Abstract
Background
Bivalves play an important role in the ecosystems they inhabit and represent an important food source all over the world. So far limited genetic research has focused on this group of animals largely due to the lack of sufficient genetic or genomic resources. Here, we performed de novo transcriptome sequencing to produce the most comprehensive expressed sequence tag resource for Zhikong scallop (Chlamys farreri), and conducted the first transcriptome comparison for scallops.
Results
In a single 454 sequencing run, 1,033,636 reads were produced and then assembled into 26,165 contigs. These contigs were then clustered into 24,437 isotigs and further grouped into 20,056 isogroups. About 47% of the isogroups showed significant matches to known proteins based on sequence similarity. Transcripts putatively involved in growth, reproduction and stress/immune-response were identified through Gene ontology (GO) and KEGG pathway analyses. Transcriptome comparison with Yesso scallop (Patinopecten yessoensis) revealed similar patterns of GO representation. Moreover, 38 putative fast-evolving genes were identified through analyzing the orthologous gene pairs between the two scallop species. More than 46,000 single nucleotide polymorphisms (SNPs) and 350 simple sequence repeats (SSRs) were also detected.
Conclusion
Our study provides the most comprehensive transcriptomic resource currently available for C. farreri. Based on this resource, we performed the first large-scale transcriptome comparison between the two scallop species, C. farreri and P. yessoensis, and identified a number of putative fast-evolving genes, which may play an important role in scallop speciation and/or local adaptation. A large set of single nucleotide polymorphisms and simple sequence repeats were identified, which are ready for downstream marker development. This transcriptomic resource should lay an important foundation for future genetic or genomic studies on C. farreri.
Introduction
Bivalves represent one of the oldest and evolutionarily most successful classes of invertebrates. They comprise 30,000 extant species which can adapt to a variety of marine and freshwater environments, although the molecular basis underlying these adaptations is still poorly understood. Bivalves play an important role in the ecosystems they inhabit [1]. For example, they act as extensive contributors to the transfer of mineral (e.g. calcium) and organic matter in benthic habitats. Moreover, bivalves serve as an important food source all over the world. In contrast with their ecological and economic significance, relatively less research attention has been paid to these animals. Moreover, most of bivalve studies carried out so far are biased towards a few well-studied species such as oysters and mussels. Obviously, future research on a broader range of bivalve species is very much encouraged to make a better understanding of bivalve adaptation and speciation.
The Pectinidae family, also known as scallops, consists of more than 300 extant species [2] and constitutes one of the most conspicuous groups of bivalves [3]. Among approximately 40 scallop species distributed along the coast of China, Zhikong scallop (Chlamys farreri, Jones et Preston 1904) represents one of the most important shellfish cultured in the north of China. Genetic breeding programs have recently been initiated for genetic improvement of this scallop species, and much research effort has been devoted to identify genes or genetic loci responsible for economically important traits such as rapid growth and disease resistance. For example, a number of growth- and immune-related genes have been cloned and characterized [4]–[10], and several growth-related quantitative trait loci (QTL) have also been identified [11]. However, none of these studies carried out so far has reached to the systems biology level, which is largely due to the lack of sufficient genetic or genomic resources for this scallop species. For example, as of 08/26/2012, there are only 3716 expressed sequence tags (ESTs) publicly available in the GenBank database for C. farreri, which are clearly far from representing the whole transcriptome of C. farreri.
Fortunately, the recent advent of next-generation sequencing (NGS) technologies that enables rapid and cost-effective large-scale sequencing shows great potential for expanding EST databases for potentially any non-model organisms, thus paving the way for functional genomics on scallops. In comparison with other NGS platforms such as Solexa and SOLiD, 454 sequencing technology can produce much longer reads, and therefore has been favorably chosen for de novo transcriptome sequencing in some ecologically and economically important bivalve species such as mussels [12], [13], clams [14], [15] and pearl oysters [16], [17]. Aside from gene discovery, many studies have demonstrated that transcriptome sequencing also represents an efficient way to discover genetic variations, e.g. single nucleotide polymorphisms (SNPs) and simple sequence repeats (SSRs), and help locating adaptive genes that are under natural selection. Meanwhile, the SNPs and SSRs discovered from transcriptome sequences, could be further developed to gene-based markers which are useful genetic tools in the studies on population genetics, QTL mapping, and pedigree assignment, etc [18].
Recently, our group has released a large amount of transcriptomic data for Yesso scallop (Patinopecten yessoensis) [19], providing the first NGS-based large-scale transcriptome resource available for scallops. This resource is valuable not only for gene discovery and molecular marker mining, but also for comparative transcriptomic analysis. Currently, almost nothing is known about the genetic bases underlying scallop adaptation and speciation. P. yessoensis is phylogenetically close to C. farreri [20] but differs remarkably in morphology and thermal preference. Transcriptome comparison between P. yessoensis and C. farreri may provide new insights into the processes of scallop adaptation and speciation.
In this study, we performed de novo transcriptome sequencing of C. farreri using the 454 GS FLX platform. A library representing diverse life stages and adult tissues of C. farreri was sequenced to identify groups of genes involved in a broad range of biological processes. Approximately 20,000 genes were identified which can serve as an important basis for further gene expression profiling studies. In comparison with the P. yessoensis transcriptome, 38 putative fast-evolving genes were identified. In addition, a large number of SSRs and SNPs were detected and are ready for marker development.
Materials and Methods
Sample collection and RNA preparation
All the experiments on scallops were conducted following the institutional and national guidelines. Embryos (blastulae and gastrulae), larvae (trochophore and D-shaped larvae) and adults of C. farreri were collected from the hatchery of Xunshan Group Co., Ltd (Shandong, China) in 2008. In addition to the total soft tissues, adductor muscle, male and female gonads were also independently dissected from sex-matured adults. All the samples were flash frozen in liquid nitrogen and stored at −80°C until use.
