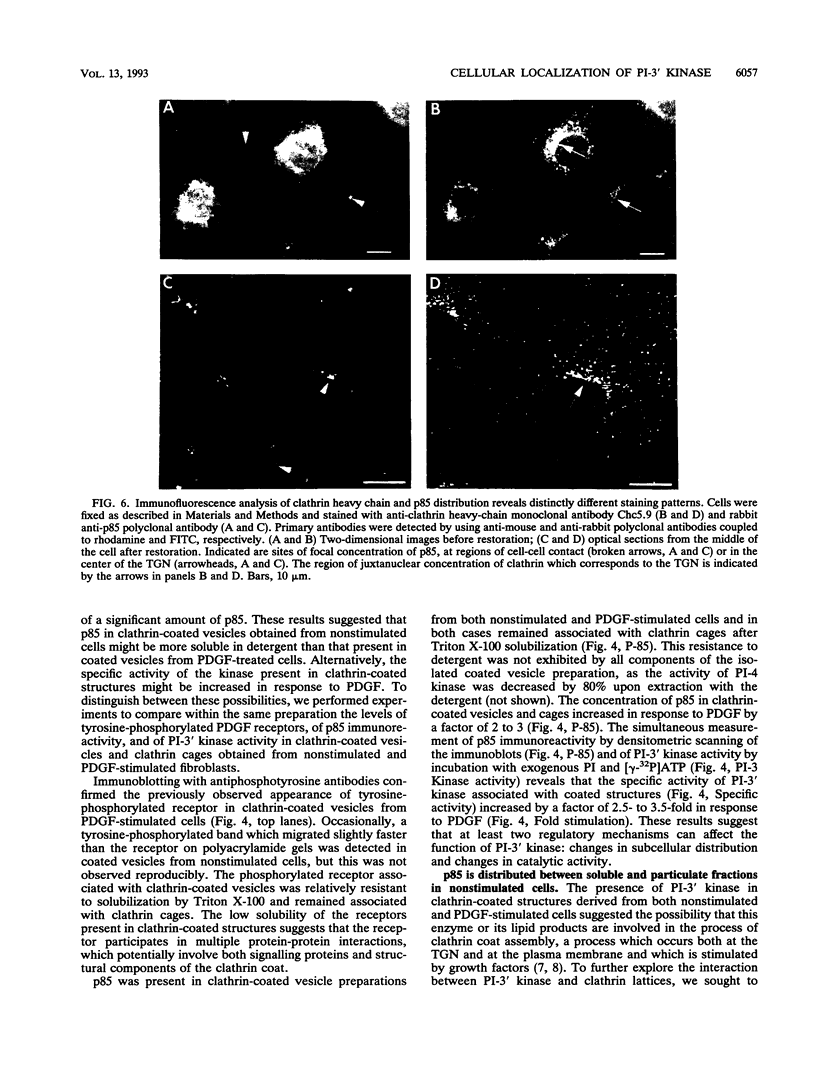
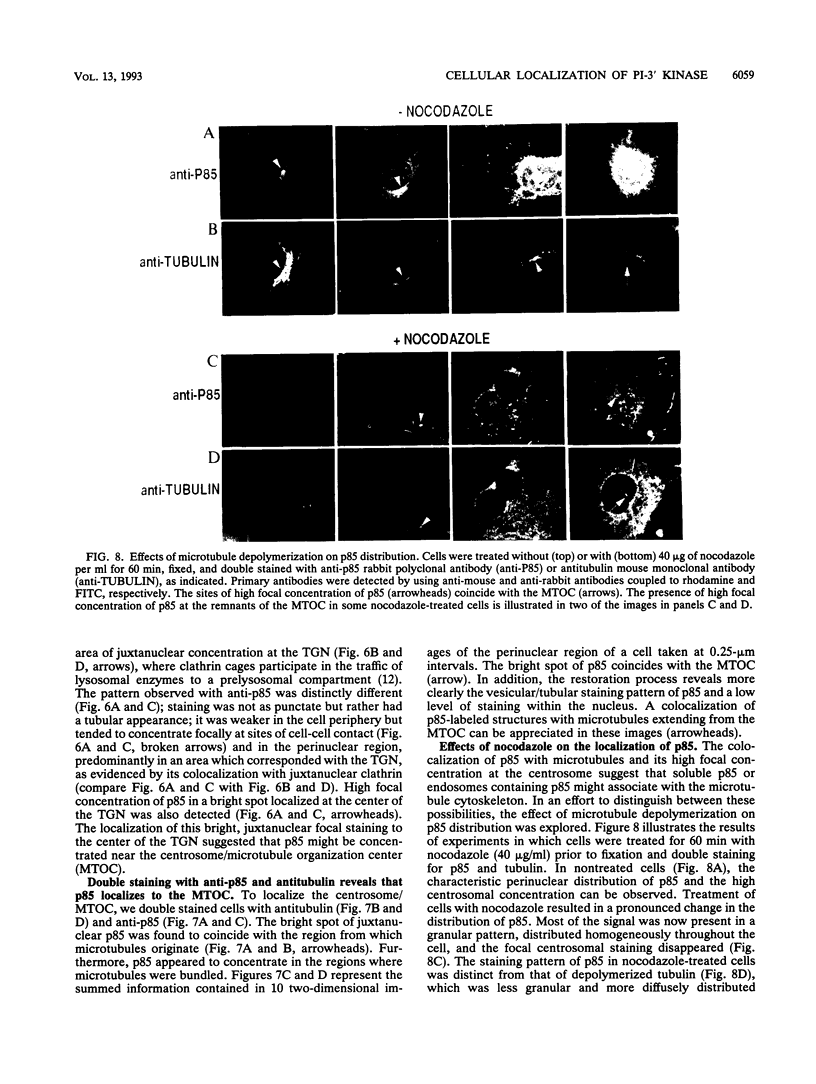
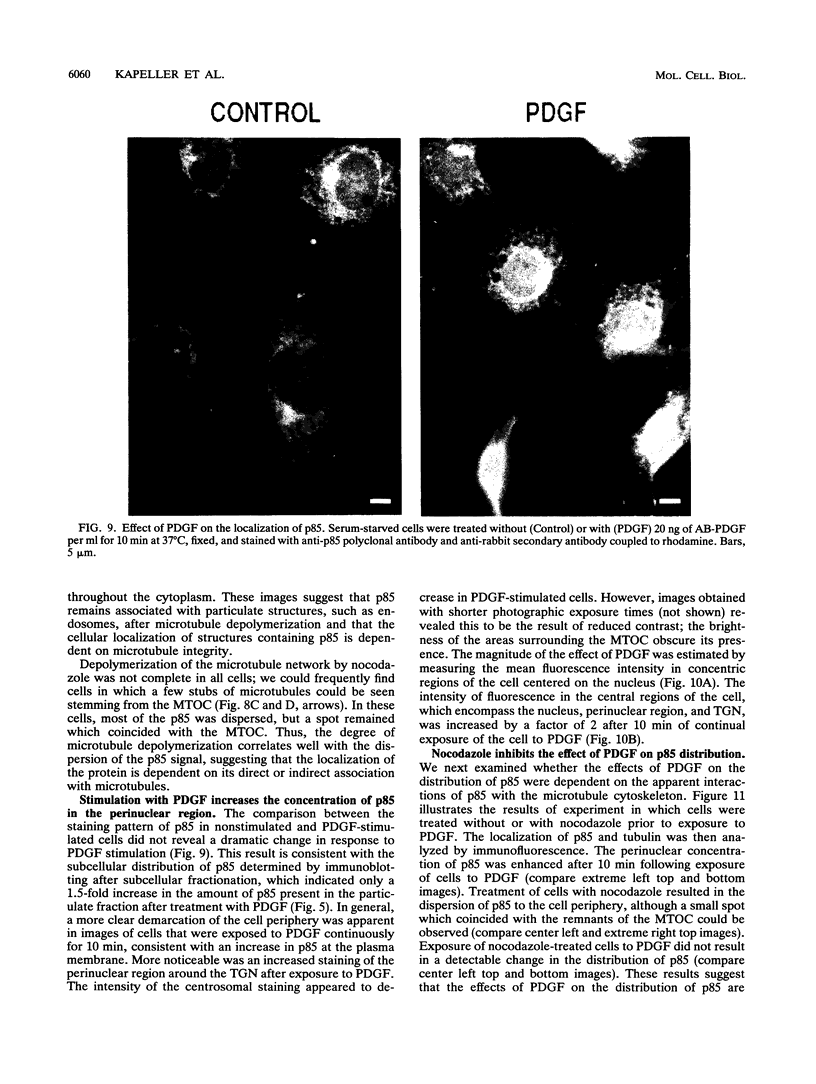
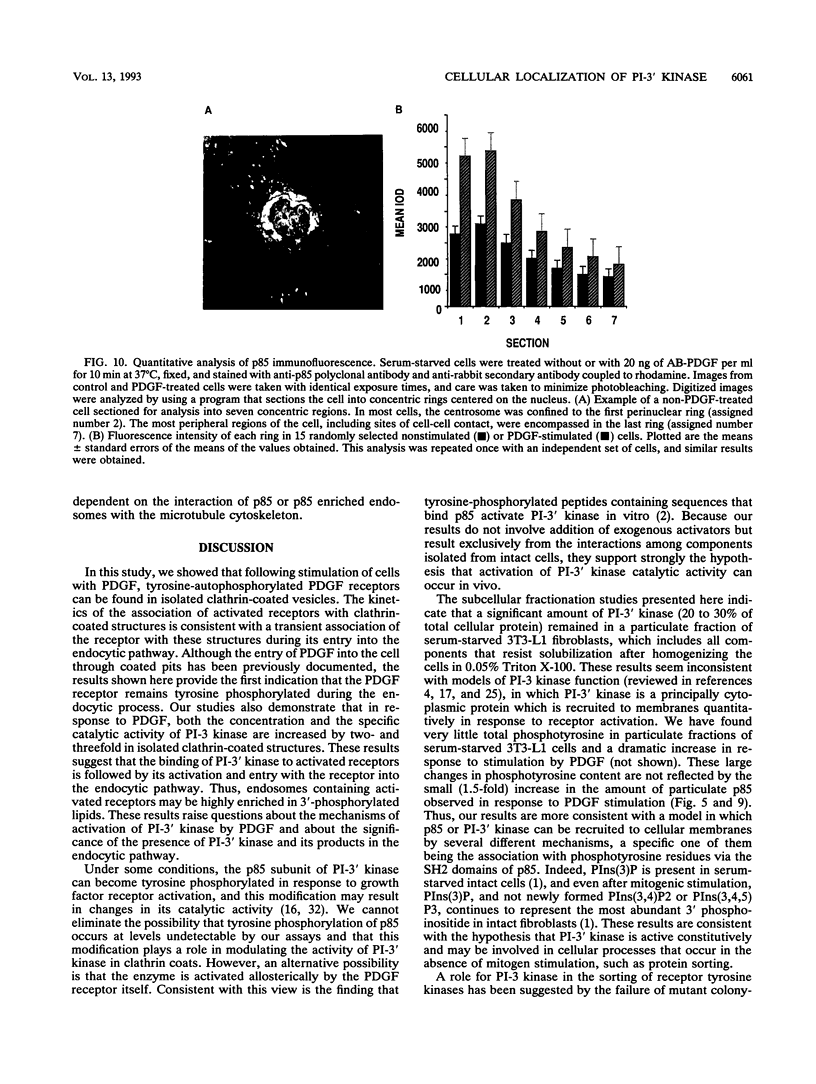
Phosphatidylinositol (PI)-3' kinase catalyzes the formation of PI 3,4-diphosphate and PI 3,4,5-triphosphate in response to stimulation of cells by platelet-derived growth factor (PDGF). Here we report that tyrosine-phosphorylated PDGF receptors, the p85 subunit of PI-3' kinase (p85), and activated PI-3' kinase are found in isolated clathrin-coated vesicles within 2 min of exposure of cells to PDGF, indicating that both receptor and activated PI-3' kinase enter the endocytic pathway. Immunofluorescence analysis of p85 in serum-starved cells revealed a punctate/reticular staining pattern, concentrated in the perinuclear region and displaying high focal concentration at the centrosome. In addition, partial coalignment of p85 with microtubules was observed after optical sectioning microscopy and image reconstruction. The association of p85 with the microtubule network was further evidenced by the microtubule-depolymerizing drug nocodazole, which caused a redistribution of p85 from the perinuclear region to the cell periphery. Interestingly, the most significant effect of PDGF on the distribution of p85 was an increase in the staining intensity of this protein in the perinuclear region, and this effect was eliminated by prior treatment of cells with nocodazole. These results suggest that PDGF receptor-p85 complexes internalize and transit in association with the microtubule cytoskeleton. In addition, the high concentration of p85 in intracellular structures in the absence of PDGF stimulation suggests additional roles for this protein independent of its association with receptor tyrosine kinases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger K. R., Serunian L. A., Soltoff S. P., Libby P., Cantley L. C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989 Apr 7;57(1):167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Myers