Abstract
Ybt1p is a class C ABC transporter (ATP-binding cassette transporter) that is localized to the vacuole of Saccharomyces cerevisiae. Although Ybt1p was originally identified as a bile acid transporter, it has also been found to function in other capacities, including the translocation of phosphatidylcholine to the vacuole lumen, and the regulation of Ca2+ homoeostasis. In the present study we found that deletion of YBT1 enhanced in vitro homotypic vacuole fusion by up to 50 % relative to wild-type vacuoles. The increased vacuole fusion was not due to aberrant protein sorting of SNAREs (soluble N-ethylmaleimide-sensitive factor-attachment protein receptors) or recruitment of factors from the cytosol such as Ypt7p and the HOPS (homotypic fusion and vacuole protein sorting) tethering complex. In addition, ybt1Δ vacuoles displayed no observable differences in the formation of SNARE complexes, interactions between SNAREs and HOPS, or formation of vertex microdomains. However, the absence of Ybt1p caused significant changes in Ca2+ transport during fusion. One difference was the prolonged Ca2+ influx exhibited by ybt1Δ vacuoles at the start of the fusion reaction. We also observed a striking delay in SNARE-dependent Ca2+ efflux. As vacuole fusion can be inhibited by high Ca2+ concentrations, we suggest that the delayed efflux in ybt1Δ vacuoles leads to the enhanced SNARE function.
Keywords: calcium efflux, fusion, homotypic fusion and vacuole protein sorting (HOPS), soluble N-ethylmaleimide-sensitive factor-attachment protein receptor (SNARE), vertex
INTRODUCTION
Maintaining eukaryotic cellular homoeostasis requires the trafficking of membrane-bound cargo through the endocytic and secretory pathways using mechanisms that are conserved in all eukaryotes [1]. The delivery of cargo to its destination is finalized by the fusion of two membranes that is driven through a series of regulated stages. In the present study we used vacuoles (lysosomes) from Saccharomyces cerevisiae to examine the regulation of membrane fusion. Yeast vacuole fusion requires numerous proteins, including the Q-SNAREs {SNAP [soluble NSF (N-ethylmaleimide-sensitive factor)-attachment protein] receptors} Vam3p, Vti1p, Vam7p and the R-SNARE Nyv1p. In addition, vacuole fusion requires the Rab GTPase Ypt7p and its effector complex HOPS (homotypic fusion and vacuole protein sorting), actin, as well as the lipids ergosterol, diacylglycerol, phosphatidic acid and phosphoinositides [2].
The final catalysts of fusion are SNARE proteins, however, at the start of the cascade, SNAREs are held in inactive cis complexes on a single membrane. The vacuole fusion pathway is initiated when cis-SNARE complexes are dissociated by the NSF homologue Sec18p and its co-chaperone α-SNAP/Sec17p in an ATP-dependent mechanism [3]. Membranes with activated SNAREs then tether and dock with partner membranes in a mechanism driven by Ypt7p and its effector complex HOPS [4,5]. During the docking stage, SNARE complexes form in trans between partner membranes where one membrane donates Nyv1p and the partner membrane donates Vam3p, Vti1p and Vam7p as a preformed 3Q-SNARE complex. In conjunction with these events, vacuoles are drawn together and become tightly apposed, causing the deformation of vesicles and the formation of a specialized membrane-raft microdomain called the vertex ring where the proteins and lipids that catalyse fusion laterally accumulate to create the site of fusion [6–8].
Although the core fusion machinery has been identified, there is a growing collection of regulatory factors that modulate fusion by various means. These regulators include the protein kinase Yck3p [9] and the lipid modifiers Pah1p [10] and Vps34p [11], as well as the Na+/H+ exchanger Nhx1p [12]. Additional regulation may occur through controlling Ca2+ transport or the translocation of lipids across the membrane bilayer. The latter function is executed by polytopic membrane proteins that are referred to as lipid ‘flippases’ and ‘floppases’ that establish lipid asymmetry between the inner and outer leaflets of a membrane [13]. Some of the putative flip/floppases belong to the ABC transporter (ATP-binding cassette transporter) superfamily [14].
