Abstract
BACKGROUND
Mounting evidence suggests that overeating may be conceptualized within the same behavioral and neurobiological framework as drug addiction. One potentially important difference between overeating vs. drug abuse refers to the sensory stimulation of oral receptors by palatable foods, a feature that may be required for reinforcement during intake. Likewise, post-ingestive effects and caloric content of food also contribute to reinforcing behavior and might influence the development of compulsive eating behavior. The purpose of the current study was to establish whether intra-gastric self-administration of fat emulsions, i.e. bypassing the oral cavity, recapitulates some of the behavioral and neurobiological hallmarks of psychostimulant self-administration.
METHODS
We employed behavioral assays in mice to assess acquisition, maintenance, extinction and reinstatement of intra-gastric self-administration of lipid emulsions to determine the extent to which post-oral fat self-administration recapitulates psychostimulant self-administration. Striatal dopamine efflux during behavioral tasks was determined by brain microdialysis coupled to chromatographic-electrochemical analyses.
RESULTS
We show that, in direct analogy to drug self-administration, 1) decreases in fat dose concentration were met with compensatory increases in response rates aimed at maintaining constant hourly caloric intake; 2) rates of responding markedly increased during both extinction and progressive ratio schedules of reinforcement; 3) Elevations in striatal dopamine levels observed during maintenance were markedly attenuated during extinction sessions, only to be restored upon reinstatement.
CONCLUSION
Our data thus supports the contention that stimulation of oral receptors by caloric foods may not be required for the expression of certain addiction-related neurobehavioral markers.
Keywords: Addiction, Dopamine, Fat, Gut, Self-Administration, Striatum
Introduction
It has been proposed that hyperpalatable, densely caloric foods share several behavioral and neurobiological features with drugs of abuse [see throughout this issue and e.g. 1, 2–4]. One potentially critical distinction between overeating and drug abuse refers to the fact that, during the ingestion of hyperpalatable caloric foods, a multitude of intra-oral receptors are strongly stimulated [including gustatory, olfactory and somatosensory sensors, 5]. In other words, it remains undetermined whether addiction-like alimentary patterns depend primarily on the flavorful properties, or instead on the caloric content, of hyperpalatable foods. The resolution of this dichotomy is of importance not only for the design of experimental models of food addiction, but also for establishing whether the prescription of low-calorie alternatives, such as artificial sweeteners and fat substitutes, are likely to restrain overeating.
In this study we aimed at determining the extent to which intra-gastric alimentary behaviors that bypass the oral cavity are capable of recapitulating some of the distinguishing features associated with the maintenance and extinction of intra-venous psychostimulant self-administration in rodents, namely, i) learned behavioral responses that trigger drug infusions appear to be primarily controlled by systemic drug levels [6, 7]; ii) compared to maintenance of self-administration, rates of responding dramatically increase during extinction and progressive ratio schedules of reinforcement [6, 8–10]; iii) compared to maintenance, extracellular brain dopamine levels markedly decrease during extinction, only to recover upon reinstatement [6, 8]. Dopamine sampling was obtained from the dorsal aspect of the striatum, where dopaminergic tone is required for the normal expression of goal directed behaviors in general [11] and feeding in particular [12, 13].
Methods and Materials
Detailed information on: Subjects, Surgical procedure for implantation of gastric catheters and microdialysis guiding cannulae, Stimuli and calculation of caloric densities, Behavioral apparatus, Behavioral protocols, Extinction and Progressive Ratio Schedules of Reinforcement, Two-dry sipper task and cue reinstatement, Dopamine measurements during behavioral performance, and Statistical Analyses is provided in Supplement 1.
Results
Learning to self-administer fat emulsions into the gastrointestinal tract
We started by assessing the learning process by which mice acquired the ability to self-administer fat emulsions directly into the gastro-intestinal tract. In our task, male mice were fitted with gastric catheters and trained to lick a dry spout in order to receive intra-gastric infusions of fat emulsions [14].
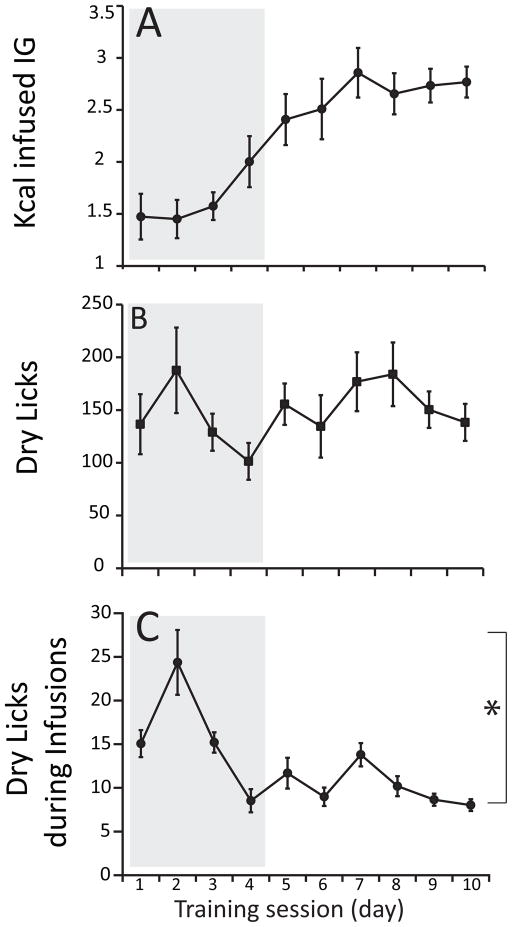
Analysis of the ten learning sessions reveals gradual and yet clear across-session increases in the amounts of calories self-infused (Figure 1A). Note that such pattern was not interrupted by the removal of standard chow from behind the spout’s orifice. The data representing the amounts of calories self-infused across sessions were fitted to a sigmoid (Boltzmann) model, which resulted in a robust approximation (R2=0.99) associated with a transition point at the fourth session (Figure 1A). Interestingly, equivalent analyses of the across-session numbers of dry licks revealed a different pattern, with dry licks instead fluctuating around an overall mean of ~150 licks per session (R2=0.36, see Figure 1B). In fact, increases in calorie self-administration during learning do not inevitably require an equivalent increase in dry licks, but rather an adaptation in the timing of the responses since dry licks produced while the pump is activated did not result in infusions, see Methods and Materials). Consistently, an analysis of the number of dry licks produced while the infusion pump was activated reveals that these non-effective responses decreased significantly across learning sessions, reaching a stable level after the fourth session (Figure 1C).
