Abstract
Yarrowia lipolytica is a genetically tractable yeast species that has become an attractive model for analyses of lipid metabolism, due to its oleaginous nature. We investigated the regulation and evolution of lipid metabolism in non-Saccharomycetaceae yeasts, by carrying out a comparative physiological analysis of eight species recently assigned to the Yarrowia clade: Candida alimentaria, Y. deformans, C. galli, C. hispaniensis, C. hollandica, C. oslonensis, C. phangngensis and Y. yakushimensis. We compared the abilities of type strains of these species to grow on 31 non hydrophobic (sugars and other carbohydrate compounds) and 13 hydrophobic (triglycerides, alkanes and free fatty acids) carbon sources. Limited phenotypic diversity was observed in terms of the range of substrates used and, in the case of short-chain fatty acids, their toxicity. We assessed the oleaginous nature of these species, by evaluating their ability to store and to synthesize lipids. The mean lipid content of cells grown on oleic acid differed considerably between species, ranging from 30% of cell dry weight in C. oslonensis to 67% in C. hispaniensis. Lipid synthesis in cells grown on glucose resulted in the accumulation of C18:1 (n-9) as the major compound in most species, except for C. alimentaria and Y. yakushimensis, which accumulated principally C18:2(n-6), and C. hispaniensis, which accumulated both C16:0 and C18:1(n-9). Thus, all species of the clade were oleaginous, but they presented specific patterns of growth, lipid synthesis and storage, and therefore constitute good models for the comparative analysis of lipid metabolism in this basal yeast clade.
Introduction
Some yeast species can store and synthesize lipids from different carbon sources. Yeast species are described as “oleaginous” if the lipids they accumulate account for more than 20- to 25% of their biomass [1]. Oleaginous yeasts are dispersed throughout the entire phylogenetic tree of basidiomycetes and hemiascomycetes. The hemiascomycetous yeast Yarrowia lipolytica has emerged as an important model for lipid metabolism studies. Like other oleaginous yeasts, it can grow on sugars, such as glucose [2], [3], and on hydrophobic substrates (HS) [4]. It can also synthesize and store lipids [5]. In addition, Y. lipolytica is highly tractable genetically, making it a good model species for biotechnological applications, particularly for single-cell oil production [6], [7], [8]. However, the amount of lipid that accumulates depends on the strain, the carbon source and growth conditions. Under optimal conditions, some wild strains of Y. lipolytica can store 36 % of their cell dry weight (CDW) as lipids [1]; similar levels are observed in fed-batch cultures with glucose/glycerol [9]; 43% of the CDW may be lipid in continuous fermentations of industrial glycerol [10] and up to 54% may be lipid in batch cultures on a stearin-based medium [11], [12]. However, in flask cultures in which nitrogen concentration is not controlled, wild strains of Y. lipolytica do not generally accumulate more than 15% of their CDW as lipids when grown in glucose medium [13], [14], [15] or in wastewater [14], [15].
Most of the lipids accumulating in Y. lipolytica are triacylglycerols rather than free fatty acids (FFA), the ratio of these two types of compounds being 5/1 (triacylglycerols/FFA) [16]. C16 and C18 compounds are the most abundant lipids stored by this yeast. However, their relative quantities depend on the growth medium used. The Y. lipolytica strain W29 ( = CBS 7405) stores mostly C18:1 (54%), C16:0 (26%), C18:2 (12%) and a little C16:1 when cultured on glucose, whereas it accumulates C18:1 (66%), C16:1 (16%), C18:2 (9%) and a little C16:0 when cultured on oleic acid [17].
We investigated the emergence of oleaginous properties in yeasts, by comparative studies of Y. lipolytica W29 and strains from the eight species recently identified as members of the Yarrowia clade: Candida (C.) alimentaria, Y. deformans, C. galli, C. hispaniensis, C. hollandica, C. oslonensis, C. phangngensis and Y. yakushimensis [18], [19], [20], [21], [22], [23]. These strains were isolated from various biotic and abiotic environments. Limited physiological data for these strains are available, mostly based on the assimilation tests used for their identification. All these strains are non fermentative, grow as a mixture of cells and hyphae and use a very limited range of sugars, mostly glucose, as carbon sources. Additional data for growth on other carbon sources have provided evidence of phenotypic diversity [24], although different tests were carried out on different strains. For example, C. hispaniensis and C. oslonensis have been reported to use galactose and sorbose, which are only weakly metabolized, if at all, by other species, whereas C. hispaniensis is the only one of the species considered able to make use of trehalose. C. galli is the only one of these species that has been reported to grow in a vitamin-free environment; the failure of the other species to do so may result from thiamin auxotrophy, as reported in Y. lipolytica [25]. Tolerance to 10% NaCl differs between species, as does maximum growth temperature, which ranges from 27°C for C. alimentaria to 37°C for C. phangngensis, with most strains of other species growing little at temperatures above 30 to 32°C. It remains unclear whether this phenotypic diversity extends to the metabolism of hydrophobic compounds and, more generally, whether the oleaginous character of Y. lipolytica is particular to this species or common to some or all members of its clade. For example, data for growth on hexadecane and lipase production are patchy or absent for these species, with the exception of Y. deformans, in which three lipases have been purified and the corresponding genes have been cloned and sequenced [26], [27], [28].
We thus investigated the capacities of the type strains of the eight species and of the French wild-type strain W29 to use various hydrophobic and non hydrophobic substrates as carbon sources. Detailed growth kinetics were obtained on glucose and oleic acid media. Lipid profiles were determined, together with the capacity to accumulate and to synthesize lipids. The knowledge about the physiology of these strains provided by this study opens up new possibilities for genetic and transcriptomic analyses within the Y. lipolytica clade. The long-term objective will be to obtain a full understanding of lipid metabolism in this group, to improve the suitability of Y. lipolytica as a tool for biotechnological applications.
Materials and Methods
Yeast strains, media and growth conditions
The strains of the Yarrowia clade investigated in this study, their origins and references are listed in Table 1. All are wild-type prototroph strains. Strain names are abbreviated as follows: YALI (Y. lipolytica W29), YAYA (Y. yakushimensis CBS10253), YADE (Y. deformans CBS2071), YAGA (C. galli CBS9722), YAOS (C. oslonensis CBS10146), YAHO (C. hollandica CBS4855), YAPH (C. phangngensis CBS10407), YAAL (C. alimentaria CBS10151), YAHI (C. hispaniensis CBS9996). All strains were cultured at 28°C, with the exception of YAAL, for which the optimal growth temperature was 21°C (Additional Figure S1). Minimal medium base (MMB) contained 0.17% (wt/vol) yeast nitrogen base without amino acids and ammonium sulfate (Difco, Paris, France), 0.5% (wt/vol) NH4Cl and 50 mM phosphate buffer pH 6.8; 1.5% (wt/vol) agar was added when necessary. The solid rich medium base (YP) contained 1% (wt/vol) yeast extract and 1% (wt/vol) peptone, together with 1.5% (wt/vol) agar. Carbon sources were added at a final concentration of 2%; hydrophobic substrates were first emulsified by sonication of a 20% mixture in the presence of 0.625% Tween 40.
