Abstract
After cerebral ischemia, disruption and subsequent reorganization of functional connections occur both locally and remote to the lesion. However, the unpredictable timing and extent of sensorimotor recovery reflects a gap in understanding of these underlying neural mechanisms. We aimed to identify plasticity of alpha-band functional neural connections within the perilesional area and the predictive value of functional connectivity with respect to motor recovery of the upper extremity after stroke. Our results show improvements in upper extremity motor recovery in relation to distributed changes in MEG-based alpha band functional connectivity, both in the perilesional area and contralesional cortex. Motor recovery was found to be predicted by increased connectivity at baseline in the ipsilesional somatosensory area, supplementary motor area, and cerebellum, contrasted with reduced connectivity of contralesional motor regions, after controlling for age, stroke onset-time and lesion size. These findings support plasticity within a widely distributed neural network and define brain regions in which the extent of network participation predicts post-stroke recovery potential
Keywords: Stroke, magnetoencephalography, plasticity, motor recovery, brain connectivity
INTRODUCTION
The unpredictable timing and extent of sensorimotor recovery after stroke reflects a fundamental gap in understanding the neural mechanisms underlying this process. Inconclusive evidence supporting many stroke rehabilitative interventions (Langhorne et al., 2009) further highlights a crucial need to define the specific pathophysiological processes underlying sensorimotor recovery.
Several researchers have attempted to refine the projected extent of motor outcomes post-stroke using variables of age, baseline clinical function, and brain structure (Jongbloed, 1986; Luft et al., 2004; Saver et al., 1999; Schiemanck et al., 2006). Of these surrogate markers, the extent of corticospinal tract integrity appears to be the most reliable predictor of recovery (DeVetten et al., 2010; Riley et al., 2011). However, these structural predictors are limited to isolated studies of tract injury and do not reflect the full extent of injury or the underlying influence of functional plasticity as a whole brain network. Advances in functional neuroimaging technology provide the opportunity to assess a baseline of residual neural activity to enhance current structural predictors. Neuroimaging studies using functional magnetic resonance imaging (fMRI) clearly show brain activity associated with movement of the upper extremity after stroke reflects a displacement of activity, excess recruitment, and/or altered hemispheric balance of activity (Calautti et al., 2007; Feydy et al., 2002; Hamzei et al., 2006; Ward et al., 2003). In general, improved sensorimotor function is paralleled by focused activity of the residual ipsilesional motor cortex (Marshall et al., 2000; Ward et al., 2003), which depends, in part, on the integrity of the corticospinal tract (Schaechter et al., 2008; Stinear et al., 2007). Poor recovery, on the other hand, is associated with greater contralesional motor cortex activation (Calautti et al., 2007). Indeed, defining the percent of spared critical functional tissue within the primary motor cortex improves the prediction of recovery (Crafton et al., 2003). Despite these insights, the process of plasticity after stroke is complex and the marked variability in the gradation and predicted extent of recovery remains largely unexplained.
In humans, resting state functional connectivity provides a promising means of assessing the intrinsic transfer of information within a widespread neural network (Fox et al., 2007; Greicius et al., 2003). The temporal correlation between neural or fMRI blood oxygenation level dependent (BOLD) oscillations in distinct brain regions is used as a quantitative index of functional connectivity within neuro-anatomical systems (Fox et al., 2005; Honey et al., 2007; Honey et al., 2009; van den Heuvel et al., 2009). Therefore, characterizing the impact of a stroke-induced lesion on local and remote resting-state connectivity can broaden the understanding of restorative mechanisms after stroke (Guye et al., 2010; James et al., 2009; van Meer et al., 2010; Wang et al., 2010). However, FMRI studies are limited not only by poor temporal resolution of the BOLD signal that renders interpretations in terms of underlying neural oscillations difficult, but also by vascular confounds introduced by stroke (D’Esposito et al., 2003).
With the temporal limitations and vascular confounds of the BOLD signal, magnetoencephalography (MEG) has emerged as a potential alternative with more precise measurements of neural interactions in a millisecond time-scale. MEG is a non-invasive imaging technique that records the magnetic fields arising from electrical activity of the brain. Recently, novel techniques have been derived for estimating functional connectivity from MEG (fcMEG) (Guggisberg et al., 2008). Such neurophysiological metrics of functional connectivity have also been proposed as useful markers of impaired brain states such as Alzheimer’s disease (Stam et al., 2002), multiple sclerosis (Cover et al., 2006), and brain tumors (Guggisberg et al., 2008).
Following stroke, MEG studies provide hints of a disrupted resting neural network with abnormally increased activity in the delta and alpha frequency bands of acute perilesional areas (Butz et al., 2004; Tecchio et al., 2005). However, it remains unknown whether ischemic disruption of functional connections, extending beyond the region of the ischemic core, can predict and account for the large variability in recovery post stroke. Moreover, prior MEG and EEG studies of subjects with stroke have analyzed cortical oscillations within sensor space as opposed to estimated brain source space such as assessed with fcMEG. Thus, interpretation of the underlying brain structures contributing to the mixture of neural oscillations in source space is highly confounded.
In the current study, we used magnetoencephalography (MEG), combined with state-of-the-art brain source reconstruction algorithms and connectivity metrics, to examine disruptions and reorganization of functional connectivity in dominant cortical oscillations in stroke patients. Based on previous evidence, our central hypothesis was that the strength and extent of functional connectivity within the residual regions of a sensorimotor network would be directly related to motor function and could be used as a prognostic indicator for the capacity for neural reorganization post-stroke. We aimed to (1) determine the relationship between sensorimotor recovery and changes in resting-state functional connectivity in the perilesional region; (2) evaluate the spatial distribution of baseline global functional connectivity as a predictor of recovery of upper extremity function; and (3) to identify the predictive value of baseline hand motor connectivity on stroke recovery using MEG task-based functional localization data to delineate a hand motor cortex region of interest (ROI).
METHODS
Participants
Fourteen subjects with a first-ever monohemisperic ischemic stroke in the territory of the middle cerebral artery affecting the motor output of the hand were included. Lesion locations were restricted to the corticospinal pathway. Exclusion criteria were as follows: indwelling metals or medical implants incompatible with MEG or MRI, pathological neurological/physical conditions, other than stroke, impairing function or resulting in pain of the impaired arm and/or other impaired brain function. Participants were assessed twice with 8-12 weeks between visits. Study procedures were approved by the institutional review board at the University of California, San Francisco. All participants provided written, informed consent according to the Declaration of Helsinki prior to study involvement.
Behavioral Assessment
Assessment tools were selected according to a framework of disability - the World Heath Organization International Classification of Functioning, Disability and Health (ICF) (World_Health_Organization, 2001). Level of motor impairment was measured using the upper extremity Fugl-Meyer (FM) score and grip strength as documented with a hand held dynamometer (Jamar). Level of activity was quantified using the timed Wolf Motor Function 6-item subtest as a measure of fine motor function. Level of participation was determined by the modified Rankin scale. Each of these clinical scores demonstrates good to excellent reliability and is valid in a stroke population (Chen et al., 2009; Dong et al., 2006; Platz et al., 2005; Wolfe et al., 1991). To normalize intersubject baseline variability, a change score for each outcome was calculated as an index of the difference between visit 1 and visit 2, divided by the score at visit 1. Directionality of change scores was such that increased scores represented improvement on all clinical tests. These measures each provided a snapshot of functional recovery with correlation between the tests ranging from r=0.37-0.7 and it was only by creating a summary score that we could adequately capture motor performance. Therefore, a composite measure of the above-mentioned change scores was calculated as the average of all scores expressed as a percent of recovery over baseline. A similar composite approach has been reported in previous studies of stroke recovery (Rehme et al., 2011b; Saur et al., 2006; Ward et al., 2003).
