Abstract
We describe the synthesis of a fluorescent deoxyguanosine derivative that co-assembles (in water) with an unlabeled analogue into a heteromeric supramolecular G-quadruplex, which forms a host-guest complex with doxorubicin as evidenced by FRET experiments.
Biological systems from cells to whole organisms remain an open frontier for supramolecular chemistry.1 Developing effective strategies for interfacing the naturally occurring supramolecules with their synthetic counterparts pose both great challenges and opportunities to improve human health. Two key elements for moving forward are the invention of biocompatible systems with the concomitant development of suitable characterization strategies. For example, while NMR still represents an indispensable tool for the characterization of most supramolecular assemblies in solution, the current trend towards increasingly complex supramolecules requires adopting techniques more commonly used in biochemistry and molecular biology.
The Förster Resonance Energy Transfer (FRET) technique has been used extensively in biophysical studies with a wide variety of biological complexes (e.g., DNA-protein, protein-protein) and for the development of biosensors.2 In particular, FRET has been used to study the thermal stability3 of the folding-unfolding of oligonucleotide based (e.g., DNA/RNA) G-quadruplexes (OGQs)4 made from G-rich sequences in the presence of suitable cations. It has also been used to study their function as host molecules when binding to proteins,5 and even small molecule ligands.6
Although the use of FRET remains somewhat limited in the field of supramolecular chemistry, it has, nonetheless, gained increasing prominence after the pioneering reports by the Rebek group.7,8 They validated the versatility of the technique for studying the kinetics and thermodynamics of self-assembled heteromeric supramolecular capsules in the presence of suitable guests. These studies relied on the use of labeled assembling subunits (e.g., calixarenes, resorcinarenes) with appropriate donor-acceptor pairs and/or by using suitable fluorescent guests. In recent years we have developed 8-aryl-2′-deoxyguanonsine (8ArG) derivatives that self-assemble into precise supramolecular G-quadruplexes (SGQs) in both organic and aquatic media.9 SGQs are coaxially stacked planar G-tetramers whose formation is promoted by cations such as potassium.10 Although NMR spectroscopy continues to be essential for studying SGQs, the need to study these systems at lower concentrations and/or in complex environments (e.g., cells, organisms) moved us to develop alternative characterization strategies. Here we report the results of our first attempts towards this goal where a fluorescent 8ArG derivative (1) and the anticancer drug doxorubicin (DOX) act, respectively, as a donor-acceptor pair in a FRET process (Fig. 1).
Fig. 1.
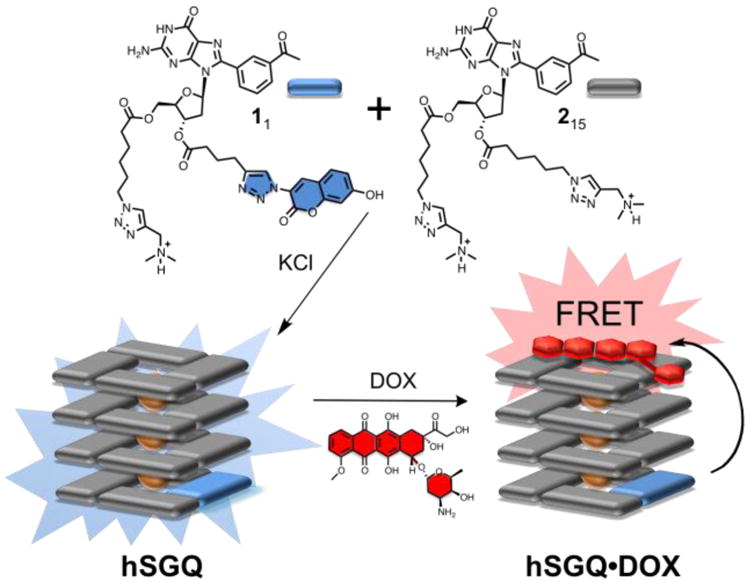
Top: Kekulé structures of the hydrophilic dG derivatives 1 (labeled with a coumarin and represented with a blue rectangle) and 2 (unlabeled and represented with a grey rectangle). Bottom: Cartoon depictions of one of the putative heteromeric hexadecamers (hSGQ), obtained after mixing 1 and 2 in the presence of KCl, and its resulting host-guest complex with the anti-cancer drug doxorubicin (hSGQ•DOX; the cartoon depicts only one of the possible configurations for the complex).