Figure 1. Learning to self-administer fat emulsions into the gastrointestinal tract.
Male mice (N=16) were fitted with gastric catheters and trained to lick a dry spout in order to receive intra-gastric infusions of fat emulsions. Training sessions lasted for 1 h and were performed daily under food (20h) deprivation. For the first four daily training sessions (delimited by the gray box), a small amount of standard chow was placed behind the spout’s orifice. After the four priming sessions the spouts containing chow were replaced by clean odorless ones and six additional learning sessions were performed. A. Analysis of the ten learning sessions reveals a gradual and yet clear increase in the amounts of calories self-infused across sessions. These ten sessions include the four initial sessions where food was placed behind the sipper to prime animals to initiate dry licking (shown within gray box). The data were fitted to a sigmoid (Boltzmann) model, which resulted in a robust approximation (R2=0.99) associated with a transition point at the fourth session. B. Interestingly, equivalent analyses of the numbers of dry licks across learning sessions revealed a different pattern, with dry licks fluctuating around an overall mean of ~150 licks per session (R2=0.36). C. Analyses of the number of dry licks produced while the infusion pump was activated reveals that these non-effective responses decreased significantly across learning sessions, reaching a stable level after the fourth session (one-way RM-ANOVA, session day effect F[9,135]=9.0, *p<0.001).
Fat self-administration responses are under the control of caloric density
Learned behavioral responses that trigger infusions of amphetamine or cocaine appear to be primarily controlled by systemic drug levels [15, 16]. Specifically, seminal work by Pickens and colleagues demonstrated that decreases in dose concentration are met with compensatory increases in response rates seemingly aimed at maintaining relatively constant hourly drug intake [7, 15, 16]. Accordingly, we have analyzed the rapidity with which animals adjust their learned responses (i.e. dry licks) in order to compensate for unpredicted changes in the caloric density of the infused emulsion.
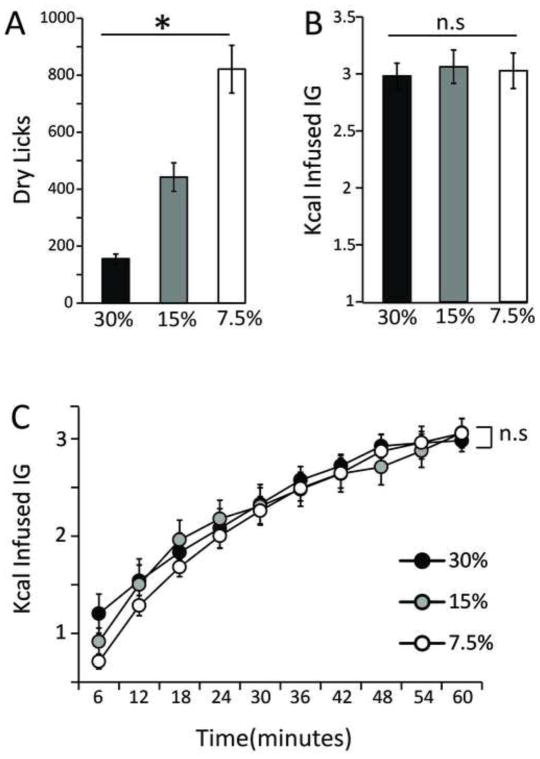
We have found that, as predicted, animals increased the numbers of dry licks in response to proportional decreases in the caloric density of the emulsion (Figure 2A). Accordingly, analysis of the total amounts of calories self-infused revealed that the changes in dry licking were aimed at compensating for the variations in the emulsion’s caloric density (Figure 2B), in overall agreement with our own previous studies [14]. However, our current study revealed a striking additional feature associated with caloric compensation, namely, the fact that the compensatory adjustments were met rapidly and are narrowly maintained throughout the entire session. In fact, analyses of the timecourses associated with the amounts of calories self-infused reveal that caloric compensation was met within the initial 6 minutes and maintained throughout the behavioral sessions (Figure 2C). In sum, these results show that, in analogy to drug self-administration, decreases in fat concentration are met with rapid increases in response rates seemingly aimed at maintaining relatively constant rates of caloric intake.
Figure 2. Fat self-administration responses are under the control of caloric density.
Previous to each daily 1 h session, the emulsion was prepared at a caloric density that had been determined from a random sequence (so that the concentration being infused could not be predicted by the animal). A. Mice (N=16) increased the numbers of dry licks in response to proportional decreases in the caloric density of the infusate (one-way RM-ANOVA infusate concentration effect F[2,30]=45.9, *p<0.0001). B. Accordingly, changes in dry licking were aimed at compensating for the variations in the infusate’s caloric density, so that overall caloric intake is maintained irrespective of the infusate’s density (one-way RM-ANOVA infusate concentration effect F[2,30]=0.25, p=0.77). C. The compensatory adjustments were rapidly met and closely maintained throughout the entire session. Analysis of the timecourses associated with the amounts of calories self-infused (split into 6-minutes bins) reveal that caloric compensation is met within the initial 6 minutes and is maintained throughout the behavioral sessions (two-way RM-ANOVA, effect of infusate concentration F[2,450]=1.3, p=0.26; effect of time F[9,450]=53.4, p<0.001; concentration × time effect F[18,450]=0.4, p=0.98). n.s. = statistically non-significant.
