Abstract
Introduction:
Retrospective research suggests smokers with posttraumatic stress disorder (PTSD) lapse more quickly after their quit date. Ecological momentary assessment (EMA) research is needed to confirm the presence of early smoking lapse in PTSD and form conceptualizations that inform intervention.
Methods:
Smokers with (n = 55) and without (n = 52) PTSD completed alarm-prompted EMA of situational and psychiatric variables the week before and after a quit date, and self-initiated EMA following smoking lapses. Blood samples at baseline and on the quit date allowed assessment of dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEA(S)).
Results:
PTSD was related to shorter time to lapse (hazard ratio [HR] = 1.677, 95% CI: 1.106–2.544). Increased smoking abstinence self-efficacy was related to longer time to lapse (HR = 0.608, 95% CI: 0.430–0.860). Analyses of participants’ real-time reports revealed that smokers with PTSD were more likely to attribute first-time lapses to negative affect (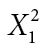 = 5.412, p = .020), and trauma reminders (Fisher’s exact p = .003**). Finally, the quit date decrease in DHEA(S) was related to shorter time to lapse (HR = 1.009, 95% CI: 1.000–1.018, p < .05).
= 5.412, p = .020), and trauma reminders (Fisher’s exact p = .003**). Finally, the quit date decrease in DHEA(S) was related to shorter time to lapse (HR = 1.009, 95% CI: 1.000–1.018, p < .05).
Conclusions:
Results provide evidence of shorter time to first smoking lapse in PTSD, and add to evidence that early lapse occasions are more strongly related to trauma reminders, negative affect, and cravings in smokers with PTSD.
INTRODUCTION
Effective smoking cessation approaches in the general population have had limited success in people with posttraumatic stress disorder (PTSD) (Hapke et al., 2005). Despite low quit rates, smokers with PTSD report desire and intent to quit smoking (Kirby et al., 2008). In addition to increased rates of relapse (i.e., return to baseline smoking levels) following quit attempts, there is initial evidence that smokers with PTSD tend to lapse (i.e., smoke a cigarette) more quickly during quit attempts (Zvolensky et al., 2008). One study found that 94% of smokers with PTSD self-reported lapse within the first week of the quit attempt, compared to 80% of those with no Axis I psychiatric disorder (Zvolensky et al., 2008). These data are based on retrospective report of lapse at the end of the quit week, with no nonpsychiatric comparison group, but they suggest the initial stage of smoking cessation is especially problematic for smokers with PTSD. Early lapse is a significant problem, as evidence suggests that even among nonpsychiatric smokers, those who lapse within the first week of a quit attempt have a greater than 90% chance of fully relapsing to smoking within 6 months (Kenford et al., 1994). Understanding smoking lapse, particularly in psychiatric populations, is vital from both a scientific and a clinical perspective. A better understanding of the processes and factors that provoke lapse could help drive the development of more effective treatments (Piasecki et al., 2000).
Self-efficacy, or the confidence that one has the ability to perform the behaviors necessary to bring about a desired outcome, is a key component of behavior change models (Bandura, 1997) with relevance to smoking cessation (DiClemente, Prochaska, & Gibertini, 1985; Gwaltney et al., 2001). There is some evidence that PTSD is associated with lower perceived self-efficacy (Ferren, 1999), but it is currently unknown whether PTSD is associated with lower self-efficacy for smoking cessation. Studies of relapse situations have consistently documented an association between negative affect and relapse (Piasecki, Jorenby, Smith, Fiore, & Baker, 2003; Piasecki, Kenford, Smith, Fiore, & Baker, 1997), although retrospective report of psychological states experienced during relapse limits inferences. Studies using ambulatory monitoring in smokers not assessed for psychiatric disorders, however, have also supported the relationship between negative affect and relapse (Shiffman et al., 1996).
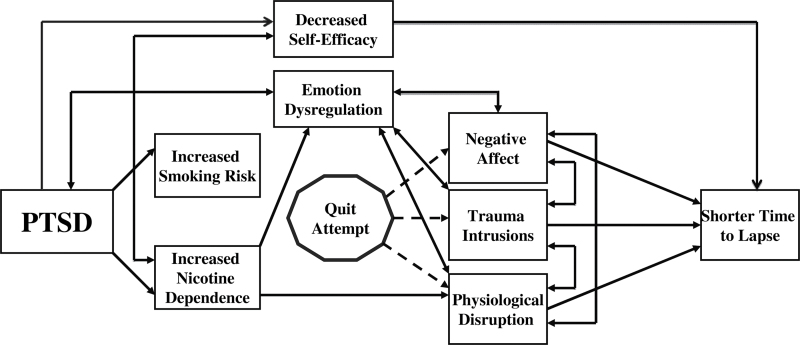
In addition to important roles of self-efficacy and affective states, physiological factors could lead to increased smoking lapse in PTSD. The hypothalamic-pituitary-adrenal (HPA) axis has been implicated in models of smoking onset and maintenance in PTSD (Rasmusson, 2006). Smoking behavior appears to be associated with changes in dehydroepiandrosterone (DHEA) and its sulfated metabolite DHEA(S), which are neuroendrocrine hormones on the HPA axis. Smoking a cigarette results in a significant increase in DHEA/DHEA(S) (Mendelson, Sholar, Goletiani, Siegel, & Mello, 2005), and regular smokers exhibit increased baseline DHEA/DHEA(S) levels (Baron, Comi, Cryns, Brinck-Johnsen, & Mercer, 1995). Physiological models of resilience suggest that DHEA/DHEA(S) has important protective effects against stress (Kroboth, Salek, Pittenger, Fabian, & Frye, 1999). This proposal is supported by the positive effects of DHEA/DHEA(S) on coping in individuals with PTSD (Rasmusson et al., 2004; Yehuda, Brand, Golier, & Yang, 2006) and on decreased negative affect in individuals with depressive disorders (Wolkowitz et al., 1999). The contributions of self-efficacy, affective states, and physiological disruption to smoking lapse are illustrated in Figure 1.