Table 1. Characteristics of the strains used in this study.
| Species | Strain number | Abbreviation | Place of isolation | Country | Reference |
| Yarrowia lipolytica | CBS 7504 = W29 | YALI | Parisian sewer | France | [25] |
| Yarrowia deformans | CBS 2071 | YADE | Fingernail | Austria | [23] |
| Candida galli | CBS 9722 | YAGA | Chicken liver | Georgia, USA | [18] |
| Yarrowia yakushimensis | CBS 10253 | YAYA | Termite gut | Japan | [23] |
| Candida oslonensis | CBS 10146 | YAOS | Kiwifruit yogurt | Norway | [20] |
| Candida phangngensis | CBS 10407 | YAPH | Seawater | Thailand | [21] |
| Candida hollandica | CBS 4855 | YAHO | Back of a cow | The Netherlands | [20] |
| Candida alimentaria | CBS 10151 | YAAL | Cured ham | Norway | [20] |
| Candida hispaniensis | CBS 9996 = Y−5580 | YAHI | Spondylus buprestoides larva | Spain | [19] |
Abbreviations: CBS, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; = W29, other common name used; = Y−5580, name from the NRRL collection.
Growth tests
Drop tests were performed with the 13 hydrophobic substrates (HS) listed in Table 2. Both solid rich medium and solid minimal medium were supplemented with 2% emulsified hydrophobic substrates, with the exception of alkanes, for which a paper inserted into the plate lid was soaked with alkane daily, to offset the effects of evaporation. Precultures were grown on plates of minimal medium containing 0.17% (wt/vol) yeast nitrogen base without amino acids and ammonium sulfate (Difco, Paris, France), 0.5% (wt/vol) (NH4)2SO4 and 1% glucose. We plated 3 µl of each of a set of five-fold dilutions, corresponding to 2 to 1250 cells. Pictures were taken daily or every two days over a period of three weeks. Growth was considered to be delayed, weak or slow with respect to the strain with the best growth in the study [29]. API ID 32 C galleries (Biomérieux, France) of 32 cupules (31 different carbon substrates plus one control), were used to evaluate the assimilation of a set of carbon sources (Additional Table S1).
Table 2. List of hydrophobic substrates used.
| Class of hydrophobic substrate | Substrate | Number of carbon atoms and insaturation number |
| Triglycerides | tributyrin | C4 |
| triolein | C18:1 | |
| Methylates | methyl hexanoate | C6 |
| methyl decanoate | C10 | |
| methyl myristate | C14 | |
| methyl palmitate | C16 | |
| methyl oleate | C18:1 | |
| Free fatty acids | hexanoic acid | C6 |
| oleic acid | C18:1 | |
| erucic acid | C22:1 | |
| Alkanes | decane | C10 |
| dodecane | C12 | |
| hexadecane | C16 |
Microtiter plate culture analysis
Growth tests were performed in 100 µl cultures in 96-well plates, with constant shaking, in the presence of 2% arabinose, fructose, glucose, N-acetyl-glucosamine, ribose, saccharose or xylose as the carbon source. Precultures were grown on minimal medium plates, as for the growth tests. Growth was monitored by measuring the optical density at 600 nm (OD600 nm) at 10-minute intervals, with a microtiter plate reader (Biotek, Colmar, France). For each strain and set of conditions, we used two biological replicates.
Growth curves and parameter determination
The nine strains were grown in MMB plus 0.15% yeast extract, with 2% glucose or 2% oleic acid, in 500-ml Erlenmeyer baffled flasks, to improve the dispersion of alkanes and oxygen supply [30]. Cells from overnight YPD cultures were used to inoculate the culture at an initial OD600 of 0.5. Biomass production was followed by measuring OD600nm every two or three hours. Cells grown in the presence of oleic acid were washed twice with 0.5% bovine serum albumin and then once with 0.9% NaCl before OD determination.
We used a custom-developed R software script [31] to derive parameters from the growth curves: length of the lag phase λ, growth rate corresponding to the maximum slope (µmax) and maximum cell density.
Lipid analysis
For fatty acid (FA) determinations, the strains were precultured and cultured under the same conditions and in the same media as for growth curve experiments. Two biological replicates were carried out. Samples of yeast cells were taken at three different growth stages and centrifuged at 3000 g for 5 min. The cell pellets were washed once with a half volume of 0.9% NaCl for glucose conditions and twice with a half volume of 0.5% bovine serum albumin and then once with a half volume of 0.9% NaCl for oleic acid conditions. Washed cells were freeze-dried for 24 h at 80°C.
The FA from the equivalent of 10 OD600 units of freeze-dried harvested cells were converted into their methyl esters by the Browse method [32]. The FA methyl esters were analyzed by gas chromatography, with a Varian 430-GC instrument equipped with a flame ionization detector and a CP SIL SCB low Bleed/MS column, for which the bleed specification at 260°C was 3 pA (30 m, 0.25 mm, 0,25 µm). FA were identified by comparison with commercial FA methyl ester standards (FAME32; Supelco) and quantified by the internal standard method, involving the addition of 75 µg of commercial C17:0 (Sigma). We determined the FA compositions from chromatograms with custom-developed Python and R software scripts [31].
Phylogeny
The phylogenetic tree was constructed by the concatenation of seven markers known to be representative of species evolution in fungi [33]. These markers were MS277 (YALI0B08756g homologs), MS444 (YALI0A00264g homologs), MS456 (YALI0B18722g homologs), MS561 (YALI0B16434g homologs), FG598 (YALI0A02695g homologs), and FG610 (YALI0D11220g homologs), together with EF-1alpha (YALI0C09141g homologs). DNA was extracted as described by Hoffman and Winston [34]. PCR amplifications were performed with an Eppendorf 2720 thermocycler, ex-Taq polymerase (Takara) and the primers listed in Additional Table S2. Both strands were sequenced by GATC Biotech (Mulhouse, France).
The Staden package was used to analyze sequencing reads [35]. We used MUSCLE [36] to align sequences, and columns of gap-containing residues were removed manually. A final alignment of 2204 amino acids was obtained after sequence concatenation. Phylogenetic trees were constructed either by neighbor-Joining in ClustalX [37], or by maximum likelihood, with PHYML [38] and a JTT substitution model corrected for heterogeneity between sites by a Γ-law distribution, with four different categories of evolution rates. The proportion of invariable sites and the α-parameter of the Γ-law distribution were optimized according to the data. A bootstrap value was calculated from 100 replicates.