Rates of dry licking during extinction and progressive ratio schedules of reinforcement
Psychostimulant self-administration studies revealed that, compared to during maintenance of self-administration, rates of responding dramatically increase during extinction and progressive ratio schedules of reinforcement [6, 8–10, 17], the latter being employed as a measure of motivation to obtain the reward [18]. Such a pattern is a strong indicator of the robust reinforcing properties of these drugs when self-infused intravenously. We have therefore inquired whether such patterns are reproduced during intra-gastric fat self-administration. In addition, we also employed progressive ratio schedules of reinforcement during licking of IntraLipid drops, in order to contrast the reward potency of intra-gastric infusions against oral delivery of fat (see Methods and Materials for schedule details).
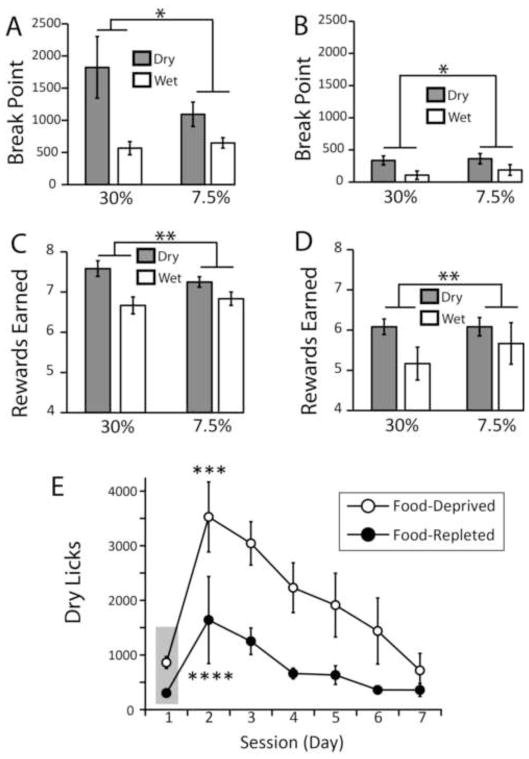
In order to analyze the impact of reward devaluation on motivation to self-administer fat, we assessed dry and wet licking responses during progressive ratio sessions in both food-deprived and food-replete (5-day food repletion) mice. Intriguingly, we observed larger break point values associated with dry compared to wet licks. This effect did not depend on emulsion density and was observed in both food-depleted (Figure 3A) and food-replete (Figure 3B) mice. It is noticeable however that food deprivation was associated with significantly larger break points during both dry and wet progressive ratio tasks (cf. Figure 3A vs. 3B). The same results are obtained when the numbers of rewards earned are analyzed instead (Figures 3C–D). We therefore conclude that, under the conditions of our experiments, intra-gastric infusions of caloric emulsions are perceived as more rewarding than the oral delivery of small drops of the same emulsions.
Figure 3. Rates of dry licking during extinction and progressive ratio schedules of reinforcement.
The rewarding properties of oral and intra-gastric fat self-administration were assessed by employing progressive ratio schedules of reinforcement to both food-deprived and food-replete (5-day food repletion) mice. A. In deprived animals, we observed significantly larger break point values associated with dry compared to wet licking (two-way mixed effects ANOVA, between-group effect of route of administration F[1,16]=4.75, *p=0.04); an effect that did not depend on emulsion concentration (7.5 vs. 30%, F[1,16]=1.0, p=0.32). B. Similar results were obtained with food-replete animals; however, food repletion significantly decreased break point values associated with both dry and wet licks (overall effect of feeding state F[1,16]=18.5, p=0.001). C. The same results are obtained if, instead of break points, numbers of rewards earned are considered instead, in both food deprived (two-way mixed effects ANOVA, between-group effect of route of administration F[1,16]=10.5, **p=0.005) and D. food-replete mice (overall effect of feeding state F[1,16]=65.0, p<0.001). E. Further experiments were performed in both food depleted (N=7) and replete (N=7) mice during six daily extinction sessions that followed one baseline fixed ratio session performed on the first session day (delimited by gray area). We observed marked increases in dry licking on the first day of extinction in both depleted (one-way RM-ANOVA, session effect F[6,36]=10.2, p<0.0001; post-hoc t-test between day 2 first extinction session vs. day 1 baseline session, t[6]=5.6, ***p<0.01) and replete (F[6,36]=2.57, p=0.035; post-hoc t-test between day 2 first extinction session vs. day 1 baseline session, t[6]=2.9, ****p<0.05) mice. This was followed by a gradual across-session decrease in response rates.
Further experiments were performed in both food-depleted and replete mice during six daily extinction sessions that followed one baseline fixed ratio session. During extinction sessions, dry licks resulted only in sham infusions. We observed marked increases in dry licking numbers on the first day of extinction (at least 300%) in both groups, although the effect was once again smaller in replete compared to depleted animals (Figure 3E). This overall pattern was followed by a gradual across-session decrease in response rates in both groups. In sum, these results show that, in analogy to drug self-administration, rates of responding dramatically increase during extinction and progressive ratio schedules of reinforcement.