MEG/MRI acquisition
Neuromagnetic activity was recorded in a magnetically shielded room using a whole-head 275-axial magnetometer system (Omega 2000; MEG International Services Ltd. (MISL), Coquitlam, BC, Canada) at a sampling rate of 1200Hz. During recording, all subjects were asked to lie supine and keep their eyes closed, while remaining awake during a 4 minute recording session. From this 4 minute recording session, a single 60s segment of the sensor data absent of any artifacts (e.g. sensor noise caused by eye blink, EMG noise, heartbeat, etc.) was selected for source reconstruction and subsequent functional connectivity analysis. Artifact detection was performed visually by removing channels with excessive scatter and removing trials with MEG sensor signal amplitude exceeding 10 pT. The spatiotemporal beamformer, described later as our means of source localization and timing of the MEG signal, are endowed with noise rejection capabilities arising from power lines, electronic equipment, and biological noise such as heartbeat and eye blink. Therefore, for data analysis subsequent to source reconstruction, we did not need to include it as a regressor in our analyses (Nagarajan et al., 2007; Zumer et al., 2007).
For each participant, a high resolution, 3D T1-weighted MP-RAGE sequence was obtained on a 3T Siemens Allegra whole body scanner with the following parameters: TR=2300ms, TE=2.98ms, field of view=256mm, 160 axial slices, slice thickness=1mm, in-plane resolution=256mm. To identify lesion location, lesion volume, and perilesional area, a T2-weighted turbo spin echo sequence was also acquired (TR = 5300ms, TE = 99ms, field of view = 210mm, axial slices=38, slice thickness=3mm, in-plane resolution=256mm). Lesion volume (cm3) and perilesional area were calculated by a neuroradiologist (MB) (Ganesan et al., 1999; Ritzl et al., 2004). A region of interest was drawn around the area judged to be abnormal by manually tracing the outer edge of the hyperintense lesional tissue at each slice of the T2-weighted images. The outer criterion was the border at which the voxels with gray values of normal brain tissue exhibited a stepwise increase to those of the hyperintense infarct lesion. The neuroradiologist was blinded to subject numbers. Lesion masks were then registered in Montreal Neurological Institute (MNI) space using tools from FMRIB Software Library, FSL to create an overlay image of all lesions (Figure 1).
Figure 1. Group lesion overlap maps.
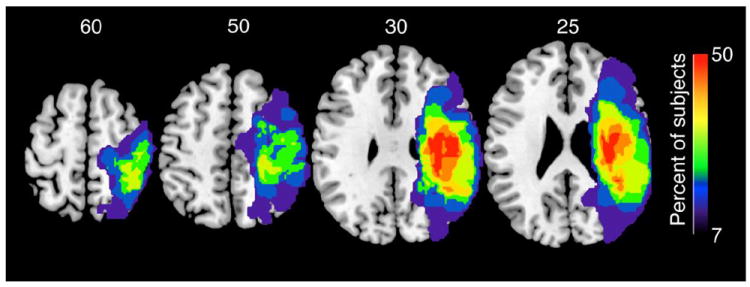
Lesions of all 14 subjects were combined to produce the conjunction maps overlaid on a standardized structural image. Color bar represents the percent of subjects with a lesion at each voxel.
Functional connectivity analysis
A well-established adaptive spatial filtering technique (“adaptive beamformer”) was used to reconstruct a tomographic volume of electromagnetic neural activity at each brain voxel from the MEG sensor data (Dalal et al., 2008). In order to generate these volumes, a fourth order Butterworth filter was applied to the MEG data and a spatial covariance matrix was calculated for the 60s segment of artifact-free sensor data. The lead-field matrix, corresponding to the forward solution for a unit dipole at a particular location, was computed for each brain voxel (generated from each subject’s T1-weighted MRI). Co-registration of structural MRI scans and MEG data was achieved by localizing three fiducials attached to the subjects head (nasion and preauricular points) before and after each MEG scan and marking the same points on the subject’s structural MRI. A spatial weighting matrix was then obtained to estimate signal power in each voxel. A timecourse of activity within each voxel could next be calculated as the linear combination of the spatial weighting matrix of the sensor data matrix. Thus, all sensors contributed to all voxel time series estimates from which functional connectivity is derived.
Functional connectivity was quantified using imaginary coherence (IC), a technique known to overcome spurious correlations in EEG/MEG data due to sensor cross talk and volume conduction artifact (Guggisberg et al., 2008; Nolte et al., 2004) and preserves interactions in the data with a nonzero time lag. A 3D grid of voxels covering the entire brain was created for each subject based on a head model of co-registered structural MR scans. For reconstruction of source time series, we used a broad 1-20hz filter, from which a narrow band of 8-12Hz was selected to capture each subject’s alpha peak for calculation of IC. This bandpass filter setting was chosen to enable sufficient stability of the spatial filter while optimizing the weighting matrix for the narrow band of interest. Mean connectivity at each voxel of interest was estimated by averaging the strength of all temporal correlations (i.e. associated connections), as determined by IC, within the whole-brain sensor array. This metric of mean IC at each voxel will be referred to as fcMEG from this point forward. The units derived from the fcMEG analysis represent the strength of connectivity at a single location with the entire brain. Prior to group analysis, each fcMEG map was spatially normalized to a standard stereotactic atlas (MNI) by applying a transformation matrix derived from each subject’s normalized MRI to the fcMEG volume with the toolbox SPM2.
It should be noted that while abnormalities may exist in other frequency bands (e.g. theta, beta) following stroke, the signal to noise ratio was highest in the 8-12Hz range. Therefore, this peak was readily identified in each individual, consistent with previous reports that alpha power can be isolated in ~95% of individuals (Nunez et al., 2001). Fluctuations in the alpha range are regarded as within the brain’s “resting state” as alpha power is modulated by levels of awareness and visual attention (Nunez et al., 2001).
Because we were interested in assessing subjects at two time points, we report here the results of a test-retest reliability study of fcMEG. Twenty healthy control subjects (mean age = 39, SD= 12) were tested twice with 2-8 weeks between sessions. Reliability was checked for both two recording segments (60s) within a scan session (within-session reliability) and across the baseline and 2-8 week follow up session (cross-session reliability). An intraclass correlation coefficient (ICC) across all elements (voxels) within a specific lobe in each hemisphere was calculated. Results from a fixed-effects model of consistency across averaged measures were used (Cronbach’s alpha; ICCC,K; McGraw and Wong, 1996). Results indicated high reliability for both within-session (mean ICCC,K =0.61) and cross-session (mean ICCC,K=0.64) measurements (Hinkley et al., 2011). ICC values for other frequency bands were found to be significantly lower, hence our focus on alpha-band functional connectivity here.