The ABC transporter superfamily is present in all organisms and is responsible for actively transporting a wide range of substrates across lipid bilayers. Loss-of-function mutations in ABC transporters result in a number of inherited human diseases, including the lung and digestive disorder cystic fibrosis, the cholesterol transport disorder Tangier’s disease and the elastic tissue disorder pseudoxanthoma elasticum [15]. ABC transporters are formed from two homologous halves that contain an NBD (nucleotide-binding domain) and an MSD (membrane-spanning domain) consisting of six transmembrane spans. Human ABC transporters are divided into seven sub-families (ABCA–ABCG). Many of the best characterized ABC transporters belong to the Class C subfamily (ABCC). With the exception of CFTR (cystic fibrosis transconductance regulator), ABCC family members, also known as MRPs (multidrug resistance-associated proteins), contain an N-terminal extension composed of five transmembrane motifs and a short cytosolic loop. The yeast vacuole harbours five ABCC transporters: the yeast cadmium factor, Ycf1p [16,17]; two bile acid and pigment transporters, Bpt1p and Ybt1p [18,19]; and two less well-characterized transporters, Vmr1p and Nft1p [15]. Ybt1p was first described as an ATP-dependent bile acid transporter [19] and is similar in structure to the human MRP transporter. Ybt1p is highly expressed on the yeast vacuole [20] and also plays a part in ade2 pigment transport [21]. Recently a novel function was described for Ybt1p showing that it translocates phosphatidylcholine from the outer leaflet of the vacuole to the inner leaflet for degradation and choline recycling [14].
MATERIALS AND METHODS
Reagents
Reagents were dissolved in PS buffer (20 mM Pipes/KOH, pH 6.8, and 200 mM sorbitol). Antibodies against Vam3p [22], Sec17p [23], Nyv1p [24], Sec18p [25] and Ypt7p [4] were described previously. The recombinant proteins His6–Gyp1-56 [8], GDI (guanine-nucleotide-dissociation inhibitor) [26], GST (glutathione transferase)–FYVE [27], MED [MARCKS (myristoylated alanine-rich C-kinase substrate) effector domain] [6], His6–MTM1 (myotubularin 1) [28] and GST–Vam7p [29,30] were prepared as described previously and stored in PS buffer with 125 mM KCl.
Strains
BJ3505 [MATα pep4::HIS3 prb1-Δ1.6R his3-200 lys2-801 trp1Δ101 (gal3) ura3-52 gal2 can1] and DKY6281 (MATα leu2-3 leu 2-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801) were used for fusion assays [31]. BJ3505 CBP (calmodulin-binding peptide)–Vam3 nyv1Δ was used for trans-SNARE complex isolation [32] (Table 1). YBT1 was deleted from BJ3505, DKY6281 and BJ3505 CBP–Vam3 nyv1Δ by homologous recombination. YBT1 was deleted from BJ3505 and DKY6281 with the kanMX6 cassette using PCR products amplified from pFA6a-kanMx6 [33] with homology flanking the YBT1 coding sequence with the forward primer 5′-GTGTGCGCATCTGCAAAGAACGTACGTTGTGACTAATGAACGGATCCCCGGGTTAATTAA-3′ and the reverse primer 5′-TCAGTAAAAGTTCATTGGATCAGATTTCCTTCAAAGACGCGAATTCGAGCTCGTTTAAAC-3′. The PCR product was transformed into BJ3505 and DKY6281 by standard lithium acetate methods and plated on YPD medium [1% (w/v) yeast extract, 2 % (w/v) peptone and 2 % (w/v) glucose] containing G418 (250 μg/μl) to generate BJ3505 ybt1Δ::kanMX6 (RFY28) and DKY6281 ybt1Δ::kanMX6 (RFY29). YBT1 was deleted from BJ3505 CBP–Vam3 nyv1Δ with the hghMX4 cassette using PCR product amplified from pAG32 [34] with homology flanking the YBT1 coding sequence with the forward primer 5′-GTGTGCGCATCTGCAAAGAACGTACGTTGTGACTAATGAAATAGGCCACTAGTGGATCTG-3′ and reverse primer 5′-TCAGTAAAAGTTCATTGGATCAGATTTCCTTCAAAGACGCTCAGCTGAAGCTTCGTACGC-3′. The PCR product was transformed into BJ3505 CBP–Vam3 nyv1Δ by standard lithium acetate methods and plated on to YPD medium containing hygromycin (250 μg/ml) to generate BJ3505 CBP–Vam3 nyv1Δ ybt1::hghMX4 (RFY30). For vacuole localization studies YBT1 was fused in-frame to GFP (green fluorescent protein) by homologous recombination. DKY6281 was transformed with a PCR product amplified from pFA6a-GFP-kanMX6 [33] with the forward primer 5′-TAAAAAAGCCTTTGTGGAAAAATTGAACTCTAAAAAGGACCGGATCCCCGGGTTAATTAA-3′ and the reverse primer 5′-ATATATATATATATATATATATACTTTAGCATCGAAACAGGAATTCGAGCTCGTTTAAAC-3′ with homology flanking the stop codon of the YBT1 gene to make RFY31 (DKY6281 YBT1::GFP).
Table 1.