Our strategy relies on the use of the coumarin-labeled 8-(m-acetylphenyl)-2′-dG derivative 1 as the donor component. We have shown9b the m-carbonylphenyl group is an essential element for the high fidelity formation of hexadecameric SGQs. Of the potential fluorophores available, we selected the coumarin moiety because its small size was expected to minimize non-specific interactions and poor aqueous solubility after its conjugation with the 8ArG fragment. Furthermore, in order to maximize the hydrophilicity, we chose to co-assemble 1 with the unlabeled analogue 2 to form heteromeric supramolecular G-quadruplexes (hSGQ) (Fig. 1) with a putative statistical distribution of both subunits. The solvent-exposed (outer) G-tetrads provide a suitable platform to interact with planar aromatic molecules, a fact that has been exploited in the development of ligands for OGQs.4,6,11 Specifically, the Neidle group have reported that the anthraquinone-containing drug daunomycin interacts with OGQs via end-stacking interactions.11 We selected the closely related anthraquinone DOX because of the latter preceden and also because its absorbance spectrum (acting as an acceptor) complements very well the emission of the coumarin moiety in 1 (donor), which would enable the desired FRET studies (ESI†, Figs. S12–S13). Molecular modeling reveal that the donor and acceptor groups could coexist at a distance below the limit required (∼10 nm) to observe FRET even in a configuration (one of the multiple possible) where the coumarin moiety and the DOX are at the opposite ends of the hSGQ (Fig. 2c and ESI†, Fig. S24).11b
Fig. 2.
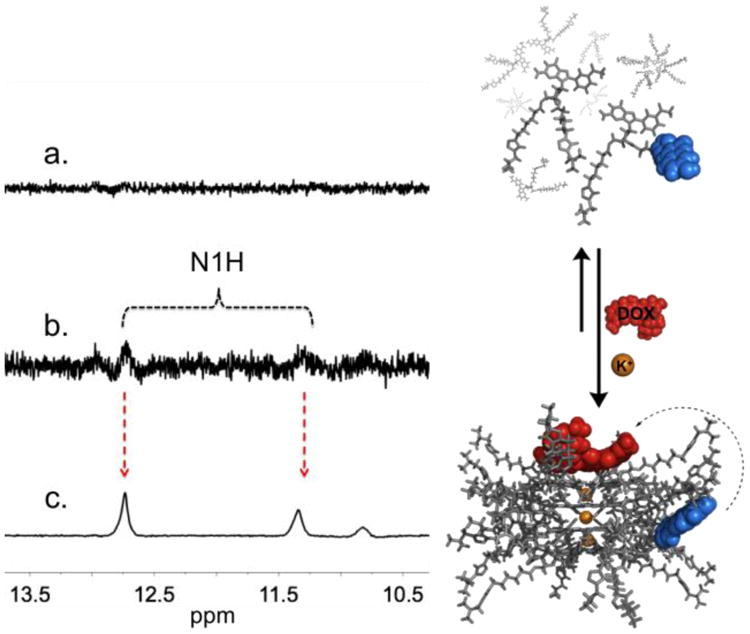
Partial 1H NMR spectra (500 MHz, 10% D2O in LiPB, pH 7.4, 298.2 K) of 5 mM sample with: (a) a mixture of 0.06 equiv of 1 and 0.94 equiv of 2; (b) same as (a) but with 0.06 equiv of DOX, where the N1H peaks (corresponding to the formation of a hexadecamer) begin to show; (c) same as b but with 1 M KCl showing a clearly defined double set of N1H signals. The right panel illustrates the equilibrium between (top) monomeric subunits of 1 and 2 and loosely bound aggregates (LBA); and (bottom) one the potential complexes between DOX and the various possible heteromeric supramolecular G-quadruplexes (hSGQ•DOX).