Total RNA was separately extracted from each sample by following the protocol previously described in Hu et al. [21]. The quantity and quality of total RNA was analyzed using an Ultrospec™ 2100 pro UV/Visible Spectrophotometer (Amersham Biosciences, Uppsala, Sweden) and gel electrophoresis. Equal amount of RNA from blastulae, gastrulae, trochophore larvae and D-shaped larvae was mixed as the embryo and larval RNA pool. Another four RNA samples for cDNA libraries construction were the total soft tissues of adults, adductor muscle, female gonad and male gonad. The technical details of these cDNA libraries were summarized in Table 1, and similar information was also displayed for P. yessoensis, which was retrieved from our previous study [19].
Table 1. Summary of the C. farreri and P. yessoensis cDNA libraries used for 454 sequencing.
| Species | Developmental stages/adult tissues | No. of individuals used for library construction | Normalization | |
| C. farreri | Library 1 | Blastulae, Gastrulae, Trochophore, D-shaped larvae | ∼1,000 for each stage | Yes |
| Library 2 | Total soft tissues | 30 | Yes | |
| Library 3 | Adductor muscle | 30 | No | |
| Library 4 | Male gonad | 30 | No | |
| Library 5 | Female gonad | 30 | No | |
| P. yessoensis | Library 1 | Blastulae, Gastrulae, Trochophore, D-shaped larvae | ∼1,000 for each stage | Yes |
| Library 2 | Adductor muscle | 40 | No | |
| Library 3 | digestive gland | 40 | No | |
| Library 4 | Male gonad | 40 | No | |
| Library 5 | Female gonad | 40 | No |
cDNA library construction and 454 sequencing
Five 454 libraries (Table 1) were prepared by following the protocol as described in Meyer et al. [22]. Library 1 and 2 were normalized using the Trimmer-Direct cDNA normalization kit (Evrogen, Moscow, Russia) to decrease the prevalence of abundant transcripts. Library 3∼5 were not normalized in order to facilitate the identification of candidate tissue-specific transcripts. Approximately, 5 µg of the mixed libraries was sequenced using the Roche Genome Sequencer FLX system (Roche, Basel, Switzerland). During the construction of each library, adaptors with a unique barcode (a 3-base sequence) were ligated to both end of the cDNAs to distinguish the sequencing reads from those of other libraries. The reads were subsequently assigned to their corresponding libraries using Perl script [23].
Sequence analysis and assembly
The raw 454 reads were first pre-processed by trimming adaptors and eliminating very short sequences (less than 100 bp). The pre-processed sequences were then subject to assembling using the program Newbler v2.5 (Roche) (cDNA assembly mode). Default assembly parameters were used with the minimum overlap length of 40 bp and the minimum sequence identity of 90%. The assembly program Newbler could account for alternative splicing by creating a hierarchical assembly composed of contigs, isotigs, and isogroups. It has been shown that this program is more efficient in assembling 454 reads than other assembling programs [24]. For transcriptome comparison, the 454 transcriptome sequences of P. yessoensis were retrieved from the NCBI SRA database under the accession no. SRA027310. To make a fair and reliable comparison, the clean reads of P. yessoensis were reassembled and annotated by following the same procedure described for C. farreri.
Functional annotation
In order to avoid redundant annotations, only the longest isotig from each isogroup was selected and compared against the Swiss-Prot database using BlastX with an E-value threshold of 1e-6. For those isotigs without significant matches, tBlastX search with an E-value threshold of 1e-6 was conducted against the Nt database for further annotation. To increase computational speed, all Blast searches were limited to the top 10 significant hits for each query. Gene names were assigned to each isotig based on the best BLAST hit (highest score). The top 10 hits extracted from the BlastX results were used for gene annotation and GO analysis (level 3) using the program Blast2GO [25]–[27], a software package that assigned GO terms to query sequences, and produced a broad overview of groups of genes in the transcriptome cataloged for each of the three ontology vocabularies, i.e., biological processes, molecular functions and cellular components. The data presented herein represent a GO analysis at level 3, illustrating general functional categories.
In addition, to obtain an overview of gene pathways networks, KEGG analysis was performed using the online KEGG Automatic Annotation Server (KAAS) (http://www.genome.jp/kegg/kaas/). The bi-directional best hit (BBH) method was used to obtain KEGG orthology assignments.
Ka/Ks analysis based on the putative orthologous sequences
The identification of putative orthologous ESTs between C. farreri and P. yessoensis was performed using the bidirectional best hit (BBH) approach [28]. Pairs of putative orthologous genes were identified based on the reciprocal best matches with an E-value threshold of 1e-6. To reduce the risk of comparing paralogs, we only retained those orthologous pairs by requiring both genes in each pair must show the best matches to the same protein when comparing against the SwissProt database (BlastX, E-value<1e-6) [29]. Since multi-gene families can confound the analysis, we required that for each orthologous gene pair, the annotated gene name must appear only once across all annotated isogroups within each transcriptome; otherwise, it will be excluded from further analysis. Coding sequences (CDSs) of the filtered orthologous gene pairs were determined from the BlastX results. CDSs with unexpected stop codons were removed. Ka (non-synonymous) and Ks (synonymous) values were calculated based on the orthologous CDSs using KaKs_Calculator [30]. Pair-wise approximate analyses were performed using the Yang and Nielsen method [31]. GO enrichment analysis was conducted through hypergeometric test for orthologous pairs that showed Ka/Ks values significantly deviated from 1, in order to find functionally coherent gene-sets that are statistically over-represented.