Yeast strains used in the present study
| Strain | Genotype | Source |
|---|---|---|
| BJ3505 | MAT α pep4::HIS3 prb1-Δ1.6R his3-200 lys2-801 trp1Δ101 (gal3) ura3-52 gal2 can1 | [48] |
| DKY6281 | MAT α pho8::TRP1 leu2-3 leu 2-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 | [49] |
| BJ3505 CBP–Vam3 nyv1Δ | BJ3505, CBP-VAM3::Kanr nyv1Δ::natr | [32] |
| RFY28 | BJ3505, ybt1Δ::kanMX6 | The present study |
| RFY29 | DKY6281, ybt1Δ::kanMX6 | The present study |
| RFY30 | BJ3505 CBP-VAM3 nyv1Δ ybt1Δ::hghMX4 | The present study |
| RFY31 | DKY6281, YBT1::GFP | The present study |
Vacuole isolation and in vitro vacuole fusion
Vacuoles were isolated by floatation as described previously [31]. Standard in vitro fusion reactions (30 μl) contained 3 μg each of vacuoles from BJ3505 and DKY6281 backgrounds, fusion reaction buffer (20 mM Pipes/KOH, pH 6.8, 200 mM sorbitol, 125 mM KCl and 5 mM MgCl2), ATP-regenerating system (1 mM ATP, 0.1 mg/ml creatine kinase and 29 mM creatine phosphate), 10 μM CoA and 283 nM IB2 (inhibitor of protease B). Reactions were incubated at 27 °C and Pho8p activity was assayed in 250 mM Tris/HCl, pH 8.5, 0.4 % Triton X-100, 10 mM MgCl2 and 1 mM p-nitrophenyl phosphate. Fusion units were measured by determining the p-nitrophenolate produced in min−1 · μg−1 pep4Δ vacuole and absorbance was detected at 400 nm.
GST–Vam7p SNARE complex isolation and bypass fusion
SNARE complex isolation was performed as described previously using GST–Vam7p [29,30]. Briefly, large-scale 6× fusion reactions (180 μl) were incubated with 85 μg/ml anti-Sec17p IgG to block priming. After 15 min, 43 μg/ml anti-Vam3p IgG was added to selected reactions and incubated for an additional 5 min before adding 400 nM GST–Vam7p. After a total of 90 min, reactions were placed on ice for 5 min and 30 μl aliquots were removed to measure Pho8p activity. The remaining 150 μl reactions were sedimented (11 000 g, 10 min, 4 °C), and the supernatants were removed before extracting vacuoles with SB (solubilization buffer: 20 mM Hepes/KOH, pH 7.4, 100 mM NaCl, 2 mM EDTA, 20 %glycerol, 0.5 %Triton X-100 and 1 mM dithiothreitol) with protease inhibitors (1 mM PMSF, 10 μM Pefabloc-SC, 5 μM pepstatin A and 1 μM leupeptin). Vacuole pellets were overlaid with 100 μl of SB and resuspended gently. An additional 100 μl of SB was added, gently mixed, and incubated on ice for 20 min. Insoluble debris was sedimented (16 000 g, 10 min, 4 °C) and 176 μl of supernatants were removed and placed in chilled tubes. Next, 16 μl was removed from each reaction as 10 % total samples, mixed with 8 μl of 3× SDS-loading buffer and heated (95 °C, 5 min). Equilibrated glutathione–Sepharose 4B beads (30 μl) were incubated with the remaining extracts (15 h, 4 °C, nutation). Beads were sedimented and washed five times with 1 ml of SB (4000 g, 2 min, 4 °C), and bound material was eluted with 40 μl of 1× SDS loading buffer. Protein complexes were examined by immunoblotting. Secondary antibodies conjugated to alkaline phosphatase were used with ECF reagent (GE Healthcare).
trans-SNARE complex assay
Analysis of trans-SNARE complex formation was conducted as described previously with some modifications [32,35]. Complex formation was compared between reactions containing vacuoles from RFY29 and RFY30 relative to those with BJ3505 CBP–Vam3p nyv1Δ and DKY6281 vacuoles. The trans-SNARE assays were performed using 16× large-scale reactions (480 μl) containing 48 μg of vacuoles each from BJ3505 CBP–Vam3 nyv1Δ and DKY6281 backgrounds and incubated at 27 °C for 60 min. After incubation, reactions were placed on ice for 5 min and 30 μl was withdrawn from each sample to assay Pho8p activity. The remaining 450 μl samples were centrifuged (13 000 g, 15 min, 4 °C) and the supernatants were decanted. Vacuole pellets were overlaid with 200 μl of ice-cold vacuole solubilization buffer (20 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1 mM MgCl2, 0.5 % Nonidet P40 alternative and 10 % glycerol) with protease inhibitors (0.46 μg/ml leupeptin, 3.5 μg/ml pepstatin, 2.4 μg/ml Pefabloc®-SC and 1 mM PMSF) and gently resuspended. Vacuole solubilization buffer was added to a final volume of 600 μl and extracts were mixed (20 min, 4 °C, nutation). Detergent-insoluble debris was removed by centrifugation (16 000 g, 20 min, 4 °C). Supernatants were transferred to fresh tubes and 10 % of the extract was removed for input samples. The remaining extracts were brought to 2 mM CaCl2. CBP–Vam3p complexes were incubated with 50 μl of equilibrated calmodulin–Sepharose 4B (GE Healthcare) (4 °C, 12 h, nutation). Beads were collected by centrifugation (4000 g, 2 min, 4 °C) and suspended four times with the solubilization buffer followed by bead sedimentation. Bound proteins were eluted with SDS sample buffer containing 5 mM EGTA and heated at 95 °C for 5 min. The samples were used for SDS/PAGE and immunoblotting.