M. G., Jr, Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J. Phosphatidylinositol 3'-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992 Sep;11(9):3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C., Squicciarini J., Shia M., Pilch P. F., Fine R. E. Identification of a protein kinase as an intrinsic component of rat liver coated vesicles. Biochemistry. 1984 Sep 11;23(19):4420–4426. doi: 10.1021/bi00314a028. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Clark S. W., Meyer D. I. Centractin is an actin homologue associated with the centrosome. Nature. 1992 Sep 17;359(6392):246–250. doi: 10.1038/359246a0. [DOI] [PubMed] [Google Scholar]
- Connolly J. L., Green S. A., Greene L. A. Comparison of rapid changes in surface morphology and coated pit formation of PC12 cells in response to nerve growth factor, epidermal growth factor, and dibutyryl cyclic AMP. J Cell Biol. 1984 Feb;98(2):457–465. doi: 10.1083/jcb.98.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera S., Capocasale R. J. Enhanced phosphorylation of a coated vesicle polypeptide in response to insulin stimulation of rat adipocytes. J Biol Chem. 1990 Sep 15;265(26):15963–15969. [PubMed] [Google Scholar]
- Corvera S. Insulin stimulates the assembly of cytosolic clathrin onto adipocyte plasma membranes. J Biol Chem. 1990 Feb 15;265(5):2413–2416. [PubMed] [Google Scholar]
- Eriksson A., Siegbahn A., Westermark B., Heldin C. H., Claesson-Welsh L. PDGF alpha- and beta-receptors activate unique and common signal transduction pathways. EMBO J. 1992 Feb;11(2):543–550. doi: 10.1002/j.1460-2075.1992.tb05085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo J. A., Navankasattusas S., Kavanaugh W. M., Milfay D., Fried V. A., Williams L. T. cDNA cloning of a novel 85 kd protein that has SH2 domains and regulates binding of PI3-kinase to the PDGF beta-receptor. Cell. 1991 Apr 5;65(1):75–82. doi: 10.1016/0092-8674(91)90409-r. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Hasilik A., von Figura K. Possible pathways for lysosomal enzyme delivery. J Cell Biol. 1985 Dec;101(6):2253–2262. doi: 10.1083/jcb.101.