SNP and SSR discovery
Potential SNPs were detected using the program GS Reference Mapper v2.6 with default parameters (cDNA mode). SNP identification was limited to the contigs containing at least eight reads for each allele and required the minor allele frequency ≥ 25%. SciRoko program version 3.3 [32] was used to identify and localize microsatellite motifs. All types of SSRs from dinucleotides to hexanucleotides were searched using default settings (for all repeat types, minimum total length = 15 bp and minimum repeats = 3).
Results and Discussion
Sequencing and assembly
The cDNA libraries representing different developmental stages, including embryos and larvae, and adults tissues of C. farreri were constructed and then pooled for 454 sequencing. The normalization and pooling strategies were used to enrich mRNA transcripts with low abundance and to maximize gene representation in a broad range of developmental and cellular processes. In addition, to obtain unique genes related to growth and reproduction which are both important economic traits for scallop, non-normalized cDNA libraries for adductor muscle, female gonad and male gonad were prepared separately (Table 1). A single run of 454 sequencing generated 1,224,989 reads. After removal of polyA tails, adaptor sequences and small reads (<100 bp), 1,033,636 (84.4%) high-quality reads remained with an average length of 310 bases. These high-quality reads have been deposited in the NCBI Short Read Archive (SRA) database with the accession number SRA030509. An overview of the sequencing and assembly statistics is presented in Table 2.
Table 2. Summary statistics of the transcriptome assembly for C. farreri and P. yessoensis.
| C. farreri | P. yessoensis. | |
| Raw reads | 1,224,989 | 970,422 |
| Clean reads | 1,033,636 | 740,491 |
| Assembled reads | 865,128 | 612,549 |
| Contigs | 26,165 | 13,306 |
| Contig size N50 | 848 bp | 898 bp |
| Average length of contigs | 646 bp | 688 bp |
| Mean no. of reads per contig | 32 | 45 |
| Isotigs | 24,437 | 12,015 |
| Isotig size N50 | 1,062 bp | 1,121 bp |
| Average length of isotigs | 868 bp | 933 bp |
| Mean no. of contigs per isotig | 1.4 | 1.6 |
| Isogroups | 20,056 | 10,147 |
| Mean no. isotigs per isogroup | 1.2 | 1.2 |
Assembly of the high-quality reads produced 26,165 contigs with an average length of 646 bp (N50 = 848 bp). Approximately 84% of the high-quality reads were incorporated into these contigs. The average coverage of contigs was 32. Size distribution of these contigs is shown in Fig. 1A. More than 63% of the contigs were >500 bp. Contigs were then assembled into 24,437 isotigs with an average length of 868 bp (N50 = 1,062 bp). The size distribution of isotigs is shown in Fig. 1B. The average contig coverage for each isotig was 1.4. About 28.3% of the isotigs were >1,000 bp. The isotigs were further grouped into 20,056 isogroups. The remaining 168,506 reads that did not overlap with other sequences were considered as singletons. Although many singletons could represent useful lowly expressed transcripts, it is also possible that some are artifacts derived from cDNA synthesis, sequencing and contamination [22]. PCR validation or re-sequencing is necessary to verify the validity of these singletons. Hence these singletons were excluded from the following analyses.
Figure 1. Overview of the de novo assembly of the C. farreri transcriptome.
(A) Size distribution of contigs. (B) Size distribution of isotigs. Assembly of the high-quality reads produced 26,165 contigs with an average length of 646 bp (N50 = 848 bp). Contigs were further assembled into 24,437 isotigs with an average length of 868 bp (N50 = 1,062 bp).
Functional annotation
Functional annotation (Table S1) of the C. farreri transcriptome was first carried out by the BlastX search against the well-annotated Swiss-Prot database with an E-value cut-off of 1e-6. Of 20,056 isogroup, 7,830 (39.0%) had significant matches in total, corresponding to 6,736 unique accessions. Among these accessions, 763 were matched by 1,857 different queries without overlap (2.4 queries matched each subject, on average). Sequences that lacked matches were subsequently compared against the Nt database (tBlastX) for further identification, and 1,498 additional isotigs returned a significant hit (E-value<1e-6). A large portion of the C. farreri transcriptome (53.5% of the isogroups) had no annotation information. The poor annotation efficiency was comparable to those reported in other de novo transcriptome sequencing studies based on the NGS platforms [22], [33]–[36]. This could be largely due to the insufficient sequences in public databases from phylogenetically closely related species to date [18]. Some of these sequences might represent novel proteins, unique to scallops, fast evolving genes or untranslated regions as well.
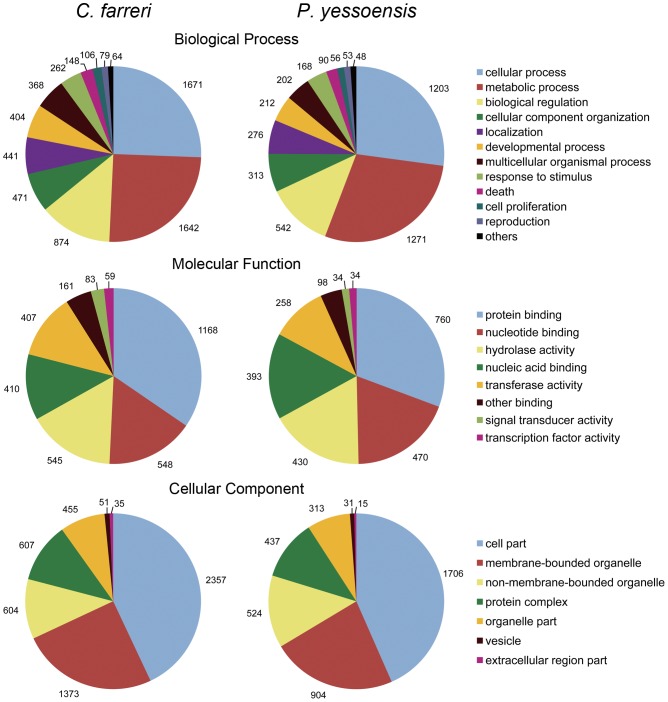
To classify the C. farreri genes based on their putative function, gene ontology (GO) analysis [37] was performed. Of the 7,830 Swiss-Prot annotated isotigs, 5,955 (76.1%) were assigned to one or more GO terms, with a total of 27,081 GO assignments. On average, about 5 GO terms were assigned to each of the annotated isotigs. Genes involved in the binding (GO:0005488) and catalytic activity categories (GO:0003824) were highly represented in molecular function. Within cellular component, the most represented GO categories were cell (GO:0005623) and organelle (GO:0043226). Regarding biological process, cellular process (GO:0009987) was the most represented, followed by metabolic process (GO:0008152) (Fig. 2).