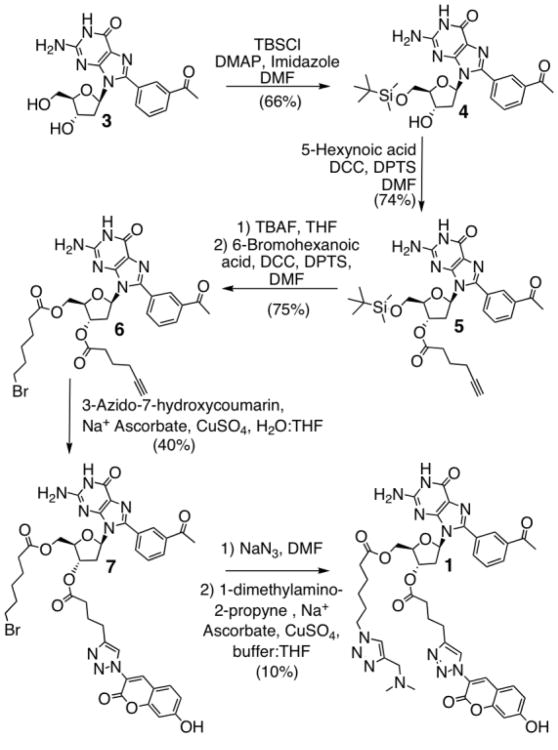
The synthesis of 1 begins with the selective protection of the 5′ hydroxyl group of 3 to generate the tert-butyldimethyl silyl ether 4. Esterification of the 3′ hydroxyl group with 5-hexynoic acid afforded the alkynyl-containing 5, which was converted to 6 after deprotection and subsequent esterification with 6-bromohexanoic acid. Coupling 6 and 3-azido-7-hydroxycoumarin,12 using the copper-catalyzed Huisgen 1,3-dipolar cycloaddition (“click”) reaction, yields compound 7. Finally, displacement of the bromine with NaN3 followed by a second click reaction furnished the desired fluorescent derivative 1 (Scheme 1).
Scheme 1.
Synthesis of coumarin labeled dG derivative 1.
Self-assembly studies were performed by mixing dye 1 (1 equiv) with 2 (15 equiv)9b in a lithium phosphate buffer (LiPB). As expected, the 1H NMR of 1 shows no detectable discrete assembly since lithium only promotes the formation of loosely bound aggregates (LBA) or thermodynamically unstable SGQs (Fig. 2a) (500 MHz, 10% D2O in LiPB, pH 7.4, 298.2 K).13 Nevertheless, the simple addition of the anti-cancer drug DOX (1 equiv) seemed to be enough to induce the stabilization of some LBA and even a small amount of hexadecamers as evidenced by the appearance of signature peaks (e.g., double sets of N1H peaks; Fig. 2b). Furthermore, those signature pairs of peaks increase significantly (to a total of 65%; Fig. 2c) after the addition of KCl (1 M), indicating the formation of robust and discrete hexadecamers, as previously reported for 2 under similar conditions.9b
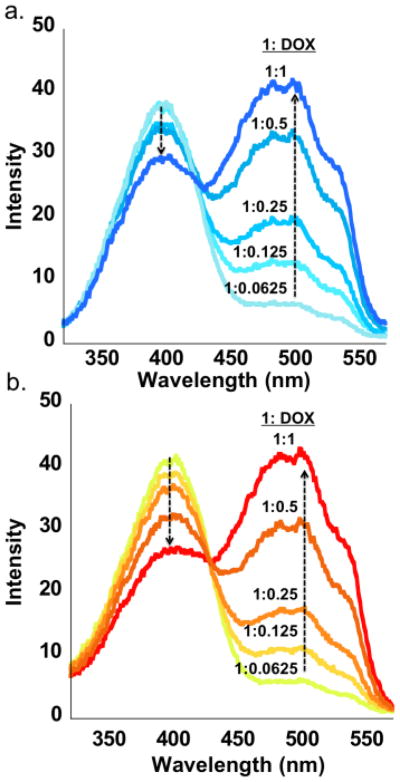
Photophysical characterization of 1 and DOX confirmed that the two compounds could serve as a donor-acceptor pair, due to the excellent overlap between the emission of the former (477 nm) and the absorbance of the latter (490 nm) (ESI†, Fig. S12-S13). FRET titration measurements were performed to study the changes in the emission intensity of the donor (1) following the addition of the acceptor (DOX). Incremental addition of DOX (0.0625–2 equiv) to samples with KCl (hSGQ•DOX = [11215•3K+]•DOX) and without potassium (LBA•DOX) (5 mM, LiPB pH 7.4) showed a linear quenching of the donor emission upon excitation of both samples at 390 nm (ESI†, Fig. S15-S16). The excitation spectra (emission at 590 nm) demonstrate a stronger quenching for hSGQ•DOX when compared to LBA•DOX (Fig. 3). A plot of the excitation intensity of 1 (390 nm) as a function of the concentration of DOX showed a more linear decrease (R2 = 0.98) and a steeper slope for the sample with KCl than for the sample without it (R2 = 0.90) (ESI†, Graph S4). A more linear quenching suggests that the addition of DOX is the main cause for the quenching of 1 while a less linear quenching is suggestive of other factors such as non-specific aggregation. The steeper slope in the presence of KCl hints at a more more efficient energy transfer as would be expected for a well-defined supramolecular complex like hSGQ•DOX.