Figure 1.
Model of posttraumatic stress disorder (PTSD) and smoking onset and maintenance.
Further research is needed to characterize the smoking lapse process and investigate lapse occasions using real-time ecological momentary assessment (EMA) methods to minimize retrospective recall bias in psychiatric and situational lapse antecedents. In this study, we hypothesized that (a) the presence of PTSD, lower self-efficacy, and quit date decrease in DHEA/DHEA(S) would be related to shorter time to lapse, (b) smokers with PTSD would be more likely than those without PTSD to cite negative affect and trauma reminders as causes of a smoking lapse, and (c) relative to baseline levels, DHEA/DHEA(S) would decrease on the quit date and would interact with PTSD, such that the decrease in DHEA/DHEA(S) would be larger in the PTSD group.
METHODS
Participants
Participants were smokers with PTSD (n = 52) and a group with no current Axis I psychiatric disorders (n = 55). Eligibility criteria included smoking at least 10 cigarettes daily for the past year, willingness to make a smoking cessation attempt, and age between 18–65. Participants meeting criteria for current alcohol or other substance abuse or dependence, current psychotic disorder (including schizophrenia), or bipolar disorder with active manic symptoms were excluded from both groups. Additionally, potential participants were excluded from either group if they used non-cigarette forms of nicotine, had major unstable medical problems or major respiratory disorders, used bupropion or benzodiazepines, or were residing in a homeless shelter. Participants were eliminated from the comparison group if they met criteria for major depressive disorder, panic disorder, specific phobia, generalized anxiety disorder, obsessive compulsive disorder, bipolar disorder, dysthymia, or an eating disorder.
Procedures
Participants completed a screening session, two smoking cessation counseling sessions based on the National Cancer Institute Fresh Start program (Lando, McCovern, & Barrios, 1990), and 2 weeks of electronic diary (ED) monitoring. Following the quit date, participants returned to the laboratory every other day for bioverification of smoking abstinence by providing expired carbon monoxide (CO) and saliva to be tested for cotinine level.
Screening Session and Diagnostic Assessment
Each participant provided sociodemographic information, smoking history, and completed the Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991). Psychiatric disorders were assessed using the Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 1994) and the Clinician Administered PTSD Scale (CAPS; Blake et al., 1995). Current diagnoses were determined by a 1-month timeframe for PTSD, major depressive episode, and anxiety disorder and a 3-month timeframe for current substance abuse or dependence. Each rater was trained using SCID and CAPS standardized training (i.e., manual, videotapes, and co-rating training with a trained rater). Interrater reliability as determined by Fleiss’ kappa (Fleiss & Cohen, 1973) for diagnoses based on videotapes of patient interviews was excellent (κ = 0.96).
EMA Procedures
EMA data were collected with an ED system on a PalmOne Treo 600 handheld computer (PalmOne, Inc.). EMA data collection procedures were designed for evaluation of the predictors of lapse and participant attributions of lapse. Participants kept the ED with them for 7 days prior to and 7 days after the designated quit date, for a total of 14 days of monitoring. Diary entries were time-stamped to ensure temporal accuracy (i.e., participants could not delay or clump entries) and to assess protocol adherence. Including all skipped and missed alarms, a total of 72.9% of the ED readings occurring between the beginning of the quit attempt and the first lapse were adherent to the protocol, as described below.