Figure 2. GO comparison between the C. farreri and P. yessoensis transcriptome.
GO analysis was performed at the level 3 for three main categories (cellular component, molecular function and biological process). Note, for the two species, the starting materials used for cDNA library preparation and their normalization histories largely resemble each other, though not completely equivalent (see Table 1 for the details of libraries in comparison).
As an alternative approach of categorizing the annotated isogroups based on biochemical pathways, KEGG analysis based on enzyme commission (EC) numbers was performed for all annotated sequences using the KEGG Automatic Annotation Server (KAAS) [38]. EC numbers were assigned to 2,062 isogroups which were involved in 229 different pathways. The isogroups involved in these pathways are summarized in Table 3. Of these 2,062 isogroups with KEGG annotation, 38.7% were classified into the genetic information processing pathways, with most of them involved in translation, folding, sorting and degradation, and transcription. The isogroups classified into metabolism accounted for 33.9% of the KEGG annotated sequences. The well-represented metabolic pathways were amino acid metabolism, carbohydrate metabolism, and lipid metabolism. About 22.7% of the isogroups were classified into organism systems, such as immune system, endocrine system, and nervous system. Cellular processes were represented by 22.6% of the KEGG annotated isogroups. The transport and catabolism, cell growth and death, and cell communication were well represented. Additionally, 13.3% of the isogroups involved environmental information processing, including signal transduction, signaling molecules and interaction, and membrane transport.
Table 3. KEGG biochemical mappings for C. farreri transcriptome.
| KEGG categories represented | Unique sequences (Number of enzymes) |
| Metabolism | 698 (572) |
| Amino Acid Metabolism | 146 (123) |
| Carbohydrate Metabolism | 134 (107) |
| Lipid Metabolism | 134 (108) |
| Energy Metabolism | 124 (112) |
| Nucleotide Metabolism | 102 (83) |
| Glycan Biosynthesis and Metabolism | 77 (64) |
| Metabolism of Cofactors and Vitamins | 72 (61) |
| Metabolism of Other Amino Acids | 64 (45) |
| Xenobiotics Biodegradation and Metabolism | 63 (41) |
| Metabolism of Terpenoids and Polyketides | 15 (13) |
| Biosynthesis of Other Secondary Metabolites | 13 (12) |
| Genetic Information Processing | 799 (692) |
| Translation Replication and Repair | 303 (263) |
| Folding, Sorting and Degradation | 276 (232) |
| Transcription | 162 (136) |
| Replication and Repair | 99 (86) |
| Environmental Information Processing | 275 (228) |
| Signal Transduction | 215 (179) |
| Signaling Molecules and Interaction | 61 (50) |
| Membrane Transport | 10 (8) |
| Cellular Processes | 465 (390) |
| Transport and Catabolism | 224 (173) |
| Cell Growth and Death | 152 (114) |
| Cell Communication | 106 (86) |
| Cell Motility | 50 (43) |
| Organismal Systems | 469 (390) |
| Immune System | 158 (128) |
| Endocrine System | 137 (114) |
| Nervous System | 133 (109) |
| Digestive System | 93 (71) |
| Development | 63 (54) |
| Excretory System | 54 (47) |
| Circulatory System | 46 (36) |
| Environmental Adaptation | 21 (18) |
| Sensory System | 18 (13) |
| Total | 2,062 (1,726) |
For scallops, growth and reproduction are economically important traits, thus genes involved in these processes are of particular interest to the scallop researchers for the purpose of genetic improvement. Genes encoding different groups of growth factors, such as EGF, TGF, IGF and FGF, as well as their receptors were identified. Regarding reproduction, genes encoding DEAD-box family members (e.g. vasa, PL10 and eIF4A) that are involved in the germ cell development and reproductive regulation [39]–[41], and Piwi-like proteins that are responsible for maintaining the stability of germline cell division rate were identified [42]. The cDNAs from adductor muscle and gonad were not experimentally normalized, so as to assess the transcripts putatively related to the function of these tissues. In adductor muscle, structural or muscle-related genes such as actin, myosin, tubulin, and troponin were highly expressed. Transcripts of myostatin which were proved to be an important regulator of muscle growth and development in vertebrates were only found in the cDNAs from scallop adductor muscle. In male and female gonad, the most highly expressed gene was sperm-specific H1/protamine-like protein and collagen-like protein, respectively. Other putative sex-specific transcripts encoding sperm-specific proteins, vitellogenins and estradiol dehydrogenase, etc. [43]–[45], were also discovered. Further GO analysis also identified sequences classified into terms associated with growth and reproduction (Table 4).