Mice are capable of directing ingesting behavior by using non-oral cues
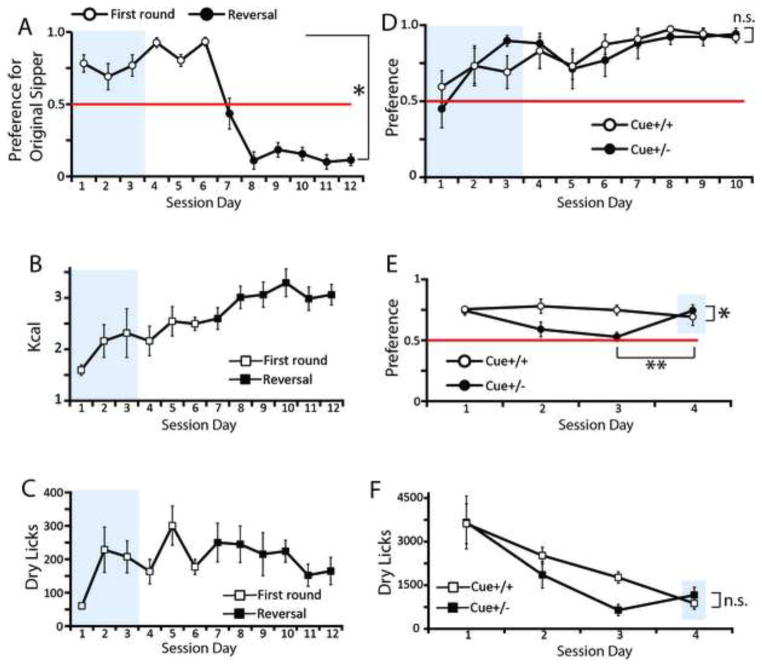
We have further assessed the ability of mice to direct their behavior towards the goal of obtaining intra-gastric calories by performing experiments where two symmetrically positioned dry sippers were available (of which only one was associated with programmed consequences). Mice rapidly and robustly acquired a behavioral preference for the active sipper, a pattern that was sustained over several days (Figure 4A). Furthermore, when contingencies were reversed by switching the positions of the active and inactive sippers, mice updated their behavioral responses by appropriately reversing sipper preference (Figure 4A). It was also clear that, as with the one-sipper case, the data representing the amounts of calories self-infused across the two-sipper sessions could be fitted to a sigmoid model (R2=0.89, Figure 4B) whereas the across-session numbers of dry licks could not (R2=0.56, see Figure 4C).
Figure 4. Mice are capable of directing ingesting behavior by using non-oral cues.
Additional experiments were performed where mice were simultaneously presented with an active and one inactive dry sipper. A. Mice rapidly acquired and sustained behavioral preferences for the active sipper over session days, a pattern that was reversed after the switching of sipper positions. The figure shows the preference levels for the originally active sipper previous to (dark symbols) and after reversal (clear symbols) of contingencies (the horizontal red line indicates the 0.5 level of indifference; two-way RM-ANOVA contingency × session day effect F[5,30]=4.0, *p=0.006). B. Likewise the one-sipper case (see Figure 1B), the data representing the amounts of calories self-infused across the two-sipper sessions was fitted to a sigmoid model (R2=0.89). C. However the across-session numbers of dry licks could not be fitted to a sigmoid model (R2=0.56). D. One group of mice (“Cue+/+”, N=6) was presented with an auditory cue triggered by licking the active sipper during both training and extinction phases, while a second group (“Cue+/−”, N=6) was presented with the auditory cue only during the training phase. Both groups rapidly acquired preferences for the active sipper (the horizontal red line indicates the 0.5 level of indifference; two-way RM-ANOVA session day effect F[9,90]=4.7, p<0.001; session day × group effect F[9,90]=0.63, p=0.76). Note: For panels A to D, during the first three daily training sessions (delimited by the gray box), a small amount of standard chow was placed behind the spout’s orifice. E. During extinction, while mice in the Cue+/+ group maintained preferences significantly above chance, mice in the Cue+/− group did not; however, during cue-driven reinstatement, mice in the Cue+/− group recovered preferences for the previously active sipper (two-way RM-ANOVA session day × group effect F[3,30]=3.52, *p=0.02). In fact, reinstatement of cue presentation produced significant increases in preferences for the previously active sipper compared to the last day of non-cued extinction (within-group Cue+/− paired t-test between session days 3 and 4, t[5]=3.4, **p=0.02). Gray area indicates cue-reinstatement test after extinction sessions. F. Reinstatement of cue presentation did not however modify dry licking patterns (session day × group effect F[3,30]=1.3, p=0.26; within-group Cue+/− paired t-test between session days 3 and 4 t[5]=1.9, p=0.11).
In a variation of the above task, a 1sec auditory cue was triggered concomitantly to activation of the infusion pump upon the detection of dry licks to the active sipper. Mice were arbitrarily divided in two groups; one group of mice (named “Cue+/+”) was presented with the auditory cue during both training and extinction phases, while a second group (“Cue+/−”) was presented with the auditory cue during the training, but not during the extinction, phase. Therefore, during 10 training sessions, all mice detected the auditory cue upon activation of the infusion pump. Both groups rapidly acquired, as above, preferences for the active sipper (Figure 4D). Extinction tests were immediately performed after the 10 daily training sessions. During extinction, while mice in the Cue+/+ group maintained sipper preferences above chance, mice in the Cue+/− group did not (Figure 4E). After three daily extinction sessions, the auditory cue was reestablished for the Cue+/− mice (cue reestablishment was also tested in extinction). Now, during cue-driven reinstatement, mice in the Cue+/− group recovered robust preferences for the previously active sipper (new Figure 4E) without modifying their dry licking patterns (Figure 4F). Overall, we conclude that mice are capable of directing their behavior towards obtaining fat calories by efficiently making use of non-oral signals such spatial cues and auditory stimuli.