Statistical Analysis
Analysis 1: Plasticity of perilesional functional connectivity in relation to recovery
We first defined a perilesional region of interest (ROI) by manually delineating the lesion in 3 planes using each subject’s structural scans. Voxels located within 2 cm of this region were also included within the perilesional ROI. We then used two-tailed paired T-tests to compare the mean fcMEG at each voxel within this ROI with the corresponding contralateral voxel within a homotopic ROI. The homotopic ROI is a reflection of the perilesional ROI about the axis of symmetry. Resulting functional maps demonstrated either a relative increase or reduction in perilesional fcMEG. A false discovery rate (FDR) corrected p-value of 0.05 was used.
Next, we calculated the percent of perilesional voxels demonstrating either significant increases or decreases in fcMEG with respect to the contralesional hemisphere. Our index of plasticity (i.e. change in fcMEG) was then derived by subtracting the percentages obtained in visit one from percentages in visit two, normalized to values of visit one. Correlations were then run between individual fcMEG change scores and composite recovery scores.
Finally, as a means to differentiate connectivity changes specific to the perilesional ROI from connectivity changes specific to the homotopic ROI in the contralesional hemisphere, we calculated the fcMEG percent change score within the individual ROIs and correlated this value with recovery scores. Because of the known relationship between time from stroke onset and recovery potential (Duncan et al., 1992), partial correlation coefficients were computed for the above correlation analyses after the variable of time from stroke onset was removed. A two-tailed FDR corrected p-value of <0.05 was considered statistically significant.
Analysis 2: Predictive value of voxel-wise global functional connectivity
To identify the relationship between initial measures of global functional connectivity and behavioral outcomes, we used a voxel-wise partial correlation mapping approach after adjusting for time from stroke onset. Using the residuals from a simple linear regression, partial correlation coefficients were calculated between the global mean fcMEG of each voxel at visit one and the composite recovery score. Images with left hemisphere lesions were flipped along the mid sagittal axis so that the right represented the ‘lesioned hemishere’ in all subjects. To further ensure that results were not influenced by individuals in later post-stroke stages, when recovery slows down, we conducted a subgroup analysis on the 11 subjects who entered the study at less than 4 months. In addition, because of the potential influence of handedness on the extent of motor recovery, a sub-analysis was conducted using only the 9 subjects in whom the non-dominant hand was affected. In order to determine the full spatial extent of our correlation maps, a low FDR corrected threshold of p < 0.1 was used.
Analysis 3: Predictive value of functional connectivity seeded from ipsilesional motor cortex
Following whole-brain, data-driven global functional connectivity analysis, we used a functional localizer to select a more specific region of interest (ROI) of the ipsilesional motor cortex. We aimed to identify which cortical fields are connected to this area and how the strength of this connectivity relates to recovery. The ROI was based on a separate MEG run in which subjects were asked to depress a button every 3 s using the affected index finger for 100 trials. Details of the MEG time-frequency analysis for this motor task have been outlined previously (Dalal et al., 2008). Results of this analysis are shown in Figure 6. The ROI was defined as a cube (2cm3) centered on the voxel of maximal power change within the beta frequency band in the motor cortex at the onset of button press. Beta band was selected as this frequency range is where the majority of activation shifts were found to occur during a motor task. Because of the tendency to activate secondary motor regions during stroke, the point of maximal activation occurred in the premotor cortex in 1 subject and somatosensory cortex in 2 subjects. In the case of the three subjects who could not complete the button press task, the ROI was centered on a normalized stereotactic coordinate over the hand knob region of M1. In the remaining 8 subjects, maximal activation occurred in M1. From this motor cortex ROI, a virtual sensor was generated and fc was then estimated from that virtual sensor and all the other voxels in the brain.
Figure 6.
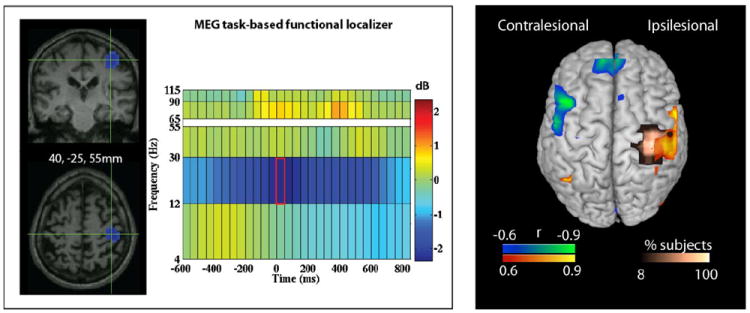
Task-Based Localizer Method and Results of Correlation Analysis. A. Illustrative figure of the 3D activation and associated time-frequency plot of the voxel of maximal power change in the beta frequency band during affected finger button press. A 2cm cube ROI was then centered on each subject-specifc voxel of maximal power change for the correlation analysis. B. Results of the correlation analysis between baseline resting fcMEG within each functionally-defined cube ROI and recovery scores. Gold indicates spatial location of ROI for all subjects combined. Blue indicates negative correlations. Red indicates positive correlations.
Adjustments for lesion size, baseline motor performance, and age did not significantly influence the relationship between recovery and perilesional changes and baseline fcMEG (p>0.05). Therefore, these variables were not included in a regression equation for Analysis 1 or 2. It should also be noted that all of our group analyses assumed a random effects model for subjects.
RESULTS
Participant characteristics
Subject characteristics and initial clinical presentation are shown in Table 1. Three women and 11 men with a mean age of 61 (SD 11) years participated. Lesions involved cortical and subcortical regions related to the motor network including the corticospinal tract (Figure 1). Subjects demonstrated moderate to moderately-severe sensorimotor deficits of the affected upper extremity at the initial measurement as indicated by a mean upper extremity Fugl-Meyer score of 27 (SD 14) out of a possible 66 points. All subjects received outpatient rehabilitation between imaging sessions, which occurred for an average of 1.5 hours, 3 days per week.