Results
Use of non hydrophobic carbon sources
Carbon assimilation tests have been performed for identification of the different species of the Yarrowia clade [18], [19], [20], [21], [22], [23]. However, to obtain a homogeneous set of data, we assessed the ability of the nine strains to use 31 compounds. API galleries were initially used, for 30 compounds. The results were compared with the data available from the CBS (http://www.cbs.knaw.nl/). For 11 carbon sources, our observations were not consistent with those of the CBS for some strains, possibly reflecting differences in experimental procedures (API galleries, microtiter plate or agar plate tests) or carbon source concentration. Thus, liquid cultures in microtiter plates were used for these substrates, with glucose and sucrose used as positive and negative controls, respectively. These experiments confirmed that YALI and YAPH did not grow on arabinose, that YAGA grew on glycerol, and that YALI and YADE grew on N-acetyl-glucosamide. We also tested fructose as a carbon source under the same conditions (Additional Table S1).
Finally, only three of the 31 compounds (fructose, glycerol and glucose) supported the growth of all species and none of the species could grow on 21 other carbon sources. For the remaining seven compounds, differences in growth were observed between species. YAHI, which belongs to the most divergent species, was unique in being able to grow on trehalose and unable to use lactate and erythritol. Growth on mannitol, N-acetyl-glucosamine, potassium gluconate and sorbitol varied between strains.
Growth capacities on hydrophobic substrates
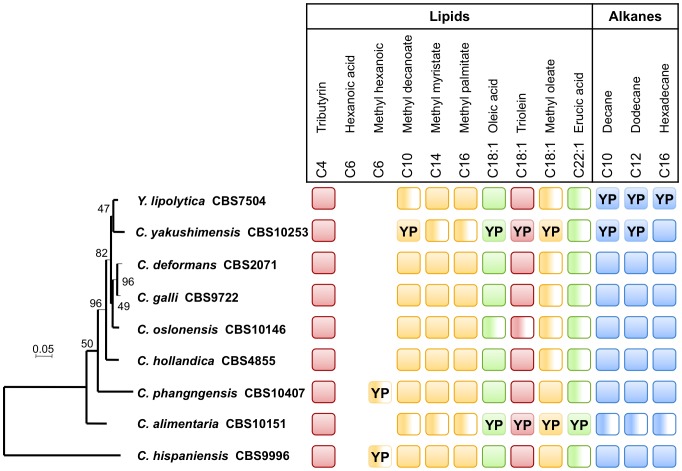
We assessed growth on lipids and alkanes by carrying out drop tests for 13 HS, initially on MMB (Table 2). Three of the 10 lipid sources tested supported the growth of all species (tributyrin, methyl myristate and methyl palmitate), two were not used by any of the species (hexanoic acid and methyl hexanoic, both C6 compounds) and the remaining five were used by some, but not all species. The strains differed in their ability to grow on the three alkanes (Figure 1). Thus, all strains were able to grow on at least some hydrophobic substrates. However, some displayed growth delay or an absence of growth on specific substrates. YAAL could not grow on C18:1 whatever the type of compound supplied (triglyceride, methyl or fatty acid) or on C22:1. On all other media, growth was delayed or weak, except in the presence of tributyrin. Similarly, YAYA did not grow on the three types of C18:1 or on C10 (methyl decanoate). On all other lipids except tributyrin, growth was delayed or weak and hexadecane (C16) was the only alkane of the three tested that supported the growth of this strain. Surprisingly, YALI formed no colonies on alkane minimal media under these growth conditions.
Figure 1. Growth capacities of Yarrowia strains on 13 hydrophobic substrates as a function of their phylogenetic position.
Squares represent growth capacity on minimal and rich (YP) media supplemented with triglycerides in red, methylates in yellow, free fatty acids in green and alkanes in blue. The squares marked YP reflect the capacity to grow on the YP base used as a carbon source, in the presence of the corresponding HS. Half-colored squares indicate slow or weak growth. The absence of a square indicates an absence of growth on both YP and minimal media. The phylogenetic tree was constructed by the concatenation of seven markers (2204 amino acids) and its robustness was estimated with a bootstrap of 100 replicates.
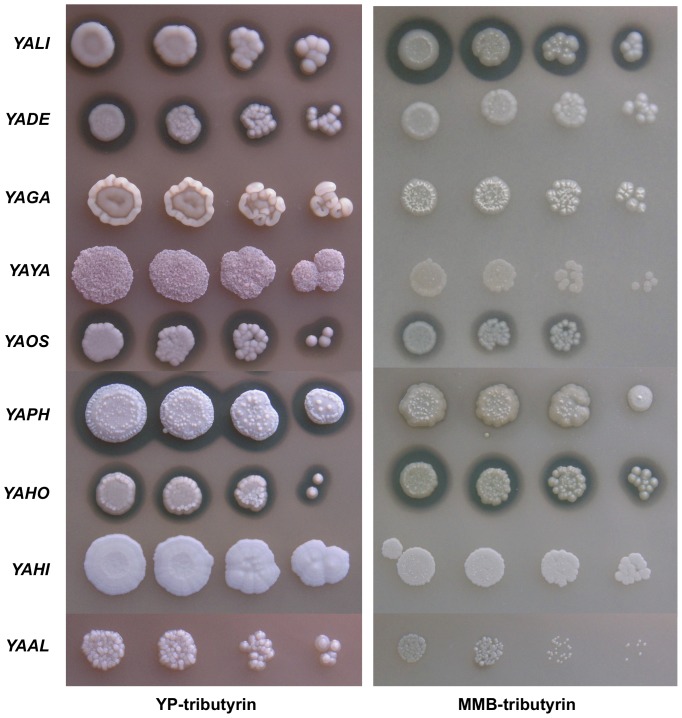
There are three possible reasons for the inability of a strain to grow on a given substrate: the substrate may not be taken up by the cells, it may not be metabolized or it may have a toxic effect, preventing growth. We tested the hypothesis of toxicity by repeating the drop tests on a rich medium (YP, yeast extract plus peptone) supplemented with hydrophobic compounds (Figure 2, Figure 3 and additional Figure S2). Under these conditions, all strains could grow in the presence of all the hydrophobic substrates tested, with two exceptions, as described below. The results of these tests indicated that some hydrophobic substrates neither supported nor inhibited cell growth. The two exceptions were the two C6 compounds (hexanoic acid and methyl hexanoic acid), which strongly inhibited all strains, although YAPH and YAHI were able to grow on YP-methyl hexanoic after 20 days. Similarly, on MMB-methyl decanoate, no growth defect was observed at low cell density, whereas growth was strongly inhibited at high cell density. No such growth inhibition was observed on YP-methyl decanoate (Figure 2).
Figure 2. Drop tests on methyl decanoate in MMB and YP media, after 9 days of culture.