Fig. 3.
Overlaid excitation spectra (590 nm) of 11215 (5 mM mixture in a LiPB pH 7.4) with increasing amounts of the acceptor DOX showing the quenching of the former (donor) with the concomitant increase intensity for the latter (acceptor) as indicated by the trend arrows; (a) sample without and (b) with 1 M KCl.
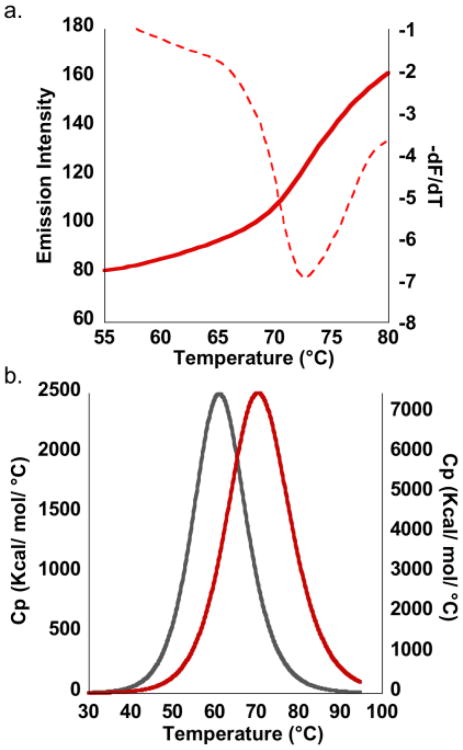
Variable temperature (VT) FRET experiments with hSGQ•DOX resulted in a sigmoidal melting profile with a calculated melting temperature (Tm) of 72.5 °C (Fig. 4a).5 In contrast, similar measurements in the absence of KCl lead to more gradual emission increments of the donor with no significant inflection points (ESI†, Fig. S18). This suggests the molecules of DOX promote the formation of random aggregates in addition to the small amounts of the hexadecamer detected in the NMR experiments (Fig. 2b), even in the absence of KCl. Addition of the potassium salt, as also seen with NMR measurements, displaces the equilibrium further towards the high fidelity formation of hexadecamers (Fig. 2, right panel).
Fig. 4.
Melting profiles for hSGQ•DOX (LiPB pH 7.4) as determined by (a) variable temperature FRET experiments, monitoring the donor emission at increasing temperatures. The reported Tm is the inflection point in the melting profile as determined by the minimum in the first derivative plot (dashed red line). (b) Overlay of the DSC endotherm for hSGQ (grey curve) and for hSGQ•DOX (red curve), showing an enhancement in the thermal stability of the hexadecamer when interacting with the drug.