TABLE 1.
| Subject | Age (yrs) | Sex | Time Since Stroke (wk) | Affected Hand | Dominant Handa | Lesion Site | Lesion Volume (cm3) | UE Fugl-Meyer (/66)b | Grip Strength (kg)b | Recovery Score (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | M | 12 | L | R | R BG/IC,F,T | 55.97 | 18 | 2 | 40.8 |
| 2 | 59 | F | 9 | L | R | R F,P | 4.27 | 19 | 1 | 46.3 |
| 3 | 55 | M | 16 | L | R | R BG/IC,P | 28.30 | 16 | 1 | 10.9 |
| 4 | 75 | M | 15 | R | R | L BG/IC | 1.60 | 25 | 1 | 26.4 |
| 5 | 79 | M | 4 | L | R | R BG/IC,F | 3.09 | 33 | 3.5 | 80.8 |
| 6 | 68 | M | 16 | L | R | R BG/IC, F | 12.62 | 39 | 13 | 27.7 |
| 7 | 79 | M | 12 | L | R | R F | 16.42 | 23 | 4 | 57.4 |
| 8 | 38 | M | 1 | L | L | R BG/IC | 6.73 | 41 | 23 | 69.8 |
| 9 | 55 | M | 1 | L | R | L BG/IC, F | 55.97 | 37 | 8 | 64.5 |
| 10 | 49 | F | 52 | L | R | R F,P | 20.22 | 13 | 0 | 0 |
| 11 | 55 | M | 32 | R | R | R BG/IC,F | 67.38 | 46 | 25 | 4.7 |
| 12 | 62 | F | 24 | R | R | L BG/IC,F,T | 7.01 | 50 | 19 | 4.6 |
| 13 | 61 | M | 8 | L | R | R F,P, T | 63.27 | 9 | 0 | -2.78 |
| 14 | 64 | M | 12 | R | R | L F, IC | 15.07 | 11 | 1 | 16.36 |
|
| ||||||||||
| mean | 61 | 15 | 25.5 | 27 | 7 | 32.0 | ||||
| SD | 11 | 13 | 24.2 | 14 | 9 | 28.1 | ||||
F=female; M=male; R=right; BG=Basal Ganglia; IC=Internal Capsule; F=Frontal lobe; P=Parietal Lobe; T=temporal lobe; UE = upper extremity; SD = standard deviation;
Handedness based on Edinburgh Handedness Inventory;
Fugl-Meyer and grip strength assessed at initial testing session.
Behavioral assessment
Significant within-group improvements were observed on all behavioral measures (paired T-tests, two-tailed). Upper extremity Fugl-Meyer score (T=-4.8, p=0.001) and grip strength (T=-2.2, p=0.045) increased, while modified Rankin (T= 2.5, p=0.026) and timed wolf-motor function 6-item dexterity subtest (T=2.3, p=0.036) were reduced in favor of recovery. Therefore, the composite recovery score comprised of all outcomes, represented improved overall group outcomes (mean±SD = 33.1± 28.2%).
Plasticity within resting-state perilesional functional connectivity
Within the perilesional area we found a normalization of fcMEG - with respect to the homotopic region of the contralesional hemisphere – that correlated with the clinical recovery of the patients, normalized to baseline performance. That is, the greater the reductions in functional underconnectivity (relative to the contralesional hemisphere) from visit 1 to visit 2, the better the composite clinical recovery score after correcting for months post-stroke (squared partial correlation, pr2= 0.66, p=0.003). Changes in the extent of overconnectivity from visit 1 to visit 2 failed to produce a significant relationship with recovery scores, although a marginal positive trend was identified (pr2= 0.23, p=0.3). To illustrate these findings, three representative subjects are shown in Figure 2a indicating the spatial distribution of perilesional connectivity changes from visit 1 to visit 2. Figure 2b depicts scatterplots relating the connectivity changes with clinical recovery across subjects. Only the relationships with changes in underconnected voxels reached significance.
Figure 2.
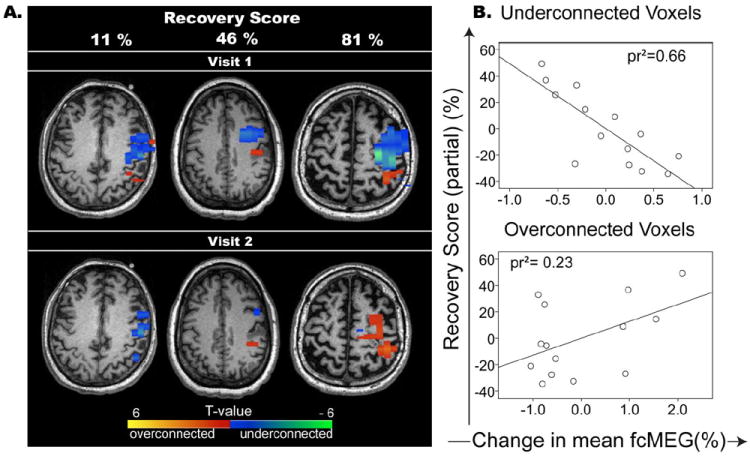
A. Perilesional functional connectivity changes in three representative subjects (subjects 3, 2, and 5, respectively) with small, moderate, and large connectivity differences between visits with respect to homotopic region of contwralesional hemishpere. Blue represents disconnected voxels and orange/red indicates hyperconnected voxels when compared to the homotopic region of the contralesional hemisphere. Color scale represents T-values of hemispheric differences. B. Scatter plots and regression lines representing the squared partial correlation (pr2) of recovery score and changes in disconnected voxels (top) and overconnected voxels (bottom) after correcting for time post stroke.
When analyzing the contralesional and perilesional regions independently, it became apparent that changes in both hemispheres contributed to the above-mentioned results. An overall reduction in fcMEG of voxels within the homotopic ROI of the contralesional hemisphere was identified from visit 1 to visit 2. The mean fcMEG difference between visit 1 and visit 2 was not significant in either ROI (paired T-test, p>0.05). However, the change in fcMEG from visit 1 to visit 2 revealed a significant partial correlation with percent recovery (pr2= 0.38, p=0.025) after correcting for time post stroke, while an increase in fcMEG of voxels within the perilesional area reveled a modest relationship (pr2=0.29, p=0.058) (Figure 3).
Figure 3.
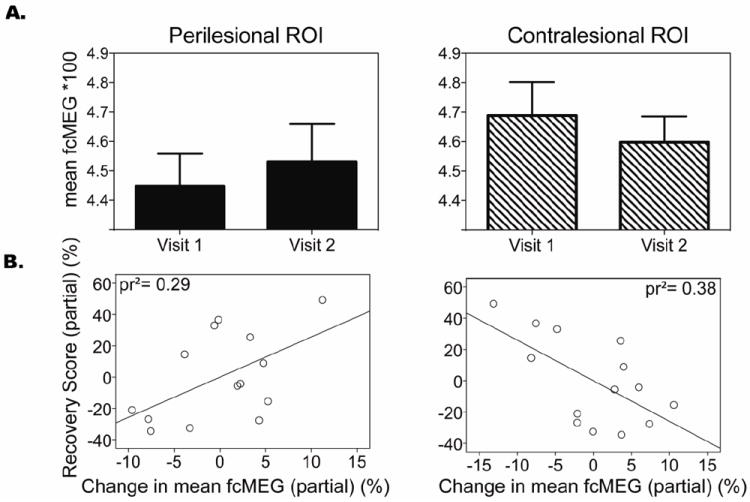
A. Differences in functional connectivity isolated to the perilesional region of interest (ROI) and homotopic contralesional ROI. B. Scatterplots and regression lines indicating the partial correlation (pr2) between changes in functional connectivity and recovery specific to perilesional and the homotopic contralesional ROIs.