At the baseline assessment session, participants watched a 20-min instructional video, received an instruction manual, and received one-on-one training in the use of the ED. Participants practiced diary entries during the session, completed 24hr of practice monitoring, then returned to the laboratory for feedback and instruction regarding their data. During the prequit phase, participants responded to random alarms throughout the day and self-initiated diary entries each time they smoked and before bed. During the postquit phase, participants responded to random alarms throughout the day, and they self-initiated entries for lapses, resisted cravings, and bedtime. Brief assessments only asked whether participants were having a craving, how long the craving lasted, and how long ago it occurred; while full assessments included smoking urge, setting, activity, mood, and PTSD symptoms. ED-initiated alarms were designed to go off randomly between 1 and 3hr after a completed assessment. Following missed or skipped alarms, the next alarm was designed to go off 30–45min later. Participants had a 2-min window after the alarm to begin the assessment. They were instructed to ignore any signal that occurred during an incompatible activity (e.g., driving) and were allowed to suspend prompting when responding would be too costly (e.g., religious services, driving). Additionally, participants were able to delay an assessment with a 5-min delay function. Finally, participants were able to inactivate alarms for 15–120min when they expected to be unavailable and for 4–11hr overnight for sleeping.
Postquit ED assessments began on the participant’s quit date and continued for 1 week. Participants were paid $25 per day for ED monitoring and could earn up to $45 in incentive pay during the postquit week for good adherence (i.e., $25 for not missing more than three alarms in any of the days between sessions; $20 for missing less than three smoking entries during the prequit phase; $20 for completing evening diary assessment each night during the postquit phase). Participants were also paid an additional $25 at each of the postquit visits for remaining abstinent by self-report and CO reading. Participants were paid a total of $750 for their complete participation.
In addition to random ED assessments, participants were asked to initiate their own assessments whenever they lapsed to smoking. This assessment began by asking for smoking duration, time since finishing smoking, and number of cigarettes smoked, followed by a full situational and psychiatric assessment (i.e., PTSD symptom clusters and affect items), then the single lapse factor item asking which of the following variables were related to the smoking lapse: location, relationships to others in the environment, type of activity, presence of positive affect, presence of negative affect, trauma reminders, and cravings. As a check against potential missed assessments, we also evaluated several other sources of information. All ED-initiated assessments specifically asked “Are you currently in the middle of smoking a cigarette?” Each night, participants were asked whether they had a smoking lapse that day on the assessment completed just before going to sleep. Each of the six participants who reported smoking abstinence, but were determined to have lapsed, exceeded the cotinine threshold for smoking abstinence. Their lapse time was assigned based on their first CO reading that exceeded the threshold (>9 ppm) for the two who did so, and the midpoint of the week was the assigned lapse time for the other four.
ED Assessments
All full assessments included common items on smoking urge, setting, activity, mood, and PTSD symptoms.
Setting
Participants reported their current setting (home, friend/family member’s home, work, car/bus, bar/restaurant, outside, or other location). They also recorded the social situation (alone, with family, strangers, coworkers, or friends) and whether others were smoking in view of them (no; yes, in my social group; yes, in view only).
Activity
Participants recorded the activity in which they were engaged (work, leisure, interaction with others, telephone, inactivity, or driving). They also recorded recent consumption of food or drink, coffee or other caffeine, alcohol, and medications.
Affect
Participants rated the severity items from the Positive and Negative Affect Schedule (Watson, Clark, & Carey, 1988; Watson, Clark, & Tellegen, 1988) along with several additional items (e.g., stress, boredom, and alertness) and items from the DSM-IV (American Psychiatric Association, 1994) criteria for nicotine withdrawal (e.g., anxiety, worry, hunger, and restlessness).
Smoking Abstinence Self-efficacy
Smoking self-efficacy was assessed daily with an ED item asking, “Confident in ability to abstain?” scored on a 4-point scale (1 = NO!!, 2 = no??, 3 = yes??, 4 = YES!!) (Shiffman et al., 2000). Smoking abstinence self-efficacy was computed by averaging self-efficacy responses from random ED assessments between the quit date and first lapse, resulting in one self-efficacy mean score for each participant who completed an assessment before lapsing.
PTSD Symptoms
Presence and severity of 13 of the 17 DSM-IV (American Psychiatric Association, 1994) PTSD symptoms were assessed using the Davidson Trauma Scale (Davidson et al., 1997). Frequency was assessed following procedures outlined in our previous work (Beckham et al., 2005). Severity was assessed on a 5-point scale with anchors ranging from “not at all” to “extremely.” This yielded a summary score for trauma-related symptoms at each measurement.
Lapse Factors
When participants indicated their first lapse, it triggered an assessment that included an item asking for their attributed cause of the lapse by stating “What factor(s) do you feel are MOST related to smoking this cigarette? (Check all that apply).” Potential lapse causes included “where you are,” “who you are with,” “what you are doing,” “positive emotion,” “negative emotion,” “trauma symptoms,” and “physical craving.” Items were scored dichotomously. Of the 94 participants who lapsed, 12 reported their first lapse on random alarm assessments, 2 reported first lapse at study visits instead of using the diary, 2 reported first lapse on the evening diary, 5 did not report a lapse but were classified as lapsers based on biological data, and 2 completed lapse readings that were invalid or incomplete. This left 71 participants who self-initiated a full, valid diary assessment of their first lapse.
Biological Measures
Expired CO
The level of CO in the breath of participants was measured with a breath CO monitor at each visit. A level exceeding 9 ppm was considering indicative of smoking.