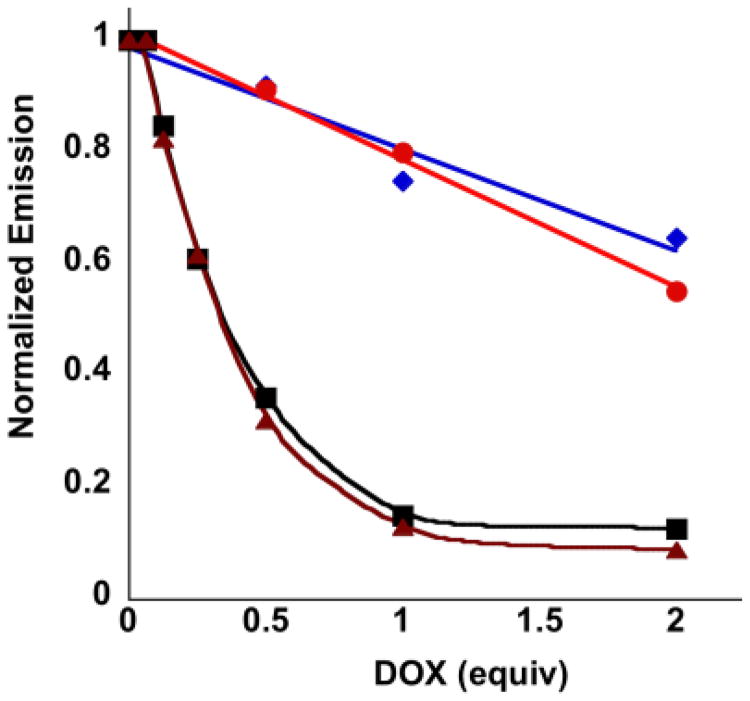
Control experiments were performed in order to evaluate the quenching due to concentration effects. The coumarin derivative 8 (ESI†, Fig. S19)14 has UV/Vis and fluorescence spectra similar to 1 but does not self-assemble because it lacks the guanine moiety. Mixing it with DOX under identical conditions to those used for the aforementioned FRET experiments shows a slight quenching of 8 (Fig. 5) with a slight further quenching after the addition of KCl (ESI†, Fig. S20). In addition, variable temperature experiments for this mixture do not show significant changes in donor emission over the relevant temperature range (ESI†, Fig. S21). These observations reveal that a simple mixture of the donor and acceptor groups is not enough for efficient energy transfer, thus highlighting the essential role of the guanine moiety.
Fig. 5.
Normalized fluorescence emission intensity (471 nm) of 8 (0.312 mM) in either LiPB (red circles) or LiPB with additional 1 M KCl (purple diamonds) as a function of the equivalents of added DOX (excited at 390 nm). The corresponding solutions of 8 mixed with 1 and 2 (5 mM) show significantly lower emissions in both LiPB (black squares) and LiPB with additional 1 M KCl (brown triangles).
Differential Scanning Calorimetry (DSC) experiments for hSGQs reveal an endothermic peak corresponding to a Tm of 61 °C, while similar measurements for the complex hSGQ•DOX indicate a significant increase in the melting temperature (ΔTm = 9 °C; Fig. 4b).15 DSC measurements lead to a value for the Tm having an excellent correlation with the one determined by the VT FRET experiments. These results underscore that the drug is interacting with and stabilizing the hSGQ in a manner reminiscent of that reported for OGQs with small molecule ligands.4,6,11.
We have recently reported the discovery of an amphiphilic hexadecameric SGQ that showed the phenomenon of thermally induced self-assembly known as Lower Critical Solution Temperature (LCST).16a At temperatures above the LCST this SGQ formed microglobules that encapsulated DOX, which we demonstrated by sedimentation experiments and fluorescence microscopy.16b Up until now, we had little evidence to demonstrate the direct interactions between DOX and the SGQs within the microglobules. The results presented here fill that gap, and also open the door to further studies with other biomedically important small molecules or macromolecular guests (e.g., DNA/RNA, proteins), in a manner reminiscent to nucleic acid based supramolecular receptors.5 The results of such studies will be reported in due course.
Supplementary Material
Acknowledgments
This research was financially supported by the NIH-SCoRE (Grant No. 5SC1GM093994-02). MMH thanks the NSF-IFN-EPSCoR (01A-0701525) and the Alfred P. Sloan Foundation for graduate fellowships. JCR thanks NIH-MARC (5 T34 GM007821) program for an undergraduate fellowship.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental details, NMR spectra and characterization for new compounds, photophysical characterization and emission data. See DOI: 10.1039/b000000x/
Footnotes should appear here. These might include comments relevant to but not central to the matter under discussion, limited experimental and spectral data, and crystallographic data.