Lipid mixing
Lipid mixing assays were conducted using Lissamine rhodamine B (Rh-PE; Invitrogen) as described previously with minor modifications [10]. Briefly, BJ3505 background vacuoles (300 μg) were incubated in 400 μl of PS buffer containing 150 μM Rh-PE (10 min, 4 °C, nutating). Samples were mixed with 15 % (w/v) Ficoll in PS buffer and transferred to a polyallomer tube (11 mm×60 mm). Samples were overlaid with 1.0 ml each of 8 %, 4 % and 0 % Ficoll. Labelled vacuoles were re-isolated by centrifugation using a SW60 Ti rotor (105 200 g, 25 min, 4 °C) and recovered from the 0–4 % Ficoll interface. Lipid mixing assays (90 μl) contained 2 μg of Rh-PE-labelled vacuoles and 16 μg of unlabelled vacuoles in fusion buffer. Reaction mixtures were transferred to a black half-volume 96-well flat-bottom microtitre plate with non-binding surface (Corning). Rhodamine fluorescence (λex = 544 nm; λem = 590 nm) was measured using a POLARstar Omega fluorescence plate reader (BMG Labtech) at 27 °C. Measurements were taken every min for 75 min, yielding fluorescence values at the onset (F0) and during the reaction (Ft). The final ten measurements of a sample after adding 0.33 % Triton X-100 were averaged and used as a value for the fluorescence after infinite dilution (FTX100). The relative total fluorescence change ΔFt/FTX100 = (Ft − F0)/FTX100 was calculated.
Microscopy
Vacuole morphology was monitored by incubating yeast cells with YPD medium containing the vital dye FM4-64 (Invitrogen). Cultures were grown overnight to saturation, diluted to ~ 0.2 D600 in YPD containing 5 μM FM4-64, grown for 1 h in a 30 °C shaker, washed with PBS, resuspended in YPD, incubated at 30 °C for 3 h, washed in PBS, concentrated by centrifugation, mixed with 0.6 % agarose, and mounted on to glass slides for observation. Images were acquired using a Zeiss Axio Observer Z1 inverted microscope equipped with an X-Cite 120XL light source, Plan Apochromat 63× oil objective (numerical aperture 1.4), and an AxioCam CCD (charge-coupled-device) camera. For vertex assembly detection, docking assays were performed. Docking reactions (30 μl) contained 6 μg of Ybt1p–GFP vacuoles, docking buffer (20 mM Pipes/KOH, pH 6.8, 200 mM sorbitol, 100 mM KCl and 0.5 mM MgCl2), ATP-regenerating system, 20 μM CoA and 283 nM IB2. PtdIns3P was labelled with 0.2 μM Cy5 (indodicarbocyanine)–FYVE and used as a marker for vertex microdomains [10]. Reactions were incubated at 27 °C for 30 min and placed on ice and stained with 3 μM FM4-64. Reactions were next mixed with 50 μl of 0.6 % low-melt agarose (in PS buffer), vortex-mixed to disrupt spurious clustering, and mounted on to slides for observation by fluorescence microscopy.
Ca2+ efflux assay
SNARE-dependent Ca2+ efflux was measured as described previously [36] with some modifications. Fusion reactions (60 μl) contained 20 μg of vacuoles isolated from BJ3505 backgrounds, fusion reaction buffer with 10 μM CoA and 283 nM IB2. In lieu of the luminescent aequorin Ca2+ detection system, we used the low-affinity fluorescent probe Fluo-4-dextran at 200 μM (Invitrogen). Reaction mixtures were transferred to a black half-volume 96-well flat-bottom microtitre plate with non-binding surface (Corning). ATP-regenerating system was added and reactions were incubated at 27 °C while monitoring Fluo-4 fluorescence (λex = 488 nm; λem = 520 nm).
Statistical analysis
Significant differences were calculated using Student’s t test and P values less than 0.05 were considered significant.