Predictive value of resting state functional connectivity
There were significant positive associations between recovery scores and mean fcMEG at visit one in ipsilesional primary motor and somatosentory cortex (BA 3,4, pr2= 0.41, p=0.01) and inferior frontal gyrus after correcting for time post stroke (BA 47, pr2=0.69, p<0.0001). In contrast, negative associations (i.e. low mean fcMEG within these regions correlates with high overall recovery) were found between contralesional sensorimotor cortex (BA 3 and 4, pr2=0.74, p<0.0001), and posterior parietal cortex (BA6, pr2= 0.57, p=0.003) (Figure 4). Both the positive and negative associations were consistently identified even when the subjects were restricted to those in the earlier, <4 month, post-stroke stage (11) and in whom the non-dominant hand was affected (n=9). Figure 5 illustrates the results for the subacute population, in which additional regions of positive correlation emerged in posterior cerebellum (r2=0.65, p=0.0028) and a relationship trend emerged in supplementary motor area (r2=0.36, p=0.05).
Figure 4.
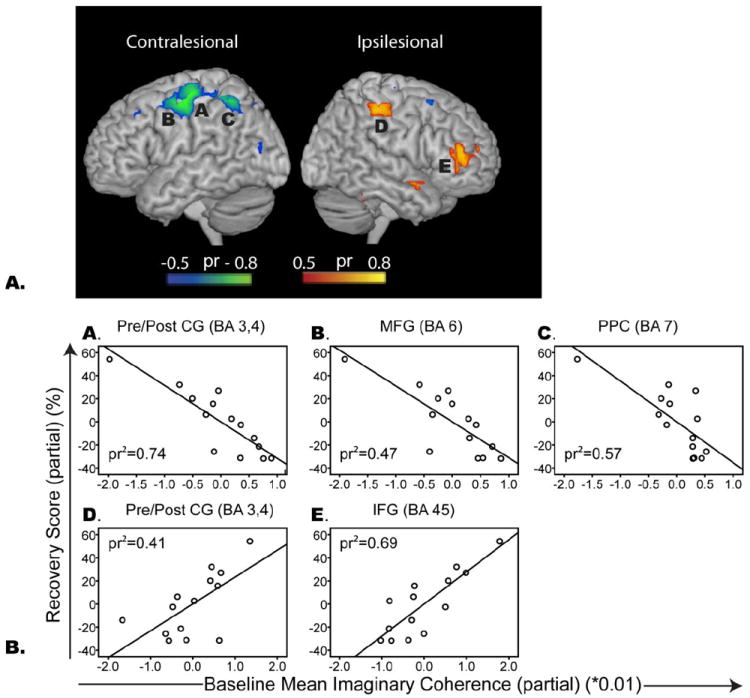
A. Spatial maps illustrating regions of high partial correlation between fcMEG at visit 1 and recovery score (corrected for time post stroke). Red/yellow regions indicate positive associations between baseline fcMEG and recovery. Blue/green regions indicate negative associations. B. Scatter plots representing the squared partial correlation (pr2) between fcMEG at visit 1 and recovery score (corrected for time post stroke). Brodmann’s Area, BA; Pre/post CG, pre/post central gyrus (primary motor and somatosensory cortex); SPL, superior parietal lobe; MFG, middle frontal gyrus; IFG, Inferior frontal gyrus (ventrolateral prefrontal cortex).
Figure 5.
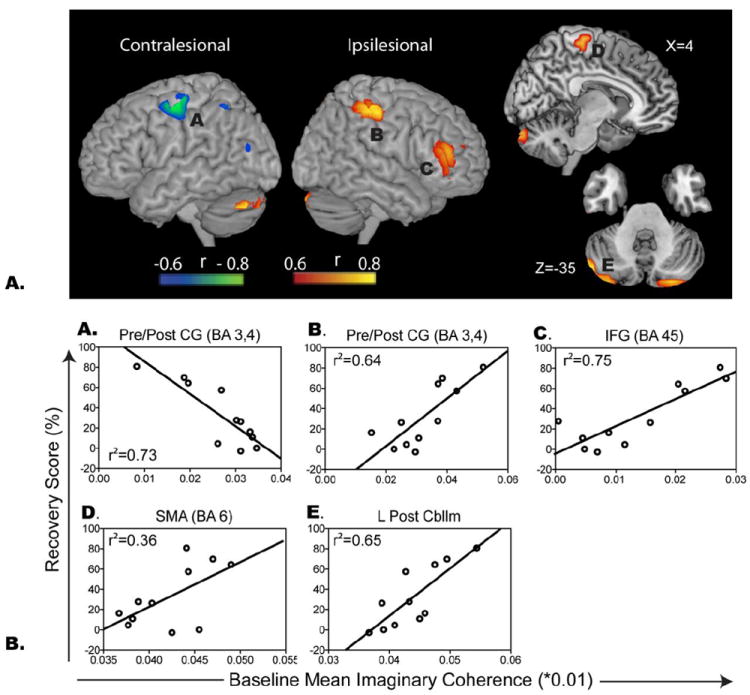
A. Spatial maps illustrating regions of correlation between fcMEG at visit 1 and recovery score restricted to subjects who were less than 4 months post-stroke. B. Scatter plots representing the relationship between fcMEG at visit 1 and recovery score. See Figure 4 for legend.
Using a functionally defined localizer based on a separate MEG motor task run, fcMEG of the ipsilesional motor cortex revealed a similar pattern of recovery-related regions of connectivity. That is, a positive relationship with ipsilesional sensorimotor cortex (BA 3 and 4, pr2=0.55, p=0.002) was identified lateral to the ROI, while a significant negative correlation with contralesional sensorimotor cortex was found (BA 3 and 4, pr2=.71, p=0.0001) (Figure 6).
DISCUSSION
This study demonstrates an important link between resting-state functional connectivity derived from oscillatory neural activity and recovery after stroke that can be clearly interpreted in terms of the underlying neuroanatomy. Although prior studies have used EEG to examine cortical oscillations in stroke, the majority of the analyses used to interrogate these interactions are based on sensor measurements, and the interpretation of the underlying brain structures contributing to these effects can be highly confounded. In contrast, in our paper, we first reconstruct resting-state brain activity from MEG data using novel reconstruction techniques that are robust to noise and interference (adaptive spatial filtering) and then perform functional connectivity assessments on these reconstructed signals using methods that are robust to spurious correlations (imaginary coherence). Equally important is our unique use of an MEG motor task to functionally define an ROI of the ipsilesional motor cortex. Although the motor cortex may be easily identified using anatomical references in healthy adults, the locations of functional activation in a lesioned motor system often varies widely across subjects. By including the results of a motor task paradigm, we effectively defined the reorganized motor output system within which resting state functional connectivity could be derived. Through the above approaches, we are able to directly demonstrate plasticity and predictive value of functional connectivity represented through alpha oscillations between cortical fields in stroke patients, and that this plasticity is primarily derived from the motor network connectivity.
The strength and extent of functional connectivity at each brain voxel was directly related to behavioral deficits and was identified as a prognostic indicator for the capacity for clinical sensorimotor recovery. More specifically, levels of functional connectivity in the perilesional and contralesional hemispheres played divergent roles in the reestablishment of function. These findings were independent of time post stroke, age, and lesion volume. More specifically, plasticity of functional connections leading to and from perilesional and remote contralesional regions was established in relation to baseline assessments. Greater initial functional connectivity, within the ipsilesional primary somatosensory cortex and prefrontal cortex, leads to better recovery 8-12 weeks later. In contrast, the lower the connectivity of the contralesional sensorimotor regions, the better the recovery. A similar focus of activity towards the ipsilesional sensorimotor regions, away from contralesional activation, has been described before in relation to stroke recovery (Calautti et al., 2007; Enzinger et al., 2008), but the underlying network dynamics of this process in the absence of the demand of task performance has not yet been characterized. Here, we discuss these findings in terms of possible contributing mechanisms.