Striatal extracellular dopamine levels during maintenance, extinction and reinstatement of intra-gastric fat self-administration
Finally, we have performed brain microdialysis concomitantly to the behavioral intra-gastric self-administration task. Previous microdialysis studies performed during cocaine self-administration revealed that, compared to during maintenance, extracellular brain dopamine levels markedly decrease during extinction, only to recover upon reinstatement [6, 8]. Accordingly, trained mice implanted with both gastric catheters and microdialysis probes in dorsal striatum were subjected to two daily 3h-long experimental sessions. On the first session, a fixed ratio reinforcement schedule (“maintenance” session) was employed. On the second day, mice were exposed to consecutive extinction (1.5 hs) and reinstatement (1.5 hs) phases.
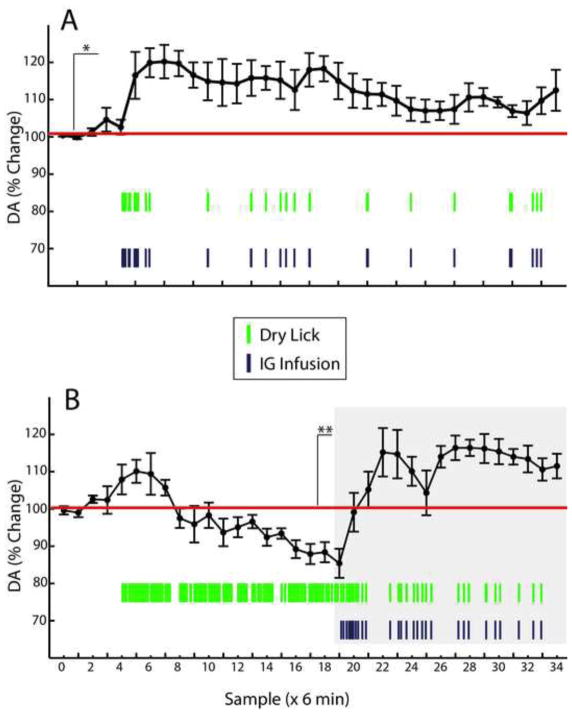
Analysis of the changes in dopamine levels with respect to pre-session baseline clearly shows that, during maintenance of intra-gastric responses, extracellular dopamine was significantly and persistently elevated throughout the sessions (~120% of baseline levels, Figure 5A). Note the rapid increases in dopamine associated with the beginning of the intra-gastric infusions (depicted as purple vertical traces in Figure 5) after baseline (samples 1–5). During extinction sessions on the other hand, we observed an initial but short-lived increase in dopamine levels (~109% of baseline levels) that was quickly reversed and followed by a steady decrease that reached levels below pre-session baseline (~90% of baseline levels, Figure 5B). Importantly, note that animals sustained frenetic dry licking rates during extinction sessions (depicted as green vertical traces in Figure 5), which unambiguously demonstrates that dry licking responses do not account for the observed increases in dopamine levels occurring during maintenance. Upon reinstatement, dopamine levels were quickly restored to an extent that these increases closely resembled those observed during maintenance (see graph under gray area in Figure 5B). Positioning of dialysis probes were confirmed histologically (Figure 6). In sum, these results show that, analogously to drug self-administration, compared to maintenance, extracellular brain dopamine levels markedly decrease during extinction, only to recover upon reinstatement.
Figure 5. Striatal extracellular dopamine levels during maintenance, extinction and reinstatement of intra-gastric fat self-administration.
Mice (N=6) implanted with both gastric catheters and microdialysis probes in dorsal striatum were subjected to two daily 3h-long experimental sessions. On the first day a fixed ratio reinforcement schedule (“maintenance” session) was employed. On the second day, mice were exposed to consecutive extinction (1.5 hs), and reinstatement (1.5 hs) phases. Pre-session baseline was calculated based on samples 1–5, during which period the dry sipper was not available. A. Analysis of the changes in dopamine levels with respect to baseline reveals that during maintenance of intra-gastric responses, extracellular dopamine was significantly elevated and remained at these levels throughout the sessions (two-way RM-ANOVA, effect of intra-gastric infusions on dopamine levels F[1,5]=15.7, *p=0.01). B. During extinction sessions, it was observed an initial but short-lived increase in dopamine levels that was quickly reversed and followed by a steady decreases (two-way RM-ANOVA, effect of intra-gastric infusions F[1,5]=1.6, p=0.26; effect of sampling time F[19,95]=9.4, p<0.001; infusions × sampling time effect F[19,95]=9.4, **p<0.001). Note that animals sustained a frenetic dry licking response during extinction sessions, which unambiguously demonstrates that licking rates do not account for the observed increases in dopamine observed during maintenance. Upon reinstatement (delimited by gray box), dopamine levels were quickly restored (two-way RM-ANOVA, effect of intra-gastric infusions F[1,5]=49.7, p=0.001). Green vertical traces = average number of dry licks during the corresponding 6-min bin; Purple vertical traces = average number of intra-gastric infusions during the corresponding 6-min bin.
Figure 6. Schematic representation of microdialysis probe locations.