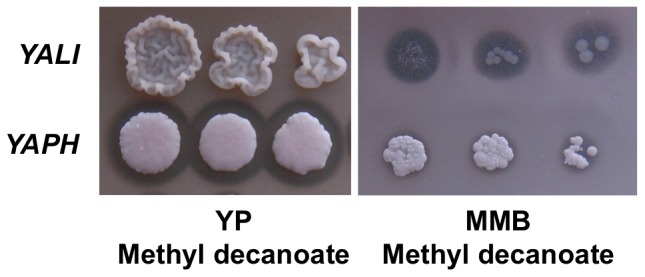
Three spots are shown, corresponding to approximately 25 to 625 cells, from right to left. Strains are represented by an abbreviation of their species name, as shown in Table 1.
Figure 3. Drop tests on YP- and MMB-tributyrin media after 5 days of culture.
Four spots are shown, with approximately 5 to 625 cells, from right to left. A halo is present around strains YALI, YADE, YAOS, YAPH and YAHO. Strains are represented by an abbreviation of their species name, as shown in Table 1.
Morphology on plates was very variable, ranging from smooth to rough colonies, and from superficial to agar-invasive forms, depending on the medium (see figures 2, 3 and data not shown). The presence of a halo around cells grown on plate containing insoluble substrates generally reflects the secretion of surfactants or of extracellular enzymes, such as lipases or esterases [39]. Only four of the 13 HS tested on MMB and YP media were associated with halo formation: tributyrin, methyl decanoate, oleic acid and triolein (Additional Table S3). Some strains formed a halo on MMB or YP tributyrin plates, whereas others did not (Figure 3). The presence of a halo was not associated with colony size and depended on the medium, i.e. MMB or YP. For instance, YAOS formed a large halo on YP-tributyrin but grew less well than YAHI, which did not form a halo.
Lipid storage capacities are variable within the Yarrowia clade
The best way to determine the storage capacity of yeasts with minimal bias due to de novo synthesis is to estimate their lipid content on a lipid-based growth medium. We determined growth characteristics, such as lag phase, generation time and maximal OD600, on the standard medium used for this purpose in Y. lipolytica: an oleic acid medium supplemented with small amounts of yeast extract to facilitate the transition from sugar to hydrophobic carbon sources [17]. (Figure S3A). All strains grew in this medium, without a lag phase (Table 3). Generation time and maximum cell growth differed between species, ranging from 2.06 h to 3.91 h and from 17.6 to 53.1 OD600, respectively. YAHI was the fastest growing species on oleic acid, reaching 53.10 OD600 units after about 23 h of growth, with a generation time of 2.06 h. By contrast, YAGA seemed be assimilate oleic acid much less efficiently, with a generation time almost twice that of YAHI. We were therefore unable to use the standard protocols for estimating lipid storage in Y. lipolytica. Thus, to ensure that the data obtained were comparable, we had to identify similar physiological stages not necessarily corresponding to the same time in culture.
Table 3. Growth parameters on oleic acid and glucose media.
| Strain | µmax (h−1) | Generation time (h) | Lag phase λ (h) | Maximum cell density (OD600) |
| Oleic acid | ||||
| YALI | 0.21 | 3.27 | - | 35.31 |
| YAYA | 0.18 | 3.73 | - | 20.35 |
| YADE | 0.25 | 2.74 | - | 17.60 |
| YAGA | 0.18 | 3.91 | - | 23.52 |
| YAOS | 0.22 | 3.12 | - | 19.98 |
| YAHO | 0.26 | 2.67 | - | 33.66 |
| YAPH | 0.32 | 2.18 | - | 46.8 |
| YAAL | 0.28 | 2.46 | - | 28.27 |
| YAHI | 0.34 | 2.06 | - | 53.10 |
| Glucose | ||||
| YALI | 0.44 | 1.56 | 2.54 | 25.00 |
| YAYA | 0.71 | 0.97 | 5.19 | 20.86 |
| YADE | 0.78 | 0.89 | 5.61 | 17.80 |
| YAGA | 0.42 | 1.64 | 3.38 | 23.71 |
| YAOS | 0.63 | 1.09 | 5.71 | 19.00 |
| YAHO | 0.53 | 1.32 | 3.85 | 27.86 |
| YAPH | 0.57 | 1.21 | 1.26 | 29.14 |
| YAAL | 0.64 | 1.08 | 8.77 | 23.2 |
| YAHI | 0.53 | 1.32 | 7.93 | 17.3 |
We estimated the maximal level of lipid storage, by determining cellular lipid content at various time points. Based on the growth curves obtained, we selected three time points between the beginning of the exponential phase and the deceleration phase. These time points corresponded roughly to the time required to obtain one fifth of the maximum OD600 (OD max), half the OD max and the OD max. An example is provided in Additional Figure S4. For lipid extraction, we collected cells for two biological replicates at about the three time points derived from the growth curve (Additional Table S4).
The total lipid content of cells grown on oleic acid is shown in Figure 4A. Three different types of behavior were observed. The first was characterized by an increase in lipid accumulation during the exponential growth phase followed by a decrease in lipid levels during the deceleration phase, possibly corresponding to lipid reconsumption. This pattern was observed in YALI, YAGA, YAAL and YAHI. The second type of pattern consisted of a continuous increase in lipid content and was observed in YAOS and YAPH. The three remaining strains, YADE, YAYA and YAHO, displayed decreases in lipid content over time. For instance, the lipid content of Y. deformans CBS2071 decreased from 34.4% to 15.2% of CDW.
Figure 4. Lipid content (% CDW) of Yarrowia strains over time.