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman P. K., Emr S. D. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Dec;10(12):6742–6754. doi: 10.1128/mcb.10.12.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman P. K., Stack J. H., Emr S. D. An essential role for a protein and lipid kinase complex in secretory protein sorting. Trends Cell Biol. 1992 Dec;2(12):363–368. doi: 10.1016/0962-8924(92)90048-r. [DOI] [PubMed] [Google Scholar]
- Hiles I. D., Otsu M., Volinia S., Fry M. J., Gout I., Dhand R., Panayotou G., Ruiz-Larrea F., Thompson A., Totty N. F. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992 Aug 7;70(3):419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- Kavanaugh W. M., Klippel A., Escobedo J. A., Williams L. T. Modification of the 85-kilodalton subunit of phosphatidylinositol-3 kinase in platelet-derived growth factor-stimulated cells. Mol Cell Biol. 1992 Aug;12(8):3415–3424. doi: 10.1128/mcb.12.8.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Phosphorylation of the PDGF receptor beta subunit creates a tight binding site for phosphatidylinositol 3 kinase. EMBO J. 1990 Oct;9(10):3279–3286. doi: 10.1002/j.1460-2075.1990.tb07527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating M. T., Williams L. T. Processing of the platelet-derived growth factor receptor. Biosynthetic and degradation studies using anti-receptor antibodies. J Biol Chem. 1987 Jun 5;262(16):7932–7937. [PubMed] [Google Scholar]
- Kedersha N. L., Rome L. H. Preparative agarose gel electrophoresis for the purification of small organelles and particles. Anal Biochem. 1986 Jul;156(1):161–170. doi: 10.1016/0003-2697(86)90168-5. [DOI] [PubMed] [Google Scholar]
- Kelly K. L., Ruderman N. B., Chen K. S. Phosphatidylinositol-3-kinase in isolated rat adipocytes. Activation by insulin and subcellular distribution. J Biol Chem. 1992 Feb 15;267(5):3423–3428. [PubMed] [Google Scholar]