Salivary Cotinine
Saliva was collected by having participants expectorate into a vial while stimulating saliva flow using methods employed in previous studies (Rose, Levin, & Benowitz, 1993). Saliva samples were frozen and later tested for cotinine assay using gas chromatography, as described by Jacob, Wilson, and Benowitz (1981).
Endocrine Testing
Blood for serum analyses was drawn between 10:00 a.m. and 2:00 p.m. on both the day of screening and the quit date; blood sample donation was voluntary. Fifty-three participants (28 with PTSD and 25 without) provided blood on both dates. Difference scores for both DHEA and DHEA(S) were calculated by subtracting quit date scores from baseline scores. Within 60min of venipuncture, samples were centrifuged at 3,000rpm for 15min and stored at −80 °C until being shipped on dry ice for analysis. Plasma DHEA samples were analyzed in duplicate using the DRG DHEA ELISA Kit, a solid phase enzyme-linked immunosorbent assay (ELISA); intra-assay coefficient of variation (CV) for DHEA was 4.5%. DHEA(S) levels were measured using the ADVIA Centaur DHEAS assay, a competitive immunoassay using direct chemiluminescent technology. class lab DHEA(S) analyses were automated and conducted singly; in-house intra- and interassay CVs for DHEA(S) were between 3.2%–6.5% and 3.3%–5.8%, respectively.
Analysis Plan
Differences in sample characteristics were evaluated using t tests for continuous variables and chi-square tests for nominal variables. To evaluate the association of PTSD diagnosis with smoking lapse, we first calculated a Fisher’s exact test to estimate the impact of PTSD on the risk of any lapse in the first week of the quit attempt, then we used Cox proportional hazard regression models to evaluate the influence of time to lapse. Though using covariates to account for baseline differences between two groups that were not randomly assigned introduces bias that obscures interpretation (Miller & Chapman, 2001), several important variables have established relationships with smoking outcomes, including age, gender, and nicotine dependence. To evaluate the potential impact of important covariates on outcomes, we first examined bivariate relationships between these variables and the outcome to see if they merited inclusion in the models before examining associations of predictors of interest with smoking outcomes. Participant perceptions of the factors most related to the first lapse for which they completed a lapse assessment were analyzed using chi-square tests to determine whether the presence of various situational variables during lapse occasions varied as a function of PTSD status.
RESULTS
Descriptive statistics for demographic, smoking, and ED variables are summarized in Table 1. The only statistically significant between-group difference in baseline variables was noted in the higher nicotine dependence reported by the PTSD group. This baseline difference is consistent with previous research on smokers with PTSD (Hapke et al., 2005). During the postquit week, participants responded to an average of 4.60 (SD = 2.05; range = 0.43–9.67) alarm-prompted assessments per day. Participants completed an average of 4.39 lapse assessments (SD = 5.79; range = 0–32) over the entire postquit week. All participants were able to achieve overnight abstinence verified through CO levels. The criterion for overnight abstinence was based on a previously validated formula (Rose & Behm, 2004) that takes into account baseline CO levels. Of the 107 participants in this analysis, a lapse in the first week following the quit attempt was observed in 94 (88%). In the PTSD group, 49 out of 52 lapsed (94%), compared to 45 out of 55 (82%) in the control group.
Table 1.
Demographic, Psychiatric, and Smoking Variables by PTSD Status
| Variable | PTSD (n = 52) | Control (n = 55) | Significance test |
|---|---|---|---|
| Age (M [SD]) | 42.52 (10.32) | 41.40 (9.57) | t(105) = −0.58 |
| Education (M [SD]) | 12.61 (1.84) | 12.94 (2.86) | t(91.4) = 0.71 |
| Gender (% female) | 29 (56%) | 23 (42%) | χ2 = 2.08 |
| Race (% minority) | 30 (58%) | 37 (67%) | χ2 = 1.05 |
| % Married | 16 (31%) | 12 (22%) | χ2 = 1.11 |
| % Veteran | 13 (25%) | 15 (27%) | χ2 = 1.50 |
| Prelapse self-efficacy | 3.56 (0.59) | 3.57 (0.58) | t(92) = 0.07 |
| Prelapse positive affect | 27.71 (11.11) | 27.04 (10.66) | t(92) = −0.30 |
| Prelapse negative affect | 14.29 (5.78) | 12.27 (4.01) | t(79.8) = −1.96 |
| Baseline nicotine dependence (FTND) | 6.04 (1.98) | 5.25 (2.07) | t(107) = −2.03* |
| DHEA pre/post difference | −0.73 (3.31) | −0.35 (2.40) | t(50) = 0.47 |
| DHEA(S) pre/post difference | −3.16 (18.55) | −7.01 (36.06) | t(33.2) = −0.47 |
Note. DHEA = dehydroepiandrosterone; DHEA(S) = dehydroepiandrosterone sulfate; FTND = Fagerström Test for Nicotine Dependence; PTSD = posttraumatic stress disorder.