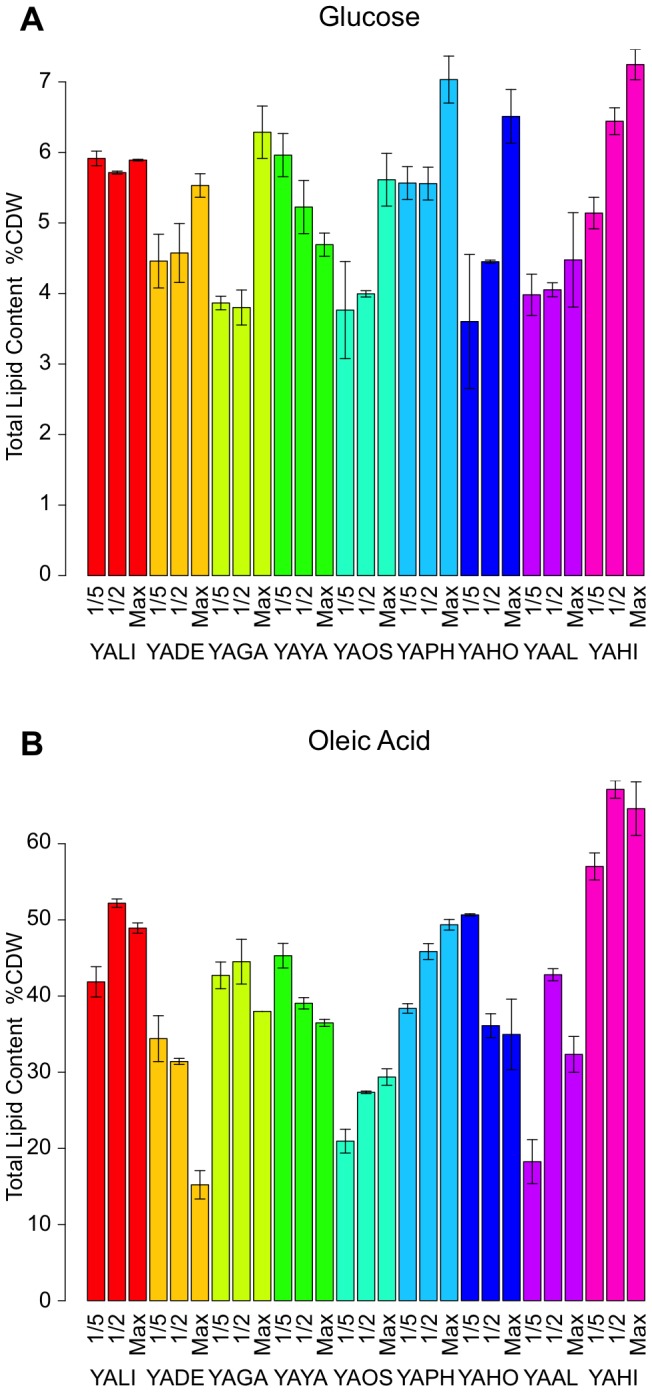
For each species, the histogram represents three different physiological states: 1/5 of maximum growth, 1/2 maximum growth and maximum growth. Error bars indicate the deviation from the mean deduced from two replicates. Analyses were performed on both glucose (A) and oleic acid (B). Strains are represented by an abbreviation of their species name, as shown in Table 1.
The maximal level of lipid storage differed considerably between species, ranging from 29.4% of CDW for YAOS to 67.1% of CDW for YAHI. YALI was the second most efficient strain for lipid storage, which accounted for 52.2% of CDW.
By contrast, overall lipid composition remained stable over time for all species (Additional Table S5). Most of the lipid was in the form of C18:1(n-9), as expected given that the main carbon source in the growth medium was oleic acid. In YALI, YAGA, YAHI and YAHO, the amount of C18:1(n-9) varied from 23.1 to 38.3 % of CDW, suggesting that oleic acid uptake was more efficient in these species than in the other strains tested (Additional Fig S5). The second most abundant compound accumulating was generally C16:1(n-7), but its level was always low and varied from 0.7 to 11.8 % of CDW.
De novo lipid synthesis
We then determined the capacity for de novo lipid synthesis of the nine strains. This capacity was estimated on a glucose medium supplemented with yeast extract: a rich medium devoid of hydrophobic compounds. As in evaluations of storage capacity, we first established growth curves (Additional Figure S3B). By contrast to what was observed on oleic acid, there was a conspicuous lag phase for all strains, lasting 1.26 to 8.77 h, and generation time was clearly shorter than on oleic acid media, but remained variable (0.89 to 1.64 h). OD max values were similar on the two media for all strains except YALI, YAPH and YAHI, for which the OD max on glucose was lower than that on oleic acid, by a factor of up to three. For instance, the OD max for YAHI was 17.3 on glucose and 53.1 on oleic acid.
We then measured lipid contents at three time points defined as described above: 1/5, 1/2 and 1 OD max (Additional Table S4). Almost all the strains presented similar patterns of total lipid content, with large increases during the deceleration phase (Figure 4B). However, slight differences in kinetics were observed. For example, in YAHI, the increase in lipid content was more progressive, remaining significant during the deceleration phase (from 5.1 to 7.2% of CDW) whereas the increase was less pronounced for YAAL (from 4.0 to 4.5% of CDW). There were two exceptions to this rule: YALI, the lipid content of which remained stable over time, and YAYA, the lipid content of which decreased gradually from 6 to 4.7% of CDW.
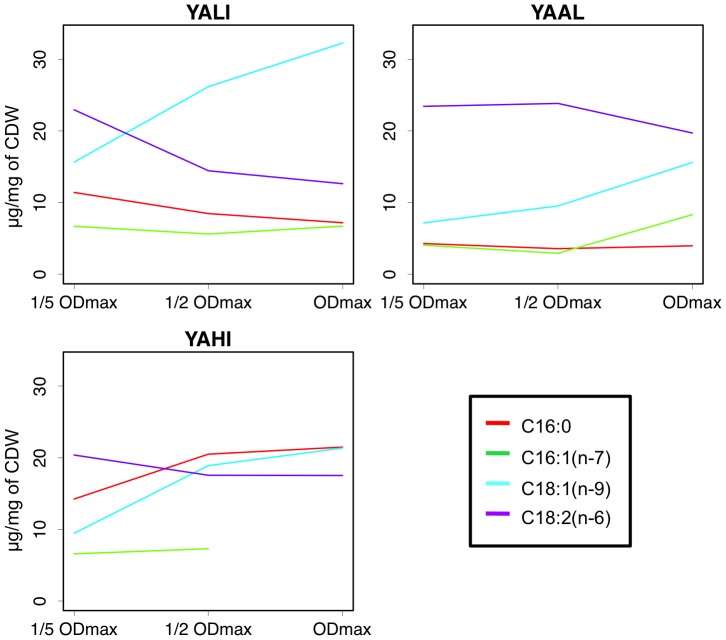
In most strains, C18:1(n-9) was the major compound that accumulated, its levels increasing over the entire time course. However, in YAAL, YAYA and YAHI, this compound was not the principal compound at OD max. In YAAL and YAYA, C18:2(n-6) was the major compound at all three time points considered. In YAHI, the increase in lipid content was due to both C16:0 and C18:1(n-9) (Figure 5, Additional Fig S5, Additional Table S5).
Figure 5. Changes in cellular lipid composition over time in strains grown on glucose media.
YALI: Yarrowia lipolytica W29, YAAL: C. alimentaria CBS10151, YAHI: C. hispaniensis CBS9996. Quantities are in mg/g CDW.
Discussion
We analyzed eight closely related species of the Yarrowia clade, to obtain preliminary data concerning their physiology and, in particular, their ability to grow on and to assimilate HS, before beginning comparative genomic and transcriptomic studies. All the data discussed below were obtained from a single isolate per species and may, therefore, not be representative of the entire species.
As expected on the basis of published findings, despite their isolation from highly diverse environments (Table 1), these strains displayed a very limited range of non hydrophobic substrate used, with low levels of variability[18], [19], [20], [21]. Glucose, fructose and glycerol were the only non hydrophobic carbon sources used by all strains of the clade. The screening of these strains for the detection of enzymatic activities useful for the exploitation of agricultural by-products, such as xylose degradation, does not therefore appear to be pertinent.