Table 4. Sequences classified into growth, reproduction and response to stimulus categories by GO analysis.
| GO terms | Number of sequences |
| growth (GO:0040007) | 47 |
| cell growth (GO:0016049) | 25 |
| regulation of growth (GO:0040008) | 5 |
| multicellular organism growth (GO:0035264) | 2 |
| negative regulation of growth (GO:0045926) | 1 |
| reproduction (GO:0000003) | 79 |
| response to stimulus (response to stimulus) | 262 |
| response to stress (GO:0006950) | 211 |
| response to external stimulus (GO:0009605) | 48 |
| behavior (GO:0007610) | 36 |
| response to abiotic stimulus (GO:0009628) | 33 |
| response to biotic stimulus (GO:0009607) | 27 |
| response to endogenous stimulus (GO:0009719) | 27 |
| immune response (GO:0006955) | 22 |
With the increasing environmental pressure on natural and farmed scallop populations largely resulting from the increasing use of coastal zones, research efforts have recently been devoted to understanding of the genetic bases of stress-resistance in scallops. In our study, both GO and KEGG analysis identified transcripts that are involved in cellular responses to environmental pressure and stimulus (Table 4 and Table 5). The GO analysis identified 198 and 262 transcripts that are related to stress responses (GO: 0006950) and stimulus responses (GO: 0050896), respectively. KEGG analysis showed that 13.3% of the isogroups belonged to environmental information processing (EIP), including signal transduction, signaling molecules and interaction, and membrane transport. In addition, 7.7% of the isogroups were involved in immune response. Further functional analysis of these genes may provide valuable information for understanding of the genetic basis underlying scallop stress-resistance and productive traits.
Table 5. Sequences classified into Immune System by KEGG analysis.
| KEGG Pathways | Number of sequences |
| Hematopoietic cell lineage | 7 |
| Complement and coagulation cascades | 9 |
| Toll-like receptor signaling pathway | 23 |
| NOD-like receptor signaling pathway | 13 |
| RIG-I-like receptor signaling pathway | 18 |
| Cytosolic DNA-sensing pathway | 21 |
| Natural killer cell mediated cytotoxicity | 21 |
| Antigen processing and presentation | 16 |
| T cell receptor signaling pathway | 22 |
| B cell receptor signaling pathway | 22 |
| Fc epsilon RI signaling pathway | 16 |
| Fc gamma R-mediated phagocytosis | 27 |
| Leukocyte transendothelial migration | 21 |
| Chemokine signaling pathway | 31 |
| Total | 267 |
Transcriptome comparison between C. farreri and P. yessoensis
Many studies have demonstrated the usefulness of next-generation sequencing in obtaining transcriptomic resources for comparative analysis in non-model organisms [46], [47]. Here, for the first time, we performed scallop transcriptome comparison by comparative analysis of the new EST data set generated for C. farreri with the one recently published for P. yessoensis [19]. To make a fair and reliable comparison, the sequencing data of P. yessoensis transcriptome was reassembled and annotated by following the same procedure as described for C. farreri. The assembled transcriptome of C. farreri and P. yessoensis contained 20,056 and 10,147 isogroups, respectively. For the two species, the starting materials used for cDNA library preparation and their normalization histories largely resemble each other, though not completely equivalent (Table 1). For example, a normalized cDNA library of total soft tissues was sequenced for C. farreri, but not for P. yessoensis. In addition, it is possible that not all transcripts have been adequately sampled from all tissues and developmental stages based on the current sequencing coverage. Despite these discrepancies, transcriptome comparison between C. farreri and P. yessoensis based on the available EST sequences remains worth doing and should represent the first step towards full understanding of transcriptome organization and evolution in the Pectinidae family.
To get an overall comparison of the transcriptome organization between the two scallop species, we first analyzed their transcriptome sequences in terms of functional annotation and relative abundance of gene ontology (GO) terms. A total of 5,955 C. farreri isogroups and 3,533 P. yessoensis isogroups were assigned to 27,081 and 16,682 GO terms, respectively. Similar transcriptome pattern in terms of GO categories and their relative frequencies were found between C. farreri and P. yessoensis (Fig. 2), suggesting the overall similar transcriptome architecture between the two species. Further analyses with batches of cDNA sequences from more tissues and embryo/larva at more developmental stages for both C. farreri and P. yessoensis are needed to compare their transcripomes more thoroughly.
To enable Ka/Ks analysis, putative orthologous gene pairs were determined through a series of stringent filtering steps. Through the BBH approach, an initial set of 5,367 putative orthologous pairs were first identified. This number was reduced to 2,847 by requiring both genes in each pair must show the best matches to the same protein when comparing against the SwissProt database (BlastX, E-value<1e-6). Since multi-gene families can confound the analysis, we further removed the orthologous pairs whose gene names appeared more than once across all annotated isogroups within each transcriptome. The final set consisted of 1,887 pairs, which were selected for further analysis.
Most of the orthologous pairs (1,709) showed a Ka/Ks ratio<1. Among these, 1,644 orthologous pairs had Ka/Ks ratios significantly<1 (Table S2). Many sequences had Ka/Ks values equal to or only slightly greater than zero, suggesting that these genes have evolved under high selective constraint [48]. On the other hand, 178 orthologous pairs had Ka/Ks ratios >1. And 38 genes, which exhibited Ka/Ks values significantly deviated from 1 (Table S2), are possibly under positive selection and may play important roles in scallop adaptation and speciation. GO enrichment analysis (p<0.05) was performed for orthologous pairs to find functionally coherent gene-sets that are statistically over-represented. Genes with Ka/Ks values significantly<1 were enriched in a variety of biological processes such as metabolic process (p = 9.6E-14), translation (p = 1.3E-09) and biosynthetic process (p = 5.8E-07) (Table S3). However, genes with Ka/Ks values significantly >1 were only enriched in cellular biosynthetic process (p = 4.6E-04) and gene expression (p = 4.8E-04) (Table S3), suggesting that these terms probably represent the most important biological processes responsible for rapid adaptive evolution.