Our first observation concerning the importance of the area surrounding the ischemic core (i.e. surviving neurons within the penumbra) is largely supported by animal models. Experimentally induced ischemic lesions in both primates and rats have convincingly shown significant structural and functional changes of the surrounding intact cortex within hours of changes in external stimuli (Kirkland et al., 2008; Kozlowski et al., 1996; Nudo and Milliken, 1996). Depending on the intensity and time window of such stimuli, resulting brain plasticity may be either maladaptive or adaptive (Kirkland et al., 2008; Kozlowski et al., 1996). In contrast, relatively little is known about the evolution of the network connectivity of surviving perilesional brain tissue in humans. Much of this limitation is due to the difficulty in using a single standardized task to activate an area surrounding widely variable lesion locations. Using a resting state paradigm, we were able to identify perilesional functional connectivity independent of lesion location. We also effectively avoided task-based confounds including selection bias towards those able to perform a standardized task, mirror movements, and increased effort leading to exaggerated activation of the contralesional hemisphere (Sehm et al., 2009; Ward et al., 2007). Future work may extend these findings to test animal models and clarify whether particular time points of intensively applied rehabilitation interventions in humans are detrimental or beneficial to perilesional connectivity patterns.
The resulting negative relationship between sensorimotor recovery and changes in the percent of perilesional functional underconnectivity suggests that, in cases of good recovery, regions of underconnectivity either normalized or became overconnected compared to the homotopic region of the contralesional hemisphere. Although this analysis accounted for changes in connectivity in both hemispheres, our data points to an overall reduction of fcMEG in the contralesional region as the primary contributor to the shift in hemispheric balance in relation to clinical outcomes. This finding is consistent with task-based neuroimaging data suggesting that recovery of affected upper extremity movement occurs as a consequence of bihemispheric reorganization of activity. Activations may initially be lateralized toward the contralesional cortex and later, as functional improvement ensues, shift back toward the ipsilesional cortex (Marshall et al., 2000; Shimizu et al., 2002). Moreover, the changes seen in the extent of underconnectivity arising from the perilesional area, while smaller than contralesional changes, are in line with a recent report of the normalization of white matter connectivity following an initial disruption at lesion border zones (van der Zijden et al., 2008). One interpretation of the reduced connectivity in the contralesional hemisphere may be that transcallosal inhibition causes more random activity (or noise) in this region. This does not seem to be the case in our own data, as levels of alpha power did not vary between the two recording sessions. Similarly, the extent of change in overconnectivity arising from the perilesional area demonstrated a positive relationship with sensorimotor recovery. The structural dynamics of perilesional neurons may provide an explanation of these results. Neurons surrounding an area of ischemia may undergo incomplete dendritic damage, leaving residual connections and neurons primed for rewiring (Dancause et al., 2005; Zhang and Murphy, 2007). Axonal sprouting and synaptogenesis have also been found to occur in this region during later stages of stroke recovery (Carmichael, 2006).
Whole brain, voxel-wise fcMEG analysis to identify brain regions predictive of sensorimotor recovery point to both positive and negative correlations between fcMEG at visit one and overall recovery, predominantly in regions remote to the lesion. The negative association of connectivity in contralesional sensorimotor regions is consistent with the notion that increases in transcollosal influences following stroke results in adaptive inhibition of the contralesional cortex in relation to recovery (Stinear et al., 2008). In the subacute subgroup, we demonstrated that the greater the density of functional connections in posterior cerebellum and SMA, the better the recovery. Prior models of motor learning point to a direct role of these regions, especially under circumstances where adaptation to sensory perturbations is critical (Morton and Bastian, 2006; Smith and Shadmehr, 2005) and during internally guided tasks (Elsinger et al., 2006). We speculate that the intrinsic coupling of these areas with subcortical and cortical regions at rest represents a protective mechanism to avoid dendritic pruning and to maintain the capacity to relearn a motor task during periods of reduced or inefficient use (Luo and O’Leary, 2005).
Following the data-driven analysis in which regions of importance were identified as part of a global recovery-related network, we sought to obtain information regarding the spatial distribution of predefined regions within the residual motor network. The similar spatial distribution between the data-driven connectivity and ipsilesional motor cortex ROI highlight the robustness of our findings and that our initial global connectivity analysis was defined by an extended motor network. Future research within our lab will attempt to dissociate specific voxels pairs in which connectivity is strongest so that directionality of information flow may be extracted.
Previous work has suggested that age, lesion volume, and initial functional deficits are predictors of long-term stroke outcomes. In the current study, relationships between each of these variables and stroke recovery were not supported, either directly or as covariates with imaging measures. Therefore, fcMEG, as a direct neurophysiological mechanism underlying stroke recovery, may be viewed as a potentially more relevant predictor of stroke outcome. In this respect, identification of fcMEG within the resulting regions of importance (i.e. ipsilesional somatosensory cortex, posterior cerebellum, SMA, and contralesional sensorimotor regions) may be used to improve the outcomes of future stroke intervention trials by allowing for a reduction of sample size and more targeted intervention protocols.
Understanding that stroke deficits are attributed to a disrupted neural network and not merely the point of injury has important implications for treatment. Clearly, clinicians must find ways to promote a bilateral shift in connectivity to aid recovery (i.e. normalization or increased fcMEG of the perilesional area paralleled by contralateral reductions of fcMEG). While intensive rehabilitation of the affected arm has proven effective in subjects demonstrating functional use of the affected hand, a bilateral training approach may be more beneficial in subjects unable to meet the functional requirements of a unilateral approach. Animal models support this approach and demonstrate adaptive plasticity of the perilesional area during bilateral training compared to the maladaptive perilesional plasticity following intensive use of the unaffected limb (Allred et al.). Moreover, the importance of connectivity within the cerebellum and somatosensory cortex highlights the need to tap into somatosensory-guided learning based rehabilitation paradigms (Shadmehr et al.).
Although the small size of our subject cohort may limit our results, the use of resting state data allowed us to study a group with moderate to moderately severe functional deficits that may have otherwise been excluded from task-based studies. Task based testing paradigms require that a higher level of hand function and/or effort be achieved prior to study participation so that resulting brain activation patterns can be compared across time and subjects (Ward et al., 2003). The resting state paradigm may also be relevant to lower extremity outcomes, which, in the past, has been a challenging association to make using traditional task-based activation protocols.