Lapse Risk and Time to Lapse From Daily ED Monitoring
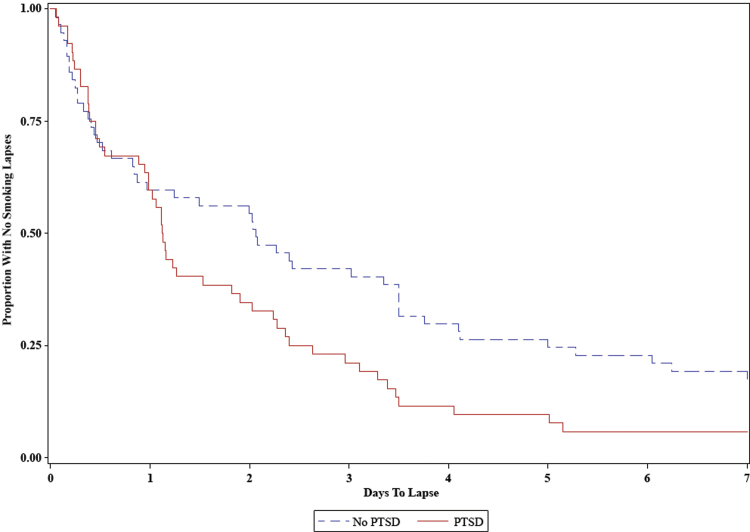
Time to lapse was not related to age (HR = 1.015, 95% CI: 0.994–1.036; OR = 0.990, 95% CI: 0.934–1.049), gender (HR = 1.016, 95% CI: 0.676–1.527; OR = 1.118, 95% CI: 0.349–3.577), or nicotine dependence (HR = 1.078, 95% CI: 0.974–1.192; OR = 0.894, 95% CI: 0.677–1.182). Participants with PTSD had a mean time to lapse of 1.82 days (SD = 1.82) compared to a mean time to lapse of 2.95 days (SD = 2.67) in participants without PTSD. In the models examining relationships of PTSD with smoking lapse, PTSD was not related to increased overall odds of lapse (Fisher’s exact two-sided p = .074), but PTSD was related to quicker time to lapse (HR = 1.677, 95% CI: 1.106–2.544; see Figure 2). The association of PTSD with quicker time to lapse was independent of the effects of nicotine dependence.
Figure 2.
Survival curves for smoking lapse in posttraumatic stress disorder (PTSD) versus non-PTSD in first week of a quit attempt.
In models of self-efficacy predicting odds of smoking lapse and time to lapse, age and gender were not significantly related to time to lapse. In the overall sample, increased self-efficacy was not significantly related to higher overall risk of lapsing (OR = 0.168, 95% CI: 0.025–1.139), but it was related to longer time to lapse (HR = 0.608, 95% CI: 0.430–0.860). Using mean self-efficacy ratings for each participant individually (as opposed to uneven number of data points contributed by each participant) did not change the pattern of results. A follow-up analysis examining a possible interaction between PTSD and self-efficacy for time to lapse revealed no significant difference.
Antecedents From Lapse Situational Assessments
Participants’ real-time attributions of causes of their first smoking lapse are summarized in Table 2. Of the situational variables tested, there were no significant associations of PTSD with location, relationships to others in the environment, type of activity, or presence of positive affect. Participants with PTSD were, however, significantly more likely those without PTSD to report that their first smoking lapse was related to negative affect and trauma reminders.
Table 2.
Odds of Attributing Lapse to a Situational Factor
| Variable | PTSD | Control | Chi-square | Phi coefficient | |
|---|---|---|---|---|---|
| Location | Yes | 14 | 8 |
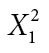 = 1.311 = 1.311 |
φ = 0.136 |
| No | 24 | 25 | |||
| Social environment | Yes | 12 | 9 |
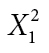 = 0.157 = 0.157 |
φ = 0.047 |
| No | 26 | 24 | |||
| Activity | Yes | 13 | 8 |
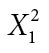 = 1.146 = 1.146 |
φ = 0.109 |
| No | 25 | 25 | |||
| Positive affect | Yes | 3 | 4 |
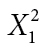 = 0.355 = 0.355 |
φ = −0.071 |
| No | 35 | 29 | |||
| Negative affect | Yes | 22 | 8 |
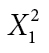 = 8.198** = 8.198** |
φ = 0.340 |
| No | 16 | 25 | |||
| Trauma reminders | Yes | 9 | 0 | Fisher’s p = .003** | n/a |
| No | 29 | 33 | |||
| Cravings | Yes | 28 | 17 |
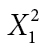 = 3.740 = 3.740 |
φ = 0.230 |
| No | 10 | 16 | |||
Note. PTSD = posttraumatic stress disorder. A positive phi coefficient indicates the extent to which that factor was more likely to be cited by individuals with PTSD as a factor in smoking lapse. Due to a cell size <5, a two-tailed Fisher’s exact test was calculated for the trauma reminders outcome.