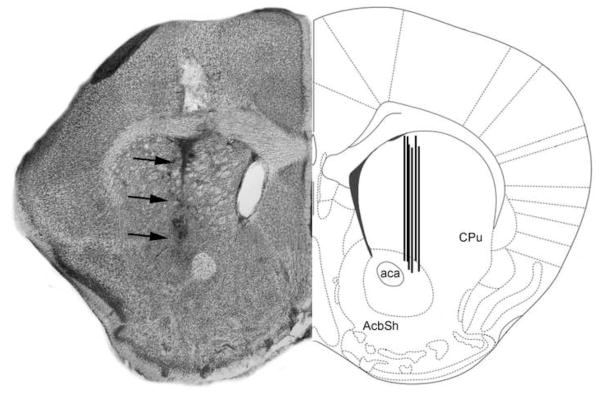
The figure shows a coronal section of the mouse brain through the dorsal striatum (caudate/putamen) region. In the left hemisphere is shown a representative Nissl-stained section revealing the tract associated with the tip of an inserted probe (dark arrows). In the right hemisphere is shown the corresponding region in a stereotaxic atlas [51]. ac = anterior commissure; Acb = Nucleus accumbens of the ventral striatum; CPu = caudate/putamen of the dorsal striatum; LV = Lateral ventricle.
Discussion
In this study, we aimed at establishing whether intra-gastric self-administration of fat emulsions recapitulates some of the behavioral and neurobiological hallmarks of self-administration of psychostimulants. We have found that, within limits, fat self-administration into the gastrointestinal tract bears striking similarities to psychostimulant self-administration, specifically the “pharmacological” regulation exerted by infusate concentration on response rates and neurochemical dynamics. Of particular interest is our observation that, as with psychostimulants such as amphetamine or cocaine, the precise behavioral regulation of fat self-administration does not require the detection of oral cues. Specifically, non-oral signals such as spatial location and auditory signs are sufficient to reinstate previously extinguished responses. Our work therefore suggests that gastrointestinal or other metabolic signals may be critical for the expression of addiction-like behaviors associated with high-calorie intake.
The use of intra-gastric self-administration allowed us to avoid any effects associated with the stimulation of oral chemosensory receptors, and directly test the contribution of post-ingestive signals to addiction-like behaviors. Overall, our study leads us to conclude that many behavioral and neurochemical patterns associated with (intra-gastric) self-infusion of caloric fat emulsions bear striking similarities with the behavioral and neurochemical patterns associated with (venous) self-infusion of drugs of abuse. Firstly, animals can rapidly learn to self-infuse emulsions, and will quickly adapt their licking rate to achieve a constant caloric rate. Consistently, learned behavioral responses that trigger infusions of amphetamine or cocaine appear to be primarily controlled by systemic drug levels [15, 16]. Thus, in direct analogy to caloric compensation, decreases in dose concentration are met with compensatory increases in response rates seemingly aimed at maintaining relatively constant hourly drug intake [15, 16]. Interestingly, and consistent with our data, analogous results were previously reported where rodents regulated cocaine self-administration by licking an empty spout [19]. Similar conclusions can be drawn from our observations that dry-lick rates increase dramatically during extinction sessions, again in direct analogy to drug self-administration [6, 10]. From a neurochemical point of view, it is also notable that rats pretreated daily with haloperidol show significant extinction-like increases in cocaine self-administration [20], as seen in mice pretreated with haloperidol prior to intra-gastric infusions of fat emulsions [14]. Finally, our behavioral studies also demonstrated that, as with psychostimulants, intra-gastric infusion rates increase significantly during progressive ratio schedules of reinforcement, an effect that persisted (although at attenuated levels) when the intra-gastric reward was devaluated by food repletion.
Importantly, the similarities between drug intravenous and fat intra-gastric self-administration extend to neurochemical analyses of dopamine fluctuations observed during these behavioral tasks. Specifically, we have observed that elevations in dopamine levels induced by the intra-gastric fat infusions produced during maintenance of the self-administration behavior were greatly attenuated during extinction sessions, only to be restored to near-maintenance levels upon reinstatement [6, 8]. Now, because the reinforcing efficacy of abused drugs appear to depend on their ability to elevate extracellular dopamine concentrations [21], it has been hypothesized [22] and subsequently shown [6] that the timing of self-infusing drug responses can be predicted with reasonable precision from fluctuations in extracellular dopamine concentrations. While it is true that the magnitude of changes in dopamine levels differs between foods and psychostimulants (as it does between different drugs of abuse as well), the relationship between fluctuations from baseline and the timing of behavioral responses (with increased dopamine levels reflecting lower response rates) remains the finding of interest relating calories to psychostimulants. Thus, an important topic for future exploration would be testing the hypothesis that the timing of intra-gastric self-infusions can be predicted from the corresponding changes in extracellular dopamine concentrations. In addition, since the presence of fat in the oral cavity induces concentration-dependent dopamine release independently of intestinal absorption [23], it will be also important to determine the extent to which gut-induced dopamine efflux is comparable to orally-induced efflux – especially in light of our current findings that oral fat sensing may not have the same reinforcing potency as gut fat sensing.
It is important to note that the dopaminergic measurements during drug self-administration referred to above were obtained from the nucleus accumbens of the ventral striatum, while our own measurements were performed on the dorsal aspect of the medial striatum. The rationale for our choice was based on ample evidence from both the human and rodent literature that dopamine release within the dorsal striatum is the critical mediator of instrumental action. More generally, integrity of the dorsal striatum is necessary for the acquisition and expression of instrumental actions [24]. Specifically, lesions to the medial part of the dorsal striatum render animals insensitive to action-outcome devaluation or degradation, whereas lesions to its more lateral aspect impair the development of habits [for a review 24]. Consistently, dynamic shifts take place involving mediodorsal to dorsolateral striatal control over reward seeking between goal-directed and habitual performance [25]. Furthermore, corticostriatal regulation of goal-directed behavior seems to be under the control of striatal dopamine release: both in vivo and in vitro preparations reveal that striatal dopamine release around the time of corticostriatal activation is a critical regulator of synaptic modification in dorsal striatum [26].