RESULTS
Ybt1p is localized to the vacuole
Ybt1p has been reported to transport bile acids into the vacuole lumen of Saccharomyces cerevisiae in an ATP-dependent manner [19]. To verify the localization of Ybt1p in yeast, we constructed a strain expressing Ybt1p–GFP. We incubated the strain with the vital dye FM4-64, which accumulates in the vacuole [37], and found that Ybt1p–GFP primarily localized to the vacuole (Figures 1a–1d), which is consistent with previous findings [14]. We next examined the vacuole morphology in ybt1Δ cells and found that the morphology and size of the vacuoles were similar to those in WT (wild-type) cells (Figures 1e–1g). As fusion regulators often accumulate at the vertices of docked vacuoles [6–8], we next examined the distribution of Ybt1p–GFP on isolated vacuoles during docking. Figure 2 shows that Ybt1p–GFP was evenly dispersed throughout the limiting membrane of the vacuole along with FM4-64. To probe for vertex microdomains, docking reactions were incubated with a Cy5-conjugated FYVE domain. The FYVE domain specifically binds to the regulatory lipid PtdIns3P [27], a critical lipid that accumulates at the site of fusion in vertex microdomains [6]. In Figure 2(c), the vertices of docked vacuoles were marked with Cy5–FYVE.
Figure 1. Ybt1–GFP is localized to the vacuole.

WT yeast cells harbouring Ybt1p–GFP were incubated with 5 μM FM4-64 to label vacuoles. Cells were washed with PBS and grown for 1 h in label-free YPD to chase the dye into the vacuole. Cells were washed with PBS and mounted for microscopy. (a and b) GFP images were acquired using a 38 HE GFP shift-free filter set and FM4-64 images were acquired using a 43 HE Cy3 (indocarbocyanine) shift-free filter set. (c) Cells were photographed using differential interference contrast (DIC). (d) Images were merged using Adobe Photoshop. (e and f) ybt1Δ yeast were incubated with FM4-64 as described above and imaged using DIC and Cy3 filters. (g) Quantification and box plot of vacuole size in WT and ybt1Δ cells. Scale bar, 4 μm.
Figure 2. Ybt1–GFP is not enriched at the vertex ring microdomain of docked vacuoles.

Purified vacuoles isolated from cells harbouring Ybt1p–GFP (a) were incubated at 27 °C for 30 min. The limiting membranes were stained with 5 μM FM4-64 (b) and vertex microdomains were labelled with Cy5-conjugated FYVE domain to label PtdIns3P (c). (d) Images were merged using Adobe Photoshop. Scale bar, 4 μm.
Ybt1p is a negative regulator of vacuole fusion
To determine the possible role of this ABCC transporter in the fusion process, we next examined the effect of deleting YBT1 on homotypic vacuole fusion. Fusion was measured by a content mixing assay in which proPho8p (pro-alkaline phosphatase) is cleaved by the protease Pep4p to yield a mature alkaline phosphatase. The level of fusion directly correlates with Pho8p activity. Equal quantities of the strains DKY6281 (PEP4 pho8Δ) and BJ3505 (pep4Δ PHO8) were mixed to assay content mixing. We deleted YBT1 from these strains to generate RFY28 and RFY29 and found that fusion was augmented and showed an approximate 50 % increase compared with WT vacuoles (Figure 3a). This suggests that Ybt1p acts as a negative regulator for vacuole fusion.
Figure 3. Vacuoles from ybt1Δ yeast show enhanced fusion.