Notes and references
- 1.Steed JW, Atwood JL. Supramolecular Chemistry. John Wiley & Sons, LTD; Chichester: 2000. [Google Scholar]
- 2.Lakowicz JR. Principles of Fluorescence Spectroscopy. Springer; New York: 2006. [Google Scholar]
- 3.Rachwal PA, Fox KR. Methods. 2007;43:291–301. doi: 10.1016/j.ymeth.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Neidle S, Balasubramanian S. Quadruplex Nucleic Acids. The Royal Society of Chemistry; Cambridge: 2006. [Google Scholar]
- 5.Margulies D, Hamilton AD. Angew Chem Int Ed. 2009;48:1771–1774. doi: 10.1002/anie.200804887. [DOI] [PubMed] [Google Scholar]
- 6.Benz A, Singh V, Mayer TU, Hartig JS. Chem Bio Chem. 2011;12:1422–1426. doi: 10.1002/cbic.201100094. [DOI] [PubMed] [Google Scholar]
- 7.Castellano RK, Craig SL, Nuckolls C, Rebek J., Jr J Am Chem Soc. 2000;122:7876–7882. [Google Scholar]
- 8.Barrett ES, Dale TJ, Rebek J., Jr J Am Chem Soc. 2007;129:3818–3819. doi: 10.1021/ja0700956. [DOI] [PubMed] [Google Scholar]
- 9.SGQs refers exclusively to homomeric assemblies as the ones described in: Gubala V, Betancourt JE, Rivera JM. Org Lett. 2004;6:4735–4738. doi: 10.1021/ol048013v.García-Arriaga M, Hobley G, Rivera JM. J Am Chem Soc. 2008;130:10492–10493. doi: 10.1021/ja8039019.Betancourt JE, Rivera JM. Org Lett. 2008;10:2287–2290. doi: 10.1021/ol800701j.Rivera-Sánchez MdC, Andujar-de-Sanctis I, Garcia-Arriaga M, Gubala V, Hobley G, Rivera JM. J Am Chem Soc. 2009;131:10403–10405. doi: 10.1021/ja9040384.Rivera LR, Betancourt JE, Rivera JM. Langmuir. 2011;24:1409–1414. doi: 10.1021/la103961m.
- 10.(a) Davis JT, Spada GP. Chem Soc Rev. 2007;36:296–313. doi: 10.1039/b600282j. [DOI] [PubMed] [Google Scholar]; (b) Davis JT. Angew Chem Int Ed. 2004;43:668–698. doi: 10.1002/anie.200300589. [DOI] [PubMed] [Google Scholar]
- 11.(a) Neidle S. Therapeutic Applications of Quadruplex Nucleic Acids. Elsevier; 2012. pp. 67–91. [Google Scholar]; (b) Clark GR, Pytel PD, Squire CJ, Neidle S. J Am Chem Soc. 2003;125:4066–4067. doi: 10.1021/ja0297988. The crystal structure of the complex daunomycin-d(TGGGGT) was used as the reference to model DOX interacting with [11215•3K+]; see ESI†, Fig. S24 for further details. [DOI] [PubMed] [Google Scholar]
- 12.Sivakumar K, Xie F, Cash BM, Long S, Barnhill HN, Wang Q. Org Letters. 2004;24:4603–4606. doi: 10.1021/ol047955x. [DOI] [PubMed] [Google Scholar]
- 13.Lithium phosphate buffer was prepared instead of a PBS, because it is known that small cations such as lithium preclude the formation of stable GQs. Hud NV, Plavec J. In: Quadruplex Nucleic Acids. Neidle S, Balasubramanian S, editors. RSCPublishing; Cambridge: 2006. pp. 100–130.
- 14.Hong V, Presolski SI, Ma C, Finn MG. Angew Chem Int Ed. 2009;48:9879–9883. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Δ Tm = Tm ([11215•3K+]•DOX)- Tm(11215•3K+)
- 16.(a) Betancourt JE, Rivera JM. J Am Chem Soc. 2009;131:16666–16668. doi: 10.1021/ja9070927. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Betancourt JE, Subramani C, Serrano-Velez JL, Rosa-Molinar E, Rotello VM, Rivera JM. Chem Commun. 2010;46:8537–8539. doi: 10.1039/c0cc04063k. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.