As Y. lipolytica uses HS efficiently, we tested the eight strains on 13 different substrates (alkanes, fatty acids and their methylated derivatives, and triglycerides) with carbon chains of different lengths (C4 to C22). Most of these substrates had never before been tested as substrates for species of this clade. We observed differences in growth on these substrates, the size of the hydrolysis halo and sensitivity to short-chain fatty acids between the species tested. These fatty acids were toxic to all strains, but YAPH and YAHI were able to grow on YP in the presence of C6 after a very long time lag.
Toxicity was clearly observed for YALI on MMB-methyldecanoate, but not on YP methyldecanoate (Figure 2). This may result from the balance between transport and degradation on the one hand and the hydrolysis of methyl-C10 by lipases/esterases to release decanoate, a compound toxic to cells, on the other. At high cell density, methyl-C10 hydrolysis by lipases may occur too rapidly, releasing too much toxic C10 for the cells to cope with, whereas, at lower cell density, the release of toxic C10 might be slow enough to prevent cell intoxication but permit cell growth. The halo was smaller for YAPH on the same medium, which thus released C10 at a rate compatible with growth. On YP supplemented with methyl-C10, both strains grew vigorously. All strains of the Yarrowia clade were able to grow efficiently on YP in the absence of any other carbon source (see additional Figure S2). On YP-methyl decanoate, no hydrolysis halo was observed with YALI, suggesting that the corresponding lipase/esterase is repressed in the presence of peptone. This putative repression was not observed with YAPH, suggesting that cell intoxication was prevented either by slow C10 uptake or by the rapid β-oxidation of C10. Further studies involving comparative genomics and transcriptomics would make it possible to test these hypotheses. These approaches might also shed light on the evolution of the POX and LIP gene networks in YAPH and YALI, in which six different acyl-CoA oxidases (Pox1p to Pox6p) of different chain length specificities have been described [40], [41], [42], together with 16 lipases and four esterases with different patterns of regulation and chain-length specificities [7].
We tried to determine whether these yeasts were oleaginous, by analyzing lipid accumulation and synthesis. However, the concept of what constitutes an oleaginous yeast remains unclear. Some authors consider that microorganisms should be able to accumulate more than 20% of CDW in the form of lipid to be considered oleaginous, whereas others consider that oleaginous organisms should be able to synthesize lipids from non HS carbon sources and to store them [8]. This is a crucial difference as some species are probably able to synthesize, but not to accumulate lipids efficiently, whereas other species may only accumulate lipids from the extracellular medium efficiently. These two capacities should thus be estimated independently, on different carbon sources, such as glucose for lipid synthesis and a model oleic acid medium previously used for YALI for the assessment of storage capacities [16].
Our results demonstrated that all strains cultured on oleic acid accumulated more than 30% of their CDW in the form of lipid at least one time point. Our findings suggest that all species of the clade are oleaginous. Most accumulated smaller amounts of lipid than YALI, but at least one, YAHI, accumulated almost 30% more lipid than YALI. However, different accumulation profiles were observed during the growth phase: some strains progressively accumulated triglycerides (YAOS and YAPH), others remobilized lipids (YADE, YAGA and YAHO), and lipid content remained essentially stable in YALI and YAAL. Lower and less variable levels of lipid accumulation were observed on glucose. Depending on the strain, the major compound accumulated was C18:1, C18:2 or C16:0. No molecules with a longer chain length were observed, by contrast to what has been reported for Saccharomyces cerevisiae and Hansenula polymorpha [43], [44]. Candida albicans can synthesize and accumulate C18:3 to levels of up to 22.7% of total fatty acids [43], but none of the yeasts of the Yarrowia clade was able to synthesize C18:3. This finding is consistent with the absence from the nuclear genomes of YAGA, YAYA, YAAL, YAPH and YAHI of a gene encoding a Δ15-desaturase, whereas homologs of YALI0C05951g (Δ9-desaturase) and YALI0B10153g (Δ12-desaturase) were identified (unpublished data). The variable levels of the various compounds may reflect differences in the activity or expression of the Δ12-desaturase (for YAAL, YAYA) or elongases (for YAHI).
The major conclusion of this work is that all species of the Yarrowia clade are oleaginous, but that they differ in their profiles of HS use and lipid accumulation. These data should facilitate the development of more robust, better performing strains for lipid accumulation and for fatty acid profile modification. Our findings also suggest that there are metabolic and genetic differences between the strains of the clade studied here, possibly reflecting species-specific differences in gene content and/or regulation. C. hispaniensis, which grew well on diverse substrates and displayed a high storage capacity, appears to be a promising model for biotechnological applications per se or as a source of genes for the improvement of Y. lipolytica. Further comparative genomic and transcriptomic studies are underway in the Yarrowia clade, to establish correlations with phenotypic variability and to obtain evidence for their genetic bases.
Supporting Information
Growth curves of YAAL on YPD at different temperatures. The growth curves at 15°C, 21°C and 25°C are shown as blue squares, green dots, and red triangles, respectively.
(PDF)
Drop tests on YP (A) and MMB (B) media with alkanes and methyl-esters of various chain lengths. Only one spot is presented for strains YALI, YADE, YAGA, YAYA and YAOS. Strains are represented by an abbreviation of their species name, as shown in Table 1.
(PDF)
Growth curves established on 2% oleic acid (A) and 2% glucose (B) media for the strains of the Yarrowia clade, over a period of 50 h. Strain names are abbreviated as follows: YALI (Y. lipolytica W29), YAYA (Y. yakushimensis CBS10253), YADE (Y. deformans CBS2071), YAGA (C. galli CBS9722), YAOS (C. oslonensis CBS10146), YAHO (C. hollandica CBS4855), YAPH (C. phangngensis CBS10407), YAAL (C. alimentaria CBS10151), YAHI (C. hispaniensis CBS9996).
(PDF)
Growth curve and time points used for YAGA cultured on glucose (2%). The experimental growth curve is shown in red. Horizontal dotted lines correspond to the maximum OD, 1/2 OD max and 1/5 of OD max. Circles, triangles and diamonds indicate the OD at the time points used for lipid accumulation tests, at 4.6 h, 11.6 h and 23.7 h of culture, respectively.
(PDF)
Changes in cellular lipid composition over time for strains grown on oleic acid (A) and glucose (B). Quantities are in mg/g CDW. For each species, three different physiological states are represented: 1/5 the OD max (1/5), 1/2 the OD max (1/2) and the OD max (max).
(PDF)
Growth capacities of the species of the Yarrowia clade on non hydrophobic carbon.
(XLSX)
Primers used to amplify the protein-coding genes.
(XLSX)
Presence of a halo on solid hydrophobic media.
(XLSX)
Characteristics of growth over time for cultures on oleic acid and glucose media: time points, optical density, lipid content and cell dry weight.