- Lees-Miller J. P., Helfman D. M., Schroer T. A. A vertebrate actin-related protein is a component of a multisubunit complex involved in microtubule-based vesicle motility. Nature. 1992 Sep 17;359(6392):244–246. doi: 10.1038/359244a0. [DOI] [PubMed] [Google Scholar]
- Nilsson J., Thyberg J., Heldin C. H., Westermark B., Wasteson A. Surface binding and internalization of platelet-derived growth factor in human fibroblasts. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5592–5596. doi: 10.1073/pnas.80.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu M., Hiles I., Gout I., Fry M. J., Ruiz-Larrea F., Panayotou G., Thompson A., Dhand R., Hsuan J., Totty N. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991 Apr 5;65(1):91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- Panayotou G., Waterfield M. D. Phosphatidyl-inositol 3-kinase: a key enzyme in diverse signalling processes. Trends Cell Biol. 1992 Dec;2(12):358–360. doi: 10.1016/0962-8924(92)90042-l. [DOI] [PubMed] [Google Scholar]
- Pazin M. J., Williams L. T. Triggering signaling cascades by receptor tyrosine kinases. Trends Biochem Sci. 1992 Oct;17(10):374–378. doi: 10.1016/0968-0004(92)90003-r. [DOI] [PubMed] [Google Scholar]
- Pierre P., Scheel J., Rickard J. E., Kreis T. E. CLIP-170 links endocytic vesicles to microtubules. Cell. 1992 Sep 18;70(6):887–900. doi: 10.1016/0092-8674(92)90240-d. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Bowen-Pope D. F., Ross R. Platelet-derived growth factor: morphologic and biochemical studies of binding, internalization, and degradation. J Cell Physiol. 1984 Nov;121(2):263–274. doi: 10.1002/jcp.1041210202. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Fine R. E., Luskey B. D., Rothman J. E. Purification of coated vesicles by agarose gel electrophoresis. J Cell Biol. 1981 May;89(2):357–361. doi: 10.1083/jcb.89.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schu P. V., Takegawa K., Fry M. J., Stack J. H., Waterfield M. D., Emr S. D. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993 Apr 2;260(5104):88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. Mitogenic response to colony-stimulating factor 1. Trends Genet. 1991 Nov-Dec;7(11-12):398–402. doi: 10.1016/0168-9525(91)90263-p. [DOI] [PubMed] [Google Scholar]