Vacuoles were harvested from WT and ybt1Δ yeast and tested for fusion activity. (a) Standard fusion reactions used equal amounts of reporter (PHO8 pep4Δ) and effector (pho8Δ PEP4) vacuoles for WT (●) and ybt1Δ (○). Error bars represent S.E.M. (n = 3). (b) Lipid mixing assays measuring fusion were employed by labelling isolated vacuoles with Rh-PE. Labelled vacuoles were incubated with an 8-fold excess of unlabelled vacuoles and dequenching was measured using a fluorescence plate reader. (c) Analysis of core fusion components. Vacuoles were isolated from WT and ybt1Δ BJ3505 and DK6281 strains. Vacuoles (5 μg by protein) were mixed with 2× SDS loading buffer, separated by SDS/PAGE and transferred on to nitrocellulose. Immunoblotting was performed using the indicated antibodies and bands were detected using ECF. (d) Quantification of Pep4p and Pho8p in WT and ybt1Δ vacuoles. (e) Measurements of vacuole diameters after incubation (27 °C, 90 min). Error bars are S.E.M. (n = 3). *P < 0.0001
As changes in fusion could be due to dysregulated Pho8p reporting, we also used a real-time lipid-dequenching assay that is independent of Pho8p activity [12,38,39]. In the present study, the outer leaflets of vacuole-limiting membranes were labelled with Rh-PE, which is self-quenched at high concentrations. When labelled donor vacuoles were incubated with an 8-fold excess of unlabelled acceptor vacuoles, Rh-PE was dequenched. WT vacuoles showed a characteristic rapid dequenching that was inhibited with anti-Vam3 IgG (Figure 3b, circles). When ybt1Δ vacuoles were examined, we found that the initial dequenching was identical with WT, after which the ybt1Δ vacuoles surpassed WT fusion starting at 15 min (Figure 3b). This illustrates that the initial rates of fusion were the same for WT and ybt1Δ vacuoles, yet the V max of ybt1Δ fusion was significantly higher. To determine whether the increase in fusion of ybt1Δ vacuoles was due to elevated levels of the fusion machinery (e.g. SNAREs), Western blot analysis was performed. No significant differences were observed in the fusion machinery of mutant vacuoles relative to their WT counterparts (Figure 3c). It should also be noted that the increased fusion was not due to defects in trafficking of Pho8p or Pep4p (Figure 3d). To further verify that the deletion of YBT1 caused an increase in fusion, visual measurements of vacuole diameters were measured. Fusion reactions containing either WT or ybt1Δ vacuoles were carried out as in Figure 3(a). After 90 min of incubation, vacuoles were placed on ice and stained with FM4-64. Aliquots of each reaction were transferred to microscope slides and images were captured for analysis. Figure 3(e) shows a box plot of vacuole diameter ranges for reactions treated with either buffer or anti-Sec17 IgG to inhibit priming, and incubated on ice or at 27 °C. We found that ybt1Δ vacuole diameters (7.13 ± 0.19 μm) were markedly larger than those of WT vacuoles (4.88 ± 0.23 μm).
To determine whether the augmented fusion seen with ybt1Δ vacuoles was regulated by the core fusion machinery, we examined the sensitivity of fusion to a panel of well-characterized fusion inhibitors that targeted SNAREs, Ypt7p and phosphoinositides. SNARE function was inhibited with antibodies against Nyv1p, Vam3p, Sec18p and Sec17p. Ypt7p function was inhibited with anti-Ypt7p IgG, the GTPase-activating protein Gyp1-56p, or the Rab GDI. PtdIns3P function was inhibited by ligation with the FYVE domain or modification with the phosphoinositide 3-phosphatase, MTM1. MED was used to bind multi-phosphorylated phosphoinositides, including PtdIns(4,5)P2. We found that both WT and ybt1Δ fusion were similarly sensitive to the panel of inhibitors, indicating that the enhanced fusion seen with ybt1Δ vacuoles was not due to an undefined mechanism (Figure 4).
Figure 4. The augmented fusion of ybt1Δ vacuoles is dependent on the standard fusion machinery.

Fusion reactions were performed using WT or ybt1Δ vacuoles. Individual reactions were treated with PS buffer, 14 μg/ml anti-Nyv1p IgG, 27 μg/ml anti-Vam3p IgG, 12 μg/ml anti-Sec18p IgG, 67 μg/ml anti-Sec17p IgG, 8 μg/ml anti-Ypt7p, 0.5 μM Gyp1-56, 0.5 μM GDI, 2 μM GST–FYVE, 10 μM MED or 2 μM His6–MTM1. Reactions were incubated for 90 min at 27 °C and tested for fusion by content mixing and Pho8p activity. Error bars are S.E.M. (n = 3).
YBT1 deletion does not alter SNARE complex formation
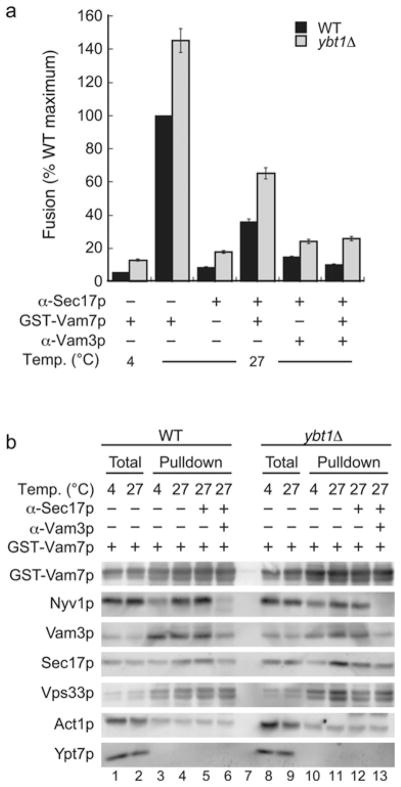
Dysregulation of fusion is often coupled with changes in SNARE complex formation. Thus we tested whether the absence of Ybt1p altered the SNARE/HOPS interactions by two methods. First, we performed a Vam7p bypass assay when SNARE priming was inhibited with anti-Sec17p IgG [29,30]. Fusion was then rescued with the addition of 400 nM GST–Vam7p and incubated for an additional 70 min. Selected reactions were treated with anti-Vam3p antibody prior to adding Vam7p. Fusion was restored when WT and mutant vacuoles were treated with exogenous GST–Vamp7p (Figure 5a). GST–Vam7p complexes were isolated and examined by immunoblotting. GST–Vam7p complexes included the SNAREs Nyv1p and Vam3p, the HOPS subunit Vps33p, the SNARE chaperone Sec17p and actin (Figure 5b). As a control we also probed for the presence of Ypt7p, which does not co-purify with the SNARE/HOPS complexes. SNARE complex formation was blocked by the anti-Vam3p antibody. We found that GST–Vam7p complexes from ybt1Δ vacuoles contained a similar SNARE makeup relative to WT vacuoles.