(XLSX)
Profile of fatty acid accumulation (% of CDW) on glucose and oleic acid media.
(XLS)
Acknowledgments
We thank Thanos Beopoulos, Vincent Sauveplane and Thierry Dulermo for technical assistance with lipid content analysis and stimulating discussions.
Funding Statement
SM has a PhD fellowship funded by the French Minister of Research via the ABIES doctoral school. This work was supported in part by the Institut National de la Recherche Agronomique (INRA) and additionally by the SAS PIVERT, in the frame of the French Institute of Excellence in the field of Low-Carbon Energies (IEED) P.I.V.E.R.T (www.institut-pivert.com) selected as an Investment for the Future ("Investissements d'Avenir"). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ratledge C (1991) Microorganisms for lipids. Acta Biotechnologica 11: 429–438. [Google Scholar]
- 2. Poncet S, Arpin M (1965) Candida species without fermentative capacity (Cryptococcoceae). Antonie Van Leeuwenhoek 31: 433–464. [DOI] [PubMed] [Google Scholar]
- 3.Barnett JA, Payne RW, Yarrow D (2000) Yeasts: Characteristics and Identification. Cambridge: Cambridge University Press. 1150 p. [Google Scholar]
- 4. Klug MJ, Markovetz AJ (1967) Degradation of Hydrocarbons by Members of the Genus Candida II. Oxidation of n-Alkanes and 1-Alkenes by Candida lipolytica . Journal of Bacteriology 93: 1847–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nyns EJ, Chiang N, Wiaux AL (1968) Comparative lipid content of Candida lipolytica grown on glucose and onn-hexadecane. Antonie Van Leeuwenhoek 34: 197–204. [DOI] [PubMed] [Google Scholar]
- 6. Nicaud JM (2012) Yarrowia lipolytica . Yeast 29: 409–418. [DOI] [PubMed] [Google Scholar]
- 7. Fickers P, Marty A, Nicaud JM (2011) The lipases from Yarrowia lipolytica: genetics, production, regulation, biochemical characterization and biotechnological applications. Biotechnol Adv 29: 632–644. [DOI] [PubMed] [Google Scholar]
- 8. Beopoulos A, Chardot T, Nicaud JM (2009) Yarrowia lipolytica: A model and a tool to understand the mechanisms implicated in lipid accumulation. Biochimie 91: 692–696. [DOI] [PubMed] [Google Scholar]
- 9.Fillaudeau L, Cescut J, Anne-Archard D, Nicaud JM, Uribelarrea JL, et al. (2009) Morphology and rheological behaviour of Yarrowia lipolytica during production of intra-cellular energetic molecules: impact of lipid accumulation and genetic modifications. 8th World Congress of Chemical Engineering, WCCE8, Montréal, Canada. [Google Scholar]
- 10. Papanikolaou S, Aggelis G (2002) Lipid production by Yarrowia lipolytica growing on industrial glycerol in a single-stage continuous culture. Bioresource Technology 82: 43–49. [DOI] [PubMed] [Google Scholar]
- 11. Papanikolaou S, Muniglia L, Chevalot I, Aggelis G, Marc I (2002) Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J Appl Microbiol 92: 737–744. [DOI] [PubMed] [Google Scholar]
- 12. Papanikolaou S, Chevalot I, Komaitis M, Marc I, Aggelis G (2002) Single cell oil production by Yarrowia lipolytica growing on an industrial derivative of animal fat in batch cultures. Appl Microbiol Biotechnol 58: 308–312. [DOI] [PubMed] [Google Scholar]
- 13. Papanikolaou S, Chatzifragkou A, Fakas S, Galiotou-Panayotou M, Komaitis M, et al. (2009) Biosynthesis of lipids and organic acids by Yarrowia lipolytica strains cultivated on glucose. European Journal of Lipid Science and Technology 111: 1221–1232. [Google Scholar]
- 14. Chi Z, Zheng Y, Jiang A, Chen S (2011) Lipid Production by Culturing Oleaginous Yeast and Algae with Food Waste and Municipal Wastewater in an Integrated Process. Applied Biochemistry and Biotechnology 165: 442–453. [DOI] [PubMed] [Google Scholar]
- 15. Hong S-P, Seip J, Walters-Pollak D, Rupert R, Jackson R, et al. (2012) Engineering Yarrowia lipolytica to express secretory invertase with strong FBA1IN promoter. Yeast 29: 59–72. [DOI] [PubMed] [Google Scholar]
- 16. Beopoulos A, Mrozova Z, Thevenieau F, Le Dall MT, Hapala I, et al. (2008) Control of lipid accumulation in the yeast Yarrowia lipolytica . Appl Environ Microbiol 74: 7779–7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mlíčková K, Roux E, Athenstaedt K, d'Andrea S, Daum G, et al. (2004) Lipid accumulation, lipid body formation, and acyl coenzyme A oxidases of the yeast Yarrowia lipolytica . Appl Environ Microbiol 70: 3918–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Péter G, Dlauchy D, Vasdinyei R, Tornai-Lehoczki J, Deák T (2004) Candida galli sp. nov., a new yeast from poultry Antonie van Leeuwenhoek 86: 105–110-110. [DOI] [PubMed] [Google Scholar]
- 19. Kurtzman CP (2005) New species and a new combination in the Hyphopichia and Yarrowia yeast clades. Antonie van Leeuwenhoek 88: 121–130. [DOI] [PubMed] [Google Scholar]
- 20. Knutsen AK, Robert V, Poot GA, Epping W, Figge M, et al. (2007) Polyphasic re-examination of Yarrowia lipolytica strains and the description of three novel Candida species: Candida oslonensis sp. nov., Candida alimentaria sp. nov. and Candida hollandica sp. nov. Int J Syst Evol Microbiol 57: 2426–2435. [DOI] [PubMed] [Google Scholar]
- 21. Limtong S, Youngmanitchai W, Kawasaki H, Seki T (2008) Candida phangngensis sp. nov., an anamorphic yeast species in the Yarrowia clade, isolated from water in mangrove forests in Phang-Nga Province, Thailand. Int J Syst Evol Microbiol 58: 515–519. [DOI] [PubMed] [Google Scholar]
- 22.Kurtzman CP (2011) Yarrowia van der Walt & von Arx (1980). In: Kurtzman CP, Fell JW, Boekhout T, editors. The Yeasts, a taxonomic study. London: Elsevier. pp. 927–929. [Google Scholar]
- 23.Groenewald M, Smith M (2013) The teleomorph state of Candida deformans Langeron & Guerra and description of Yarrowia yakushimensis comb. nov. Antonie van Leeuwenhoek: 1–6. [DOI] [PubMed] [Google Scholar]
- 24.Lachance M-A, Kurtzman CP (2011) Lachancea Kurtzman (2003). In: Kurtzman CP, Fell JW, Boekhout T, editors. The Yeasts, a taxonomic study. London: Elsevier. pp. 511–519. [Google Scholar]
- 25.Barth G, Gaillardin C (1996) Yarrowia lipolytica. In: Wolf WK, editor. Non-Conventional Yeasts in Biotechnology. Berlin: Springer-Verlag. pp. 313–388. [Google Scholar]