- Skolnik E. Y., Margolis B., Mohammadi M., Lowenstein E., Fischer R., Drepps A., Ullrich A., Schlessinger J. Cloning of PI3 kinase-associated p85 utilizing a novel method for expression/cloning of target proteins for receptor tyrosine kinases. Cell. 1991 Apr 5;65(1):83–90. doi: 10.1016/0092-8674(91)90410-z. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., Rabin S. L., Cantley L. C., Kaplan D. R. Nerve growth factor promotes the activation of phosphatidylinositol 3-kinase and its association with the trk tyrosine kinase. J Biol Chem. 1992 Aug 25;267(24):17472–17477. [PubMed] [Google Scholar]
- Sorkin A., Westermark B., Heldin C. H., Claesson-Welsh L. Effect of receptor kinase inactivation on the rate of internalization and degradation of PDGF and the PDGF beta-receptor. J Cell Biol. 1991 Feb;112(3):469–478. doi: 10.1083/jcb.112.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack J. H., Herman P. K., Schu P. V., Emr S. D. A membrane-associated complex containing the Vps15 protein kinase and the Vps34 PI 3-kinase is essential for protein sorting to the yeast lysosome-like vacuole. EMBO J. 1993 May;12(5):2195–2204. doi: 10.1002/j.1460-2075.1993.tb05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker I. U., Rickard J. E., De Mey J. R., Kreis T. E. Accumulation of a microtubule-binding protein, pp170, at desmosomal plaques. J Cell Biol. 1992 May;117(4):813–824. doi: 10.1083/jcb.117.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark B., Siegbahn A., Heldin C. H., Claesson-Welsh L. B-type receptor for platelet-derived growth factor mediates a chemotactic response by means of ligand-induced activation of the receptor protein-tyrosine kinase. Proc Natl Acad Sci U S A. 1990 Jan;87(1):128–132. doi: 10.1073/pnas.87.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]