Figure 5. Vam7p forms complexes with SNAREs and HOPS on ybt1Δ vacuoles.
(a) Large-scale fusion reactions (6×) were incubated with anti-Sec17p IgG to block priming. After 15 min, anti-Vam3 IgG was added to the indicated reactions and incubated for 5 min prior to adding 400 nM GST–Vam7p. Reactions were incubated for an additional 70 min. After incubation, reactions were placed on ice and 30 μl was removed to measure Pho8p activity. (b) The remaining fractions were extracted with SB with protease inhibitors for 20 min while on ice. Insoluble debris was sedimented by centrifugation and supernatants were removed and incubated with equilibrated glutathione–Sepharose. Beads were washed with SB and protein complexes were eluted with 2× SDS/PAGE buffer. Proteins were resolved by SDS/PAGE, transferred on to nitrocellulose and probed with antibodies against Nyv1p, Vam3p, Vam7p, Sec17p, Vps33p, actin and Ypt7p. Error bars are S.E.M. (n = 3).
We next determined whether endogenous SNAREs could form trans complexes when priming is not blocked [32,35]. YBT1 was deleted from the trans-SNARE reporter strain BJ3505 CBP–Vam3p nyv1Δ to create RFY30. Vacuoles isolated from RFY29 (pho8Δ ybt1Δ VAM3 NYV1) and RFY30 were incubated and trans-SNARE complexes were isolated through the CBP integrated into Vam3p. CBP–Vam3p complexes were isolated using calmodulin–Sepharose beads and examined by immunoblotting. As shown previously, ybt1Δ vacuoles show increased fusion relative to WT vacuoles (Figure 6a). Both WT and ybt1Δ fusion reactions were sensitive to anti-Vam3p IgG and to the inactivation of Ypt7p with the combination of Gyp1-56p and GDI. We found that ybt1Δ vacuoles formed trans-SNARE complexes in a similar manner as their WT counterparts indicating that the enhanced fusion was not due to an augmented number of SNARE complexes (Figures 6b and 6c). Similarly, the association of SNAREs with the HOPS subunit Vps33p was not altered on ybt1Δ vacuoles. The lack of a notable difference between WT and mutant vacuoles suggest that fusion was not altered due to the efficiency of complex formation.
Figure 6. trans-SNARE complex formation.

Vacuoles harbouring CBP–Vam3p in a nyv1Δ background were incubated with vacuoles harbouring a full complement of SNAREs. Large-scale reactions (16×) were incubated at 27 °C for 60 min. After incubating, reactions were placed on ice for 5 min and 30 μl was withdrawn from each sample to assay Pho8p activity (a). (b) Membranes were pelleted and then resuspended in SB containing protease inhibitors. CBP–Vam3p complexes were isolated with calmodulin–Sepharose beads and protein complexes were resolved by SDS/PAGE. Complexes were probed for Nyv1p, Vam3p, Vps11p and Vps33p by immunoblot. (c) Quantification of efficiency of Nyv1p binding to CBP–Vam3p. Error bars are S.E.M. (n = 3).