A further point of concern may be the heterogeneity of our sample with lesions of varied locations, size, and time post stroke. We selected subjects with ischemic stroke in the territory of the middle cerebral artery, which may have included somatosensory, motor, premotor or the posterior limb of the internal capsule. Controlling for the heterogeneity of lesion location is challenging in human stroke studies due to the nature of an ischemic lesion (e.g. (Marshall et al., 2009; Park et al., 2011; Rehme et al., 2011a; Wang et al.). It is promising that, even in the presence of this heterogeneity, we find a consistent, robust relationship between functional connectivity and motor recovery. Although it could be argued that lesion size may have confounded our results, however a correlation analysis between lesion size and motor recovery was not found to be significant. Finally, we are aware that the extent of motor recovery varies with the stroke study intervals. For this reason, each of our analyses were conducted as partial correlations with time post stroke included as a control, illustrating that this relationship between connectivity and recovery is independent of when the stroke occurred.
The results of the present study highlight the utility of applying fcMEG clinically to predict functional outcome in stroke. Although our study focuses on functional connectivity in a frequency range where signal is robust during spontaneous MEG recording (Guggisberg et al., 2008), novel source reconstruction methods are currently being developed to model neural interactions in suboptimal (yet neurophysiologically relevant) oscillatory bands (e.g. beta, gamma). A fine-grained understanding of how functional connectivity operates at multiple temporal levels will ultimately broaden our understanding of neural reorganization after stroke.
Highlights.
We used MEG to examine connectivity in resting cortical oscillations after stroke
Arm recovery is related to plasticity in perilesional and contralesional connectivity
Connectivity in somatosensory cortex, SMA, and cerebellum predicted recovery
Stroke affects distributed neural networks in alpha band imaginary coherence
Acknowledgments
Funding
This work was supported by the NIH grants R21 NS076171, RO1 DC4855, DC6435, DC10145, NS67962, NS64060 and by NSF grant BCS-0926196.; American Heart Association [KPW]; AMES technology Inc; and Canadian Institutes of Health Research [postdoctoral fellowship to KPW].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allred RP, Cappellini CH, Jones TA. The “good” limb makes the “bad” limb worse: experience-dependent interhemispheric disruption of functional outcome after cortical infarcts in rats. Behav Neurosci. 124:124–132. doi: 10.1037/a0018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz M, Gross J, Timmermann L, Moll M, Freund HJ, Witte OW, Schnitzler A. Perilesional pathological oscillatory activity in the magnetoencephalogram of patients with cortical brain lesions. Neurosci Lett. 2004;355:93–96. doi: 10.1016/j.neulet.2003.10.065. [DOI] [PubMed] [Google Scholar]
- Calautti C, Naccarato M, Jones PS, Sharma N, Day DD, Carpenter AT, Bullmore ET, Warburton EA, Baron JC. The relationship between motor deficit and hemisphere activation balance after stroke: A 3T fMRI study. Neuroimage. 2007;34:322–331. doi: 10.1016/j.neuroimage.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- Chen HM, Chen CC, Hsueh IP, Huang SL, Hsieh CL. Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabil Neural Repair. 2009;23:435–440. doi: 10.1177/1545968308331146. [DOI] [PubMed] [Google Scholar]
- Cover KS, Vrenken H, Geurts JJ, van Oosten BW, Jelles B, Polman CH, Stam CJ, van Dijk BW. Multiple sclerosis patients show a highly significant decrease in alpha band interhemispheric synchronization measured using MEG. Neuroimage. 2006;29:783–788. doi: 10.1016/j.neuroimage.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Crafton KR, Mark AN, Cramer SC. Improved understanding of cortical injury by incorporating measures of functional anatomy. Brain. 2003;126:1650–1659. doi: 10.1093/brain/awg159. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Dalal SS, Guggisberg AG, Edwards E, Sekihara K, Findlay AM, Canolty RT, Berger MS, Knight RT, Barbaro NM, Kirsch HE, Nagarajan SS. Five-dimensional neuroimaging: localization of the time-frequency dynamics of cortical activity. Neuroimage. 2008;40:1686–1700. doi: 10.1016/j.neuroimage.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, Nudo RJ. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVetten G, Coutts SB, Hill MD, Goyal M, Eesa M, O’Brien B, Demchuk AM, Kirton A. Acute corticospinal tract Wallerian degeneration is associated with stroke outcome. Stroke. 2010;41:751–756. doi: 10.1161/STROKEAHA.109.573287. [DOI] [PubMed] [Google Scholar]
- Dong Y, Dobkin BH, Cen SY, Wu AD, Winstein CJ. Motor cortex activation during treatment may predict therapeutic gains in paretic hand function after stroke. Stroke. 2006;37:1552–1555. doi: 10.1161/01.STR.0000221281.69373.4e. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke. 1992;23:1084–1089. doi: 10.1161/01.str.23.8.1084. [DOI] [PubMed] [Google Scholar]
- Elsinger CL, Harrington DL, Rao SM. From preparation to online control: reappraisal of neural circuitry mediating internally generated and externally guided actions. Neuroimage. 2006;31:1177–1187. doi: 10.1016/j.neuroimage.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Enzinger C, Johansen-Berg H, Dawes H, Bogdanovic M, Collett J, Guy C, Ropele S, Kischka U, Wade D, Fazekas F, Matthews PM. Functional MRI correlates of lower limb function in stroke victims with gait impairment. Stroke. 2008;39:1507–1513. doi: 10.1161/STROKEAHA.107.501999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feydy A, Carlier R, Roby-Brami A, Bussel B, Cazalis F, Pierot L, Burnod Y, Maier MA. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33:1610–1617. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Ganesan V, Ng V, Chong WK, Kirkham FJ, Connelly A. Lesion volume, lesion location, and outcome after middle cerebral artery territory stroke. Arch Dis Child. 1999;81:295–300. doi: 10.1136/adc.81.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggisberg AG, Honma SM, Findlay AM, Dalal SS, Kirsch HE, Berger MS, Nagarajan SS. Mapping functional connectivity in patients with brain lesions. Ann Neurol. 2008;63:193–203. doi: 10.1002/ana.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guye M, Bettus G, Bartolomei F, Cozzone PJ. Graph theoretical analysis of structural and functional connectivity MRI in normal and pathological brain networks. MAGMA. doi: 10.1007/s10334-010-0205-z. [DOI] [PubMed] [Google Scholar]
- Guye M, Bettus G, Bartolomei F, Cozzone PJ. Graph theoretical analysis of structural and functional connectivity MRI in normal and pathological brain networks. Magma. 2010;23:409–421. doi: 10.1007/s10334-010-0205-z. [DOI] [PubMed] [Google Scholar]
- Hamzei F, Liepert J, Dettmers C, Weiller C, Rijntjes M. Two different reorganization patterns after rehabilitative therapy: an exploratory study with fMRI and TMS. Neuroimage. 2006;31:710–720. doi: 10.1016/j.neuroimage.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Kotter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci U S A. 2007;104:10240–10245. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James GA, Lu ZL, VanMeter JW, Sathian K, Hu XP, Butler AJ. Changes in resting state effective connectivity in the motor network following rehabilitation of upper extremity poststroke paresis. Top Stroke Rehabil. 2009;16:270–281. doi: 10.1310/tsr1604-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongbloed L. Prediction of function after stroke: a critical review. Stroke. 1986;17:765–776. doi: 10.1161/01.str.17.4.765. [DOI] [PubMed] [Google Scholar]