More specifically regarding food intake regulation, dopamine receptor deficiency, which provides a neurobiological link between overeating and drug addiction [3, 27], has been reported for the dorsal aspect of striatum of human obese subjects [28, 29]; indeed, this is the site where dopamine release was observed in humans during feeding [30]. It must be noted that in obese rodents most evidence supports deficient dopamine signaling in the mesolimbic pathway. In fact, rodents overfed with fat-derived calories, as well as those sustaining genetic propensity to excessive weight gain, display decreases in both dopamine release [31, 32] and turnover [33] in ventral striatum. Moreover, genetically obese mice seem to display altered ventral striatal dopamine levels [34, 35]. However, downregulation of dopamine signaling in dorsal striatum was found to be sufficient to produce a hyperphagic phenotype in rodents presented with a cafeteria diet [13]. These data add to previous studies demonstrating that restoring dopamine signaling in dorsal striatum is sufficient to normalize food intake [12] and goal-directed behaviors [11] in dopamine-deficient mutant animals. Conversely, evidence favors preponderant roles for opioids [36] and melanin-concentrating hormone [37], more than for dopamine, in modulating ventral striatum activity during feeding. In sum, there is ample evidence to support our choice to sample dopamine release within the dorsal aspect of the striatum. In this regard, it is interesting to note that recent studies have been attributing greater importance to the role of the dorsal striatum in drug abuse [38]. Specifically, it has been recently shown that dorsal, as well as ventral, striatal lesions affect rates of intravenous cocaine and morphine self-administration in rats [39].
Consistently, the development of long-term and habitual drug intake also relies on the dorsal striatum [25, 40]. It is thus conceivable that future research will reveal that intravenous drug and intra-gastric food self-administration share the dorsal aspect of the striatum as a common circuit mediating “unsensed” rewards [41]. It is however important to stress that our choice to sample dorsal striatum dopamine should not be taken as implying that ventral dopamine signaling is less important. Current evidence suggests that these two structures cooperate in the formation of habitual responses to rewards [40], and interruption of dopaminergic signaling in ventral striatum is likely to produce disruptions in self-administration responses given its critical role in mediating psychostimulant intake [42, 43]. In sum, the picture emerging from ours and previous studies supports the involvement of a complex circuit including both the nigrostriatal and mesolimbic dopamine pathways in food reward.
Related to the above, future research must identify the physiological pathways linking the stimulation of the gastrointestinal tract to midbrain dopamine cells, and the extent to which signals produced by gut fat sensing overlap with those produced by other nutrients such as sugars [44]. It is plausible to assume that the actual pathways linking gut fat sensing to dopamine release may differ significantly from those associated with psychostimulant intake (for example effects on dopamine transporter function). Because the increases in extracellular dopamine levels have a relatively fast onset, pre-absorptive mechanisms may be critical, including a hypothetical mediation by gastrointestinal hormones [45, 46].
It is nevertheless important to also acknowledge that a direct comparison between self-administration of psychostimulants vs. nutrients does not go without limitations. First, linear relationships involving infusate concentration vs. behavioral responses can only be inferred for a limited range of doses [7]; since the densest emulsion concentration tested was 30%, we cannot at present rule out the possibility that using dose ranges that include more caloric infusates would have resulted in non-linear behavioral response curves. Appropriate dose-response curves will therefore be necessary for testing the validity of concepts such as individual variations in substance vulnerability in the context of caloric intake. Furthermore, we also note that we did not test in our study the important effects of stress and/or substance-primed reinstatement [47, 48]; neither did we assess escalation or incubation as described with drug seeking [49]. While our results demonstrating cue-induced reinstatement of intra-gastric fat infusions further suggest important similarities between psychostimulant and caloric intake, appropriate use of additional behavioral paradigms is required for establishing the extent to which our animal model data is applicable to the human case [49, 50].
In conclusion, our data support the argument that, while caloric and psychostimulant intake may share behavioral and neurochemical regulatory mechanisms [throughout this issue and e.g. 1, 2–4], stimulation of oral receptors by caloric foods may not be required for the expression of certain addiction-related neurobehavioral patterns. This finding may have implications to our understanding of the efficacy of flavor-mimicking low-calorie alternatives in curbing calorie overconsumption.
Supplementary Material
Acknowledgments
NIH grant DC009997 to IEA.