SNP and SSR discovery
For genetic improvement of C. farreri, a large number of molecular markers are usually required for fine QTL mapping and marker-assisted selection (MAS). The transcriptome data here provided a rich EST resource for genetic variants mining, such as SNP and SSR screening.
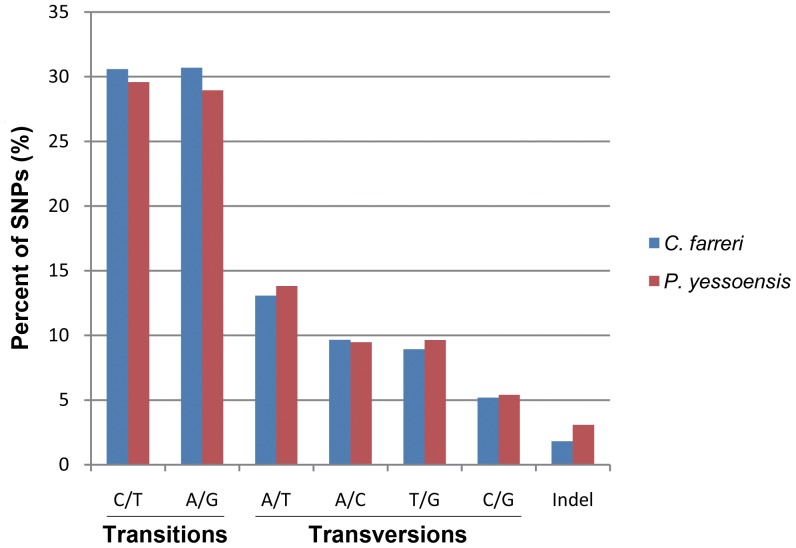
Using the GS Reference Mapper program, we were able to identify 46,527 high-quality SNPs and 866 indels from 9,989 contigs. The overall frequency of all types of SNPs including indels was one per 357 bp. To make a comparison, SNPs in the reassembled P. yessoensis transcriptome were also examined. As a result, 20,633 SNPs and 658 indels were identified. The frequency of SNPs and indels was one per 430 bp. In the two species, the distribution of each SNP type was similar, and transitions occurred more frequently than transversions (Fig. 3). The proportion of transitions in C. farreri was higher than that in P. yessoensis, while the transversions were more abundant in P. yessoensis. A/T was the most abundant transversions type and C/G was the least in both species. Indels occurred the least frequently compared with transitions and transversions in the two species. Unlike other SNP types which showed not much difference in proportion between the two scallops, the indels proportion in P. yessoensis was 1.7 times as much as that in C. farreri.
Figure 3. Classification of single nucleotide polymorphisms (SNPs) identified from the C. farreri and P. yessoensis transcriptome.
For both species, transitions occurred more frequently than transversions. The overall frequency of all types of SNPs including indels was one per 357 bp for C. farreri and one per 430 bp for P. yessoensis.
In addition, 352 and 213 SSRs were identified from the transcriptome sequences of C. farreri and P. yessoensis, respectively. Comparison between the two scallop species revealed some difference in distribution pattern of SSR motifs (Table 6B). Trinucleotide and tetranucleotide were the most frequent type of repeats in both C. farreri and P. yessoensis. Although dinucleotide repeats were the third most frequent type in C. farreri, they were the least abundant type in P. yessoensis. ATC motif represented the most abundant trinucleotide motif in both species, which was also common in other bivalves [49]. For dinucleotide and pentanucleotide repeats, AT and AAAAT was the most frequent motif in the two species. But for tetranucleotides, the most frequent motif in C. farreri was ATAC, while in P. yessoensis was AAAT. A notable difference also existed in hexanucleotides. AAGGTC was the most common hexanucleotide motif in C. farreri, but only one copy was found in P. yessoensis.
Table 6. Summary of simple sequence repeat (SSR) types in C. farreri and P. yessoensis transcriptome.
| C. farreri | P. yessoensis | |||||
| SSR Type | Number of motif | Count | Major motif | Number of motif | Count | Major motif |
| Dinucleotides | 3 | 50 | AT | 3 | 24 | AT |
| Trinucleotides | 9 | 164 | ATC | 8 | 104 | ATC |
| Tetranucleotides | 16 | 60 | ATAC | 14 | 31 | AAAT |
| Pentanucleotides | 18 | 46 | AAAAT | 18 | 29 | AAAAT |
| Hexanucleotides | 22 | 32 | AAGGTC | 19 | 25 | AACTGG |
The SNPs and SSRs identified in this study provided for the first time over thousands of putative candidate loci for marker development in C. farreri. In case that there might be sequencing errors or assembly artifacts, the bioinformatically discovered SNPs and SSRs should be further validated and evaluated for marker utility in the C. farreri natural populations. A small portion of the SNPs identified in this study have already been assessed in two separate studies, and some of them were successfully developed as gene-associated polymorphic markers [50], [51], which could be very useful in future selective breeding and population genetic studies for C. farreri.
Supporting Information
Sequences with significant BLAST matches against Swiss-Prot and NCBI Nt database.
(XLS)
Putative orthologous genes with Ka/Ks values significantly different from one (p<0.05).
(XLS)
GO enrichment analysis for putative orthologous genes with Ka/Ks values significantly different from one.
(DOC)
Acknowledgments
We thank Xunshan Aquatic Product Group Co., Ltd. (Rongcheng, China) for providing scallop samples.