- Kirkland SW, Coma AK, Colwell KL, Metz GA. Delayed recovery and exaggerated infarct size by post-lesion stress in a rat model of focal cerebral stroke. Brain Res. 2008;1201:151–160. doi: 10.1016/j.brainres.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski DA, James DC, Schallert T. Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J Neurosci. 1996;16:4776–4786. doi: 10.1523/JNEUROSCI.16-15-04776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorne P, Sandercock P, Prasad K. Evidence-based practice for stroke. Lancet Neurol. 2009;8:308–309. doi: 10.1016/S1474-4422(09)70060-2. [DOI] [PubMed] [Google Scholar]
- Luft AR, Waller S, Forrester L, Smith GV, Whitall J, Macko RF, Schulz JB, Hanley DF. Lesion location alters brain activation in chronically impaired stroke survivors. Neuroimage. 2004;21:924–935. doi: 10.1016/j.neuroimage.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Luo L, O’Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–661. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Zarahn E, Alon L, Minzer B, Lazar RM, Krakauer JW. Early imaging correlates of subsequent motor recovery after stroke. Ann Neurol. 2009;65:596–602. doi: 10.1002/ana.21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26:9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan SS, Attias HT, Hild KE, 2nd, Sekihara K. A probabilistic algorithm for robust interference suppression in bioelectromagnetic sensor data. Stat Med. 2007;26:3886–3910. doi: 10.1002/sim.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte G, Bai O, Wheaton L, Mari Z, Vorbach S, Hallett M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin Neurophysiol. 2004;115:2292–2307. doi: 10.1016/j.clinph.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Wingeier BM, Silberstein RB. Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp. 2001;13:125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, Kim YH. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 2011;42:1357–1362. doi: 10.1161/STROKEAHA.110.596155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz T, Pinkowski C, van Wijck F, Kim IH, di Bella P, Johnson G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: a multicentre study. Clin Rehabil. 2005;19:404–411. doi: 10.1191/0269215505cr832oa. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, Wang LE, Fink GR, Grefkes C. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. Neuroimage. 2011a;55:1147–1158. doi: 10.1016/j.neuroimage.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme AK, Fink GR, von Cramon DY, Grefkes C. The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal FMRI. Cereb Cortex. 2011b;21:756–768. doi: 10.1093/cercor/bhq140. [DOI] [PubMed] [Google Scholar]
- Riley JD, Le V, Der-Yeghiaian L, See J, Newton JM, Ward NS, Cramer SC. Anatomy of stroke injury predicts gains from therapy. Stroke. 2011;42:421–426. doi: 10.1161/STROKEAHA.110.599340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzl A, Meisel S, Wittsack HJ, Fink GR, Siebler M, Modder U, Seitz RJ. Development of brain infarct volume as assessed by magnetic resonance imaging (MRI): follow-up of diffusion-weighted MRI lesions. J Magn Reson Imaging. 2004;20:201–207. doi: 10.1002/jmri.20096. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Saver JL, Johnston KC, Homer D, Wityk R, Koroshetz W, Truskowski LL, Haley EC. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. The RANTTAS Investigators. Stroke. 1999;30:293–298. doi: 10.1161/01.str.30.2.293. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Perdue KL, Wang R. Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. Neuroimage. 2008;39:1370–1382. doi: 10.1016/j.neuroimage.2007.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemanck SK, Kwakkel G, Post MW, Prevo AJ. Predictive value of ischemic lesion volume assessed with magnetic resonance imaging for neurological deficits and functional outcome poststroke: A critical review of the literature. Neurorehabil Neural Repair. 2006;20:492–502. doi: 10.1177/1545968306289298. [DOI] [PubMed] [Google Scholar]
- Sehm B, Perez MA, Xu B, Hidler J, Cohen LG. Functional Neuroanatomy of Mirroring during a Unimanual Force Generation Task. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error Correction, Sensory Prediction, and Adaptation in Motor Control. Annu Rev Neurosci. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol. 2005;93:2809–2821. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- Stam CJ, van Cappellen van Walsum AM, Pijnenburg YA, Berendse HW, de Munck JC, Scheltens P, van Dijk BW. Generalized synchronization of MEG recordings in Alzheimer’s Disease: evidence for involvement of the gamma band. J Clin Neurophysiol. 2002;19:562–574. doi: 10.1097/00004691-200212000-00010. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Coxon JP, Fleming MK, Byblow WD. Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain. 2008;131:1381–1390. doi: 10.1093/brain/awn051. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Tecchio F, Zappasodi F, Pasqualetti P, Tombini M, Salustri C, Oliviero A, Pizzella V, Vernieri F, Rossini PM. Rhythmic brain activity at rest from rolandic areas in acute mono-hemispheric stroke: a magnetoencephalographic study. Neuroimage. 2005;28:72–83. doi: 10.1016/j.neuroimage.2005.05.051. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zijden JP, van der Toorn A, van der Marel K, Dijkhuizen RM. Longitudinal in vivo MRI of alterations in perilesional tissue after transient ischemic stroke in rats. Exp Neurol. 2008;212:207–212. doi: 10.1016/j.expneurol.2008.03.027. [DOI] [PubMed] [Google Scholar]
- van Meer MP, van der Marel K, Wang K, Otte WM, El Bouazati S, Roeling TA, Viergever MA, Berkelbach van der Sprenkel JW, Dijkhuizen RM. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 30:3964–3972. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer MP, van der Marel K, Wang K, Otte WM, El Bouazati S, Roeling TA, Viergever MA, Berkelbach van der Sprenkel JW, Dijkhuizen RM. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 2010;30:3964–3972. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yu C, Chen H, Qin W, He Y, Fan F, Zhang Y, Wang M, Li K, Zang Y, Woodward TS, Zhu C. Dynamic functional reorganization of the motor execution network after stroke. Brain. 133:1224–1238. doi: 10.1093/brain/awq043. [DOI] [PubMed] [Google Scholar]
- Wang L, Yu C, Chen H, Qin W, He Y, Fan F, Zhang Y, Wang M, Li K, Zang Y, Woodward TS, Zhu C. Dynamic functional reorganization of the motor execution network after stroke. Brain. 2010;133:1224–1238. doi: 10.1093/brain/awq043. [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Frackowiak RS, Thompson AJ, Greenwood RJ, Rothwell JC. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur J Neurosci. 2007;25:1865–1873. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe CD, Taub NA, Woodrow EJ, Burney PG. Assessment of scales of disability and handicap for stroke patients. Stroke. 1991;22:1242–1244. doi: 10.1161/01.str.22.10.1242. [DOI] [PubMed] [Google Scholar]
- World_Health_Organization. International Classification of Functioning, Disability, and Health. World Health Organization; Geneva: 2001. [Google Scholar]
- Zhang S, Murphy TH. Imaging the impact of cortical microcirculation on synaptic structure and sensory-evoked hemodynamic responses in vivo. PLoS Biol. 2007;5:e119. doi: 10.1371/journal.pbio.0050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumer JM, Attias HT, Sekihara K, Nagarajan SS. A probabilistic algorithm integrating source localization and noise suppression for MEG and EEG data. Neuroimage. 2007;37:102–115. doi: 10.1016/j.neuroimage.2007.04.054. [DOI] [PubMed] [Google Scholar]