*p < .05. **p < .01.
DHEA and DHEA(S) Analysis
Contrary to our hypothesis, there were no significant decreases in DHEA or DHEA(S) on the quit date, relative to baseline levels in either group (see Table 1). However, hazard regression analyses revealed that shorter time to lapse was related to a larger quit date decrease in DHEA(S) (HR: 1.009, 95% CI: 1.000–1.018, p < .05), but not in DHEA (HR: 1.001, 95% CI: 0.906–1.105, p = .99). In follow-up hazard regression analyses examining potential differences by group in the relationship of the quit date DHEA or DHEA(S) difference score and to time to lapse, no significant interactions between PTSD and either variable were detected.
CONCLUSIONS
This study found that the presence of PTSD predicted shorter time to first smoking lapse during a quit attempt. Quicker lapse in PTSD is consistent with previous research using retrospective report that indicated smokers with PTSD lapse more quickly. This study extends that finding by using EMA methods to corroborate study session self-reports and bioverification. In addition, results confirm that smoking abstinence self-efficacy is as an important variable related to short-term abstinence for smokers both with and without PTSD. Finally, the design of the study allowed the unique opportunity to examine real-time participant reports of attributed causes of first smoking lapse. In these analyses, smokers with PTSD were more likely to endorse negative affect and trauma reminders as lapse causes.
The proportions of smokers lapsing in this study (PTSD: 94%; nonpsychiatric group: 82%) are similar to those reported by Zvolensky and colleagues (PTSD: 94%; nonpsychiatric group: 80%). These results are very similar and robust to methodology differences, as the previous study examined a primarily Caucasian sample (93%), used retrospective report of smoking lapse, and investigated self-guided quit attempts, contrasted with the smoking cessation counseling provided in the study reported here.
In this study, predictors such as PTSD and self-efficacy were related to time to lapse, but not to overall risk of lapse during the first week. Since only a few participants in each group remained lapse free, there was very little variance in the outcome variable, resulting in wide confidence intervals that might have influenced the observed results. As indicated in previous studies, PTSD is associated with decreased odds of successful smoking cessation (Hapke et al., 2005), though it is possible that this difference does not emerge in the first week.
The link between trauma reminders and smoking lapse fits conceptualizations of PTSD proposing that trauma-related symptomatology may be a primary reason for smoking lapse (Beckham et al., 2007). Similarly, PTSD is marked by elevations in negative affect (Marshall-Berenz, Morrison, Schumacher, & Coffey, 2011) that are likely related to the inability of many smokers with PTSD to achieve sustained smoking abstinence (Cook, McFall, Calhoun, & Beckham, 2007). Finally, the relationship between cravings and lapse in the PTSD group is consistent with research finding worse smoking withdrawal symptoms, including stronger cravings, in the early stage of abstinence among smokers with PTSD (Dedert et al., 2012).
Analyses of the influence of the difference between baseline and quit date DHEA(S) levels revealed that a larger decrease between baseline and quit date DHEA(S) levels was related to shorter time to lapse. There was, however, no corresponding effect in DHEA. Results suggest that the decrease in DHEA(S) levels on the quit date could be a marker of higher risk for early lapse, though the small sample size means that caution is warranted in evaluating results. Future research could include more measures of DHEA/DHEA(S) and other indicators of HPA axis function throughout the early period of abstinence. Additional research is needed to evaluate DHEA/DHEA(S) as a biomarker for smoking relapse and the utility of DHEA/DHEA(S) supplementation to facilitate smoking cessation.
Study results were limited by the low rate of DHEA/DHEA(S) collection (55% agreed to donate a blood sample at both assessments). Due to the multiple types of readings participants were asked to record, future designs might focus data collection more closely around lapse occasions to reduce participant burden and increase adherence to this critical event. This study was limited by the time to follow-up, as a longer time would allow assessment of relapse (i.e., a return to sustained smoking at baseline levels). Study results are also limited by the lack of a comparison group with non-PTSD psychiatric disorders. As a result, it is unclear whether differences were due to the presence of psychopathology generally, or the effects of PTSD in particular.
Despite its limitations, this study provides important insights into the presence and process of early smoking lapse in individuals with PTSD. The short time to lapse (M = 1 day) and the influences of psychiatric symptoms and craving on early lapse suggest the utility of further research into intensive intervention for managing PTSD and withdrawal symptoms in the first week(s) of smoking abstinence. Elevated smoking abstinence self-efficacy emerged as a significant predictor of longer time to lapse in both groups, supporting its importance in quit attempts. Because theory supports the responsiveness of self-efficacy to successful experiences (Bandura, 1977), smokers might experience a boost in self-efficacy from successfully abstaining from smoking for the first week, suggesting an added benefit of intensive early intervention in the first week of a quit attempt in smokers with PTSD. For example, one study found that smoking abstinence associated with contingency management predicted increases in self-efficacy rather than self-efficacy predicting smoking abstinence (Romanowich, Mintz, & Lamb, 2009). Results of this study suggest that inclusion of smoking cessation intervention components that address PTSD symptoms and negative affect could reduce early smoking lapses. Interventions promoting smoking cessation among individuals with PTSD might also consider more intensive treatment around the time of the quit date, as this is a high risk time for smoking lapse.