Funding Statement
This work was supported by National Basic Research Program of China [973 Program, 2010CB126406], National Natural Science Foundation of China [31172384, 30972239], National High Technology Research and Development Program of China [2012AA092204, 2012AA10405, 2012AA10402, 2012AA10401], Open Project Program of the Key Laboratory of Marine Bio-resources Sustainable Utilization, SCSIO, CAS and Scholarship award for Excellent Doctoral Student granted by China Ministry of Education. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Saavedra C, Bachère E (2006) Bivalve genomics. Aquaculture 256: 1–14. [Google Scholar]
- 2.Brand AR (2006) Scallop ecology: distributions and behaviour. In: Shumway SE, Parsons GJ, editors. Scallops: biology, ecology and aquaculture.Netherlands: Elsevier BV. pp. 651–744. [Google Scholar]
- 3. Bieler R, Mikkelsen PM (2006) Bivalvia-a look at the branches. Zool J Linn Soc Lond 148: 223–235. [Google Scholar]
- 4. Hu XL, Guo HH, He Y, Wang S, Zhang LL, et al. (2010) Molecular characterization of Myostatin gene from Zhikong scallop Chlamys farreri (Jones et Preston 1904). Genes Genet Syst 85: 207–218. [DOI] [PubMed] [Google Scholar]
- 5. Li F, Huang S, Wang L, Yang J, Zhang H, et al. (2011) A macrophage migration inhibitory factor like gene from scallop Chlamys farreri: Involvement in immune response and wound healing. Dev Comp Immunol 35: 62–71. [DOI] [PubMed] [Google Scholar]
- 6. Zhou Z, Wang LL, Shi XW, Zhang H, Gao Y, et al. (2011) The modulation of catecholamines to the immune response against bacteria Vibrio anguillarum challenge in scallop Chlamys farreri . Fish Shellfish Immun 31: 1065–1071. [DOI] [PubMed] [Google Scholar]
- 7. Zhou Z, Yang JL, Wang LL, Zhang H, Gao Y, et al. (2011) A dopa decarboxylase modulating the immune response of scallop Chlamys farreri . PLoS One 6: e18596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi XW, Wang LL, Zhou Z, Yang CY, Gao Y, et al. (2012) The arginine kinase in Zhikong scallop Chlamys farreri is involved in immunomodulation. Dev Comp Immunol 37: 270–278. [DOI] [PubMed] [Google Scholar]
- 9. Shi XW, Zhou Z, Zhou Z, Yue F, Wang MQ, et al. (2012) The immunomodulation of acetylcholinesterase in zhikong scallop Chlamys farreri . PLoS One 7: e30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou Z, Ni DJ, Wang MQ, Wang LL, Wang LL, et al. (2012) The phenoloxidase activity and antibacterial function of a tyrosinase from scallop Chlamys farreri . Fish Shellfish Immun 33: 375–381. [DOI] [PubMed] [Google Scholar]
- 11. Zhan AB, Hu JJ, Hu XL, Hui M, Wang ML, et al. (2009) Construction of microsatellite-based linkage maps and identification of size-related quantitative trait loci for Zhikong scallop (Chlamys farreri). Anim Genet 40: 821–831. [DOI] [PubMed] [Google Scholar]
- 12. Craft JA, Gilbert JA, Temperton B, Dempsey KE, Ashelford K, et al. (2010) Pyrosequencing of Mytilus galloprovincialis cDNAs: tissue-specific expression patterns. PLoS One 5: e8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bettencourt R, Pinheiro M, Egas C, Gomes P, Afonso M, et al. (2010) High-throughput sequencing and analysis of the gill tissue transcriptome from the deep-sea hydrothermal vent mussel Bathymodiolus azoricus . BMC Genomics 11: 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Milan M, Coppe A, Reinhardt R, Cancela LM, Leite RB, et al. (2011) Transcriptome sequencing and microarray development for the Manila clam, Ruditapes philippinarum: genomic tools for environmental monitoring. BMC Genomics 12: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huan P, Wang H, Liu B (2012) Transcriptomic analysis of the clam Meretrix meretrix on different larval stages. Mar Biotechnol 14: 69–78. [DOI] [PubMed] [Google Scholar]
- 16. Joubert C, Piquemal D, Marie B, Manchon L, Pierrat F, et al. (2010) Transcriptome and proteome analysis of Pinctada margaritifera calcifying mantle and shell: focus on biomineralization. BMC Genomics 11: 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kinoshita S, Wang N, Inoue H, Maeyama K, Okamoto K, et al. (2011) Deep sequencing of ESTs from nacreous and prismatic layer producing tissues and a screen for novel shell formation-related genes in the pearl oyster. PLoS One 6: e21238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang S, Zhang L, Meyer E, Matz MV (2009) Construction of a high-resolution genetic linkage map and comparative genome analysis for the reef-building coral Acropora millepora. Genome Biol 10: R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hou R, Bao ZM, Wang S, Su HL, Li Y, et al. (2011) Transcriptome sequencing and de novo analysis for Yesso scallop (Patinopecten yessoensis) using 454 GS FLX. PLoS One 6: e21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waller TR (1991) Evolutionary relationship among commercial scallops (Mollusca: Bivalvia: Pectinidae). In: Shumway SE, editor. Scallops: biology, ecology and aquaculture.Netherlands:Elsevier BV. pp. 1–73. [Google Scholar]
- 21. Hu XL, Bao ZM, Hu JJ, Shao MY, Zhang LL, et al. (2006) Cloning and characterization of tryptophan 2,3-dioxygenase gene of Zhikong scallop Chlamys farreri (Jones and Preston 1904). Aquac Res 37: 1187–1194. [Google Scholar]
- 22. Meyer E, Aglyamova GV, Wang S, Buchanan-Carter J, Abrego D, et al. (2009) Sequencing and de novo analysis of a coral larval transcriptome using 454 GSFlx. BMC Genomics 10: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang S, Durban J, Juárez P, Angulo Y, Lomonte B, et al. (2011) Profiling the venom gland transcriptomes of Costa Rican snakes by 454 pyrosequencing. BMC Genomics 12: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kukekova AV, Johnson JL, Teiling C, Li L, Oskina IN, et al. (2011) Sequence comparison of prefrontal cortical brain transcriptome from a tame and an aggressive silver fox (Vulpes vulpes). BMC Genomics 12: 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, et al. (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21: 3674–3676. [DOI] [PubMed] [Google Scholar]