- 26. Bigey F, Tuery K, Bougard D, Nicaud J-M, Moulin G (2003) Identification of a triacylglycerol lipase gene family in Candida deformans: molecular cloning and functional expression. Yeast 20: 233–248. [DOI] [PubMed] [Google Scholar]
- 27. Boutur O, Dubreucq E, Galzy P (1995) Factors influencing ester synthesis catalysed in aqueous media by the lipase from Candida deformans (zach) langeron and guerra. Journal of Biotechnology 42: 23–33. [DOI] [PubMed] [Google Scholar]
- 28. Muderhwa JM, Ratomahenina R, Pina M, Graille J, Galzy P (1985) Purification and properties of the lipase from Candida deformans (zach) langeron and guerra. Journal of the American Oil Chemists Society 62: 1031–1036. [Google Scholar]
- 29.Kurtzman CP, Fell JW, Boekhout T, Robert V (2011) Methods for isolation, phenotypic characterisation and maintenance of yeasts. In: Kurtzman CP, Fell JW, Boekhout T, editors. The Yeasts, a taxonomic study. London: Elsevier. pp. 87–110. [Google Scholar]
- 30.Mauersberger S (1991) Mutants of alkane oxidation in the yeasts Yarrowia lipolytica and Candida maltosa. In: Sharyshev AA, Finogenova TV, editors. Alkane metabolism and oversynthesis of metabolites by microorganisms. Pushchino, USSR: Center for Biological Research, USSR Academy of Sciences. pp. 59–78. [Google Scholar]
- 31.R Development Core Team (2011) R: A language and environment for statistical computing. In: Computing RFfS, editor. Vienna, Austria. [Google Scholar]
- 32. Browse J, McCourt PJ, Somerville CR (1986) Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Analytical Biochemistry 152: 141–145. [DOI] [PubMed] [Google Scholar]
- 33. Aguileta G, Marthey S, Chiapello H, Lebrun MH, Rodolphe F, et al. (2008) Assessing the performance of single-copy genes for recovering robust phylogenies. Syst Biol 57: 613–627. [DOI] [PubMed] [Google Scholar]
- 34. Hoffman CS, Winston F (1987) A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli . Gene 57: 267–272. [DOI] [PubMed] [Google Scholar]
- 35. Bonfield JK, Smith K, Staden R (1995) A new DNA sequence assembly program. Nucleic Acids Res 23: 4992–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Edgar R (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thompson JD, Gibson TJ, Plewniak Fdr, Jeanmougin Fo, Higgins DG (1997) The CLUSTAL_X Windows Interface: Flexible Strategies for Multiple Sequence Alignment Aided by Quality Analysis Tools. Nucleic Acids Research 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 39. Pignede G, Wang H, Fudalej F, Gaillardin C, Seman M, et al. (2000) Characterization of an Extracellular Lipase Encoded by LIP2 in Yarrowia lipolytica . J Bacteriol 182: 2802–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang H, Le Dall M-T, Waché Y, Laroche C, Belin J-M, et al. (1999) Cloning, sequencing, and characterization of five genes coding for Acyl-CoA oxidase isozymes in the yeast Yarrowia lipolytica . Cell Biochemistry and Biophysics 31: 165–174. [DOI] [PubMed] [Google Scholar]
- 41. Luo YS, Nicaud JM, Van Veldhoven PP, Chardot T (2002) The acyl-CoA oxidases from the yeast Yarrowia lipolytica: characterization of Aox2p. Arch Biochem Biophys 407: 32–38. [DOI] [PubMed] [Google Scholar]
- 42. Luo YS, Wang HJ, Gopalan KV, Srivastava DK, Nicaud JM, et al. (2000) Purification and characterization of the recombinant form of Acyl CoA oxidase 3 from the yeast Yarrowia lipolytica . Arch Biochem Biophys 384: 1–8. [DOI] [PubMed] [Google Scholar]
- 43. Tylicki A, Siemieniuk M, Dobrzyn P, Ziolkowska G, Nowik M, et al. (2012) Fatty acid profile and influence of oxythiamine on fatty acid content in Malassezia pachydermatis, Candida albicans and Saccharomyces cerevisiae . Mycoses 55: e106–e113. [DOI] [PubMed] [Google Scholar]
- 44. Prasitchoke P, Kaneko Y, Bamba T, Fukusaki E, Kobayashi A, et al. (2007) Identification and characterization of a very long-chain fatty acid elongase gene in the methylotrophic yeast, Hansenula polymorpha . Gene 391: 16–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Growth curves of YAAL on YPD at different temperatures. The growth curves at 15°C, 21°C and 25°C are shown as blue squares, green dots, and red triangles, respectively.
(PDF)
Drop tests on YP (A) and MMB (B) media with alkanes and methyl-esters of various chain lengths. Only one spot is presented for strains YALI, YADE, YAGA, YAYA and YAOS. Strains are represented by an abbreviation of their species name, as shown in Table 1.
(PDF)
Growth curves established on 2% oleic acid (A) and 2% glucose (B) media for the strains of the Yarrowia clade, over a period of 50 h. Strain names are abbreviated as follows: YALI (Y. lipolytica W29), YAYA (Y. yakushimensis CBS10253), YADE (Y. deformans CBS2071), YAGA (C. galli CBS9722), YAOS (C. oslonensis CBS10146), YAHO (C. hollandica CBS4855), YAPH (C. phangngensis CBS10407), YAAL (C. alimentaria CBS10151), YAHI (C. hispaniensis CBS9996).
(PDF)
Growth curve and time points used for YAGA cultured on glucose (2%). The experimental growth curve is shown in red. Horizontal dotted lines correspond to the maximum OD, 1/2 OD max and 1/5 of OD max. Circles, triangles and diamonds indicate the OD at the time points used for lipid accumulation tests, at 4.6 h, 11.6 h and 23.7 h of culture, respectively.
(PDF)
Changes in cellular lipid composition over time for strains grown on oleic acid (A) and glucose (B). Quantities are in mg/g CDW. For each species, three different physiological states are represented: 1/5 the OD max (1/5), 1/2 the OD max (1/2) and the OD max (max).
(PDF)
Growth capacities of the species of the Yarrowia clade on non hydrophobic carbon.
(XLSX)
Primers used to amplify the protein-coding genes.
(XLSX)
Presence of a halo on solid hydrophobic media.
(XLSX)
Characteristics of growth over time for cultures on oleic acid and glucose media: time points, optical density, lipid content and cell dry weight.
(XLSX)
Profile of fatty acid accumulation (% of CDW) on glucose and oleic acid media.
(XLS)