FUNDING
This work was supported primarily by the National Institutes of Health Grants 5R01CA081595, 5K24DA016388, R21DA019704, and 1R21CA128965; the Department of Veterans Affairs Office of Research and Development, Clinical Science, and the Mid-Atlantic Mental Illness Research Education and Clinical Center.
DECLARATION OF INTERESTS
None declared.
ACKNOWLEDGMENTS
We would like to thank the participants who volunteered to participate in this study. The views expressed in this presentation are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the National Institutes of Health.
REFERENCES
- American Psychiatric Association (1994). Diagnostic and statistical manual of mental disorders, (4th ed.). Washington, DC: Author; [Google Scholar]
- Bandura A. (1977). Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review, 84, 191–215 [DOI] [PubMed] [Google Scholar]
- Bandura A. (1997). Health promotion from the perspective of social cognitive theory. Psychology and Health, 13, 191–215 [Google Scholar]
- Baron J. A., Comi R. J., Cryns V., Brinck-Johnsen T., Mercer N. G. (1995). The effect of cigarette smoking on adrenal cortical hormones. Journal of Pharmacology and Experimental Therapeutics, 272, 151–155 [PubMed] [Google Scholar]
- Beckham J. C., Dennis M. F., McClernon F. J., Mozley S. L., Collie C. F., Vrana S. R. (2007). The effects of cigarette smoking on script-driven imagery in smokers with and without posttraumatic stress disorder. Addictive Behaviors, 32, 2900–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham J. C., Feldman M. E., Vrana S. R., Mozley S. L., Erkanli A., Clancy C. P., et al. (2005). Immediate antecedents of cigarette smoking in smokers with and without posttraumatic stress disorder: A preliminary study. Experimental and Clinical Psychopharmacology, 13, 219–228 [DOI] [PubMed] [Google Scholar]
- Blake D. D., Weathers F. W., Nagy L. M., Kaloupek D. G., Gusman F. D., Charney D. S., et al. (1995). The development of a clinician-administered posttraumatic stress disorder scale. Journal of Traumatic Stress, 8, 75–80 [DOI] [PubMed] [Google Scholar]
- Cook J. W., McFall M. M., Calhoun P. S., Beckham J. C. (2007). Posttraumatic stress disorder and smoking relapse: A theoretical model. Journal of Traumatic Stress, 20, 989–998 [DOI] [PubMed] [Google Scholar]
- Davidson J. R. T., Book S. W., Colket J. T., Tupler L. A., Roth S., David D., et al. (1997). Assessment of a new self-rating scale for posttraumatic stress disorder: The Davidson Trauma Scale. Psychological Medicine, 27, 153–160 [DOI] [PubMed] [Google Scholar]
- Dedert E. A., Calhoun P. S., Harper L. A., Dutton C. E., McClernon F. J., Beckham J. C. (2012). Smoking withdrawal in smokers with and without posttraumatic stress disorder. Nicotine & Tobacco Research, 14, 372–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiClemente C. C., Prochaska J. O., Gibertini M. (1985). Self-efficacy and the stages of self-change of smoking. Cognitive Therapy & Research, 9, 181–200 [Google Scholar]
- Ferren P. M. (1999). Comparing perceived self-efficacy among adolescent Bosnian and Croatian refugees with and without posttraumatic stress disorder. Journal of Traumatic Stress, 12, 405–420 [DOI] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M., Williams J. B. W. (1994). Structured clinical interview for Axis I DSM-IV disorders, New York, NY: Biometrics Research Department; [Google Scholar]
- Fleiss J. L., Cohen J. (1973). The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educational and Psychological Measurement, 33, 613–619 [Google Scholar]
- Gwaltney C. J., Shiffman S., Norman G. J., Paty J. A., Kassel J. D., Gnys M., et al. (2001). Does smoking abstinence self-efficacy vary across situations? Identifying context-specificity within the Relapse Situation Efficacy Questionnaire. Journal of Consulting and Clinical Psychology, 69, 516–527 [PubMed] [Google Scholar]
- Hapke U., Schumann A., Rumpf H. J., John U., Konerding U., Meyer C. (2005). Association of smoking and nicotine dependence with trauma and posttraumatic stress disorder in a general population sample. Journal of Nervous and Mental Disease, 193, 843–846 [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127 [DOI] [PubMed] [Google Scholar]
- Jacob P., Wilson M., Benowitz N. L. (1981). Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. Journal of Chromatography, 222, 61–70 [DOI] [PubMed] [Google Scholar]
- Kenford S. L., Fiore M. C., Jorenby D. E., Smith S. S., Wetter D., Baker T. B. (1994). Predicting smoking cessation. Who will quit with and without the nicotine patch. Journal of the American Medical Association, 271, 589–594 [DOI] [PubMed] [Google Scholar]