- 26. Conesa A, Götz S (2008) Blast2GO: A Comprehensive Suite for Functional Analysis in Plant Genomics. Int J Plant Genomics 2008: 619832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, et al. (2008) High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res 36: 3420–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Overbeek R, Fonstein M, D′Souza M, Pusch GD, Maltsev N (1999) The use of gene clusters to infer functional coupling. PNAS 96: 2896–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang XW, Luan JB, Li JM, Su YL, Xia J, et al. (2011) Transcriptome analysis and comparison reveal divergence between two invasive whitefly cryptic species. BMC Genomics 12: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Z, Li J, Zhao XQ, Wang J, Wong GK, et al. (2006) KaKs_Calculator: calculating Ka and Ks through model selection and model averaging. Genomics Proteomics & Bioinfor 4: 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang Z, Nielsen R (2000) Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol Biol Evol 17: 32–43. [DOI] [PubMed] [Google Scholar]
- 32. Kofler R, Schlotterer C, Lelley T (2007) SciRoKo: a new tool for whole genome microsatellite search and investigation. Bioinformatics 23: 1683–1685. [DOI] [PubMed] [Google Scholar]
- 33. Vera JC, Wheat CW, Fescemyer HW, Frilander MJ, Crawford DL, et al. (2008) Rapid transcriptome characterization for a nonmodel organism using 454 pyrosequencing. Mol Ecol 17: 1636–1647. [DOI] [PubMed] [Google Scholar]
- 34. Wang XW, Luan JB, Li JM, Bao YY, Zhang CX, et al. (2010) De novo characterization of a whitefly transcriptome and analysis of its gene expression during development. BMC Genomics 11: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rismani-Yazdi H, Haznedaroglu BZ, Bibby K, Peccia J (2011) Transcriptome sequencing and annotation of the microalgae Dunaliella tertiolecta: pathway description and gene discovery for production of next-generation biofuels. BMC Genomics 12: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Du HX, Bao ZM, Hou R, Wang S, Su HL, et al. (2012) Transcriptome sequencing and characterization for the sea cucumber Apostichopus japonicus (Selenka, 1867). PLoS One 7: e33311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, et al. (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kanehisa M, Götz S (2000) KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 28: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marracci S, Casola C, Bucci S, Ragghianti M, Ogielska M, et al. (2007) Differential expression of two vasa/PL10-related genes during gametogenesis in the special model system Rana. Dev Genes Evol 217: 395–402. [DOI] [PubMed] [Google Scholar]
- 40. Raz E (2000) The function and regulation of vasa-like genes in germ-cell development. Genome Biol 1: 1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pause A, Sonenberg N (1992) Mutational analysis of a DEAD box RNA helicase: the mammalian translation initiation factor eIF-4A. Embo J 11: 2643–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cox DN, Chao A, Lin H (2000) piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development 127: 503–514. [DOI] [PubMed] [Google Scholar]
- 43. Agelopoulou B, Cary PD, Pataryas T, Aleporou-Marinou V, Crane-Robinson C (2004) The sperm-specific proteins of the edible oyster (European flat oyster (Ostrea edulis)) are products of proteolytic processing. Biochim Biophys Acta 1676: 12–22. [DOI] [PubMed] [Google Scholar]
- 44. Spieth J, Nettleton M, Zucker-Aprison E, Lea K, Blumenthal T (1991) Vitellogenin motifs conserved in nematodes and vertebrates. J Mol Evol 32: 429–438. [DOI] [PubMed] [Google Scholar]
- 45. Kautsky MP, Hagerman DD (1920) 17 Beta-estradiol dehydrogenase of ovine ovaries. J Biol Chem 245: 1978–1984. [PubMed] [Google Scholar]
- 46. Baldo L, Santos ME, Salzburger W (2011) Comparative transcriptomics of Eastern African cichlid fishes shows signs of positive selection and a large contribution of untranslated regions to genetic diversity. Genome Biol Evol 3: 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barakat A, DiLoreto DS, Zhang Y, Smith C, Baier K, et al. (2009) Comparison of the transcriptomes of American chestnut (Castanea dentata) and Chinese chestnut (Castanea mollissima) in response to the chestnut blight infection. BMC Plant Biol 9: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tiffin P, Hahn MW (2002) Coding sequence divergence between two closely related plant species: Arabidopsis thaliana and Brassica rapa ssp. pekinensis. J Mol Evol 54: 746–753. [DOI] [PubMed] [Google Scholar]
- 49. Wang Y, Guo X (2007) Development and characterization of EST-SSR markers in the eastern oyster Crassostrea virginica . Mar Biotechnol 9: 500–511. [DOI] [PubMed] [Google Scholar]
- 50. Jiang GD, Li JQ, Li L, Zhang LL, Bao ZM (2011) Development of 44 gene-based SNP markers in Zhikong scallop, Chlamys farreri . Conserv Genet Resour 3: 659–663. [Google Scholar]
- 51. Wang XJ, Hu XL, Li JQ, Li L, Hou R, et al. (2012) Characterization of 38 EST-derived SNP markers in Zhikong scallop (Chlamys farreri) and their cross-species utility in Yesso scallop (Patinopecten yessoensis). Conserv Genet Resour 4: 747–753. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequences with significant BLAST matches against Swiss-Prot and NCBI Nt database.
(XLS)
Putative orthologous genes with Ka/Ks values significantly different from one (p<0.05).
(XLS)
GO enrichment analysis for putative orthologous genes with Ka/Ks values significantly different from one.
(DOC)