Footnotes
Financial Disclosure
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gearhardt AN, Grilo CM, Dileone RJ, Brownell KD, Potenza MN. Can food be addictive? Public health and policy implications. Addiction. 2011;106:1208–1212. doi: 10.1111/j.1360-0443.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkow ND, O’Brien CP. Issues for DSM-V: should obesity be included as a brain disorder? Am J Psychiatry. 2007;164:708–710. doi: 10.1176/ajp.2007.164.5.708. [DOI] [PubMed] [Google Scholar]
- 3.Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011;12(11):638–51. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- 4.Puhl MD, Cason AM, Wojnicki FH, Corwin RL, Grigson PS. A history of bingeing on fat enhances cocaine seeking and taking. Behav Neurosci. 2011;125(6):930–42. doi: 10.1037/a0025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Small DM, Green BG. A Proposed Model of a Flavor Modality. 2012 [PubMed] [Google Scholar]
- 6.Ranaldi R, Pocock D, Zereik R, Wise RA. Dopamine Fluctuations in the Nucleus Accumbens during Maintenance, Extinction, and Reinstatement of Intravenous D-Amphetamine Self-Administration. J Neuroci. 1999;19:4102–4109. doi: 10.1523/JNEUROSCI.19-10-04102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickens R, Thompson T. Cocaine reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther. 1968;161:122–129. [PubMed] [Google Scholar]
- 8.Suto N, Ecke LE, You ZB, Wise RA. Extracellular fluctuations of dopamine and glutamate in the nucleus accumbens core and shell associated with lever-pressing during cocaine self-administration, extinction, and yoked cocaine administration. Psychopharmacology (Berl) 2010;211(3):267–75. doi: 10.1007/s00213-010-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuber GD, Wightman RM, Carelli RM. Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron. 2005;46(4):661–9. doi: 10.1016/j.neuron.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Olmstead MC, Munn EM, Franklin KB, Wise RA. Effects of pedunculopontine tegmental nucleus lesions on responding for intravenous heroin under different schedules of reinforcement. J Neurosci. 1998;18(13):5035–44. doi: 10.1523/JNEUROSCI.18-13-05035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson S, Sotak BN, During MJ, Palmiter RD. Local dopamine production in the dorsal striatum restores goal-directed behavior in dopamine-deficient mice. Behav Neurosci. 2006;120:196–200. doi: 10.1037/0735-7044.120.1.000. [DOI] [PubMed] [Google Scholar]
- 12.Szczypka MS, Mandel RJ, Snyder RO, Leff SE, Palmiter RD. Viral gene delivery selectively restores feeding and prevents lethality of dopamine-deficient mice. Neuron. 1999;22:167–178. doi: 10.1016/s0896-6273(00)80688-1. [DOI] [PubMed] [Google Scholar]
- 13.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13(5):635–41. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira JG, Tellez LA, Ren X, Yeckel CW, de Araujo IE. Regulation of fat intake in the absence of flavor signaling. J Physiol. 2012;590:953–972. doi: 10.1113/jphysiol.2011.218289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmer BA, Dobrin CV, Roberts DC. Brain-cocaine concentrations determine the dose self-administered by rats on a novel behaviorally dependent dosing schedule. Neuropsychopharmacology. 2011;36(13):2741–9. doi: 10.1038/npp.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettit HO, Pan HT, Parsons LH, Justice JB., Jr Extracellular concentrations of cocaine and dopamine are enhanced during chronic cocaine administration. J Neurochem. 1990;55(3):798–804. doi: 10.1111/j.1471-4159.1990.tb04562.x. [DOI] [PubMed] [Google Scholar]
- 17.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66(1):1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 18.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134(3483):943–4. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 19.Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav Neurosci. 2002;116(2):321–33. [PubMed] [Google Scholar]
- 20.Roberts DC, Vickers G. The effect of haloperidol on cocaine self-administration is augmented with repeated administrations. Psychopharmacology. 1987;93:526–8. doi: 10.1007/BF00207247. [DOI] [PubMed] [Google Scholar]
- 21.Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187:547–549. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]
- 22.Wise RA. In vivo estimates of extracellular dopamine and dopamine metabolite levels during intravenous cocaine or heroin selfadministration. Semin Neurosci. 1993;5:337–342. [Google Scholar]
- 23.Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1236–9. doi: 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- 24.Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci. 2008;28(8):1437–48. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray JE, Belin D, Everitt BJ. Double Dissociation of the Dorsomedial and Dorsolateral Striatal Control Over the Acquisition and Performance of Cocaine Seeking. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickens JR, Begg AJ, Arbuthnott GW. Dopamine reverses the depression of rat corticostriatal synapses which normally follows high-frequency stimulation of cortex in vitro. Neuroscience. 1996;70(1):1–5. doi: 10.1016/0306-4522(95)00436-m. [DOI] [PubMed] [Google Scholar]
- 27.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8(5):555–60. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 28.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357(9253):354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 29.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23(3):39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- 30.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage. 2003;19(4):1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 31.Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159(4):1193–9. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rada P, Bocarsly ME, Barson JR, Hoebel BG, Leibowitz SF. Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a fat-rich diet. Physiol Behav. 2010;101(3):394–400. doi: 10.1016/j.physbeh.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ, et al. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci. 2008;122(6):1257–63. doi: 10.1037/a0013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–22. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Opland DM, Leinninger GM, Myers MG., Jr Modulation of the mesolimbic dopamine system by leptin. Brain Res. 2010;1350:65–70. doi: 10.1016/j.brainres.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 2007;191(3):439–59. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- 37.Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, et al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25(11):2933–40. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32(10):517–24. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suto N, Wise RA, Vezina P. Dorsal as well as ventral striatal lesions affect levels of intravenous cocaine and morphine self-administration in rats. Neurosci Lett. 2011;493(1–2):29–32. doi: 10.1016/j.neulet.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57(3):432–41. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 41.Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36(2):229–40. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 42.Carboni E, Silvagni A, Valentini V, Di Chiara G. Effect of amphetamine, cocaine and depolarization by high potassium on extracellular dopamine in the nucleus accumbens shell of SHR rats. An in vivo microdyalisis study. Neuroscience & Biobehavioral Reviews. 2003;27(7):653–659. doi: 10.1016/j.neubiorev.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharm. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, De Araujo IE. Nutrient Selection in the Absence of Taste Receptor Signaling. J Neurosci. 2010;30:8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Araujo IE, Tellez LA, Ferreira JG, Ren X, Yeckel CW. The gut-brain dopamine axis: A regulatory system of caloric intake. Physiol & Behav. 2012;106:394–9. doi: 10.1016/j.physbeh.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sclafani A, Ackroff K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 2012;302(10):R1119–33. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168(1–2):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 48.Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3233–43. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305(5686):1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 50.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320(5881):1352–5. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 2. San Diego: Academic Press; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.