- Kirby A. C., Hertzberg B. P., Collie C. F., Yeatts B., Dennis M. F., McDonald S. D., et al. (2008). Smoking in help-seeking veterans with PTSD returning from Afghanistan and Iraq. Addictive Behaviors, 33, 1448–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroboth P. D., Salek F. S., Pittenger A. L., Fabian T. J., Frye R. F. (1999). DHEA and DHEA-S: A review. Journal of Clinical Pharmacology, 39, 327–348 [DOI] [PubMed] [Google Scholar]
- Lando H. A., McCovern P. G., Barrios F. X. (1990). Comparative evaluation of American Cancer Society and American Lung Association smoking cessation clinics. American Journal of Public Health, 80, 554–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall-Berenz E. C., Morrison J. A., Schumacher J. A., Coffey S. F. (2011). Affect intensity and lability: The role of posttraumatic stress disorder symptoms in borderline personality disorder. Depression and Anxiety, 28, 393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson J. H., Sholar M. B., Goletiani N., Siegel A. J., Mello N. K. (2005). Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology, 30, 1751–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. A., Chapman J. P. (2001). Misunderstanding analysis of covariance. Journal of Abnormal Psychology, 110, 40–48 [DOI] [PubMed] [Google Scholar]
- Piasecki T. M., Jorenby D. E., Smith S. S., Fiore M. C., Baker T. B. (2003). Smoking withdrawal dynamics: III. Correlates of withdrawal heterogeneity. Experimental and Clinical Psychopharmacology, 11, 276–285 [DOI] [PubMed] [Google Scholar]
- Piasecki T. M., Kenford S. L., Smith S. S., Fiore M. C., Baker T. B. (1997). Listening to nicotine: Negative affect and the smoking withdrawal conundrum. Psychological Science, 8, 184–189 [Google Scholar]
- Piasecki T. M., Niaura R., Shadel W. G., Abrams D., Goldstein M., Fiore M. C., et al. (2000). Smoking withdrawal dynamics in unaided quitters. Journal of Abnormal Psychology, 109, 74–86 [DOI] [PubMed] [Google Scholar]
- Rasmusson A. M., Picciotto M. R., Krishnan-Sarin S. (2006). Smoking as a complex but critical covariate in neurobiological studies of posttraumatic stress disorders: A review. Journal of Psychopharmacology, 20, 693–707 [DOI] [PubMed] [Google Scholar]
- Rasmusson A. M., Vasek J., Lipschitz D. S., Vojvoda D., Mustone M. E., Shi Q., et al. (2004). An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology, 29, 1546–1557 [DOI] [PubMed] [Google Scholar]
- Romanowich P., Mintz J., Lamb R. (2009). The relationship between self-efficacy and reductions in smoking in a contingency management procedure. Experimental Clinical Psychopharmacology, 17, 139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. E., Behm F. M. (2004). Extinguishing the rewarding value of smoke cues: Pharmacologic and behavioral treatments. Nicotine & Tobacco Research, 6, 523–532 [DOI] [PubMed] [Google Scholar]
- Rose J. E., Levin E. D., Benowitz N. (1993). Saliva nicotine as an index of plasma levels in nicotine skin patch users. Therapeutic Drug Monitoring, 15, 431–435 [DOI] [PubMed] [Google Scholar]
- Shiffman S., Balabanis M. H., Paty J. A., Engberg J., Gwaltney C. J., Liu K. (2000). Dynamic effects of self-efficacy on smoking lapse and relapse. Health Psychology, 19, 315–323 [DOI] [PubMed] [Google Scholar]
- Shiffman S., Gnys M., Richards T. J., Paty J. A., Hickcox M., Kassel J. D. (1996). Temptations to smoke after quitting: A comparison of lapsers and maintainers. Health Psychology, 15, 455–461 [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L. A., Carey G. (1988). Positive and negative affectivity and their relation to anxiety and depressive disorders. Journal of Abnormal Psychology, 97, 346–353 [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L. A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070 [DOI] [PubMed] [Google Scholar]
- Wolkowitz O. M., Reus V. I., Keebler A., Nelson N., Friedland M., Brizendine L., et al. (1999). Double-blind treatment of major depression with dehydroepiandrosterone. American Journal of Psychiatry, 156, 646–649 [DOI] [PubMed] [Google Scholar]
- Yehuda R., Brand S. R., Golier J. A., Yang R. K. (2006). Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatrica Scandinavica, 114, 187–193 [DOI] [PubMed] [Google Scholar]
- Zvolensky M. J., Gibson L. E., Vujanovic A. A., Gregor K., Bernstein A., Kahler C., et al. (2008). Impact of posttraumatic stress disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine & Tobacco Research, 10, 1415–1427 [DOI] [PubMed] [Google Scholar]