Ybt1p regulates the release of lumenal Ca2+ stores
From Figure 3(b) we found that mutant fusion was increased after 15 min, which coincides with the formation of trans-SNARE pairing [32]. The formation of trans-SNARE complexes is linked to the release of lumenal Ca2+ stores from the vacuole [40,41]. As Ybt1p interacts with the Ca2+ ATPase Pmc1p [42], we next examined the effect of deleting YBT1 on Ca2+ transport. Fusion reactions (2×) were prepared containing 20 μg of either BJ3505 (WT) or RFY28 (ybt1Δ) in the presence of standard fusion reaction components and the fluorescent calcium indicator Fluo4-dextran. Reactions were started by the addition of buffer or ATP-regenerating system. Priming was blocked by the addition of anti-Sec17p IgG where indicated and fluorescence was monitored. Upon the addition of ATP, Ca2+ was transported into the vacuole, causing a drop in fluorescence. Ca2+ is transported into the vacuole via the Ca2+ -ATPase Pmc1p and the Vcx1p H+/Ca2+ exchanger [43]. After approximately 15 min, when trans-SNAREs have formed, we observed an increase in fluorescence indicating that Ca2+ was being effluxed from the vacuole lumen (Figure 7a, ●), which is in keeping with previous studies using the aequorin reporter system [40]. When ybt1Δ vacuoles were observed, we found that there were four differences relative to WT reactions. First, there was a prolonged influx of Ca2+ early in the reaction, suggesting that Pmc1p and/or Vcx1p activity may be augmented. Secondly, we found that the trans-SNARE complex-dependent efflux was markedly delayed in ybt1Δ vacuoles (Figure 7b). These observations suggest that Ybt1p regulates Ca2+ transport in vacuoles and that the enhanced fusion may be linked to alterations in Ca2+ homoeostasis. In addition, the relative amount of Ca2+ in ybt1Δ vacuoles was nearly twice as much as seen with WT vacuoles. We also found that WT vacuoles reabsorb Ca2+ after docking, resulting in a return to basal ‘pre-docking’ levels of extraluminal Ca2+, whereas ybt1Δ fails to reabsorb the effluxed Ca2+.
Figure 7. Calcium efflux is delayed in ybt1Δ vacuoles.
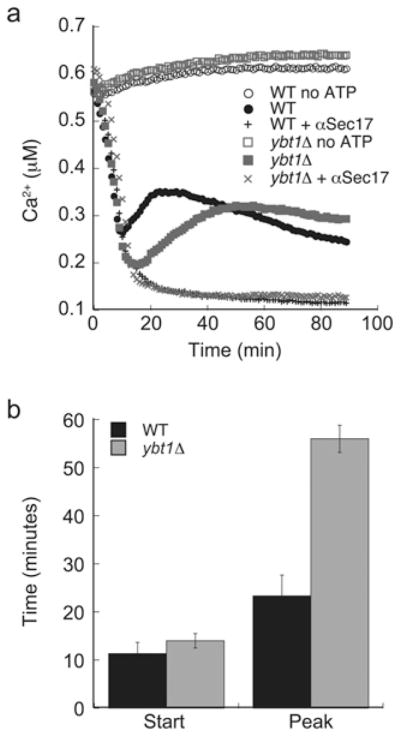
Vacuoles were harvested from WT BJ3503 and RFY28 (ybt1Δ). Fusion reactions (2×) were prepared containing 20 μg of either WT or ybt1Δ vacuoles and 200 μM Fluo-4-dextran. Immediately after addition of ATP or PS buffer, reactions were incubated at 27 °C and fluorescence was measured for 90 min. (a) A representative time course of Ca2+ efflux. (b) The average times at which the start and peaks of efflux occurred in WT and ybt1Δ vacuoles. Error bars are S.E.M. (n = 3).
DISCUSSION
In the present study we found that the ABCC transporter Ybt1p acts as a negative regulator of vacuole fusion. This was evident by the enhanced fusion observed with vacuoles from YBT1-deleted strains. Using a battery of experiments that tested different stages of the fusion pathway, we found that the defect in fusion occurred after the docking stage when trans-SNARE complexes are formed. The enhancement in fusion correlated with a delay in Ca2+ efflux, an event that is triggered by the formation of trans-SNARE complexes [40]. This suggested that Ybt1p might regulate the transport of ions across the membrane. Although Ybt1p itself does not transport Ca2+, the observed changes might be attributed in part to the interaction of Ybt1p with Pmc1p, a Ca2+-ATPase that transports Ca2+ ions into the vacuole [42]. Although the SNARE-dependent vacuolar Ca2+ efflux channel remains undefined, the connections between Ybt1p, Pmc1p and the delayed efflux during fusion suggests that the import and export of Ca2+ is linked to the fusion machinery.
Ybt1p and the SNARE machinery
The direct connection between Ybt1p and the fusion machinery remains unknown; however, the link between Ybt1p and Pmc1p suggests that an indirect mechanism is possible. Others have shown that Pmc1p interacts with free Nyv1p, the required R-SNARE in vacuole homotypic fusion [44]. The interaction between Pmc1p and Nyv1p inhibits the transport of Ca2+ into the vacuole lumen. After priming, the Q-SNARE complexes compete for Nyv1p binding to form trans-SNARE complexes, thus de-repressing Pmc1p activity and leading to the uptake of extraluminal Ca2+. In the absence of Ybt1p we observed the prolonged uptake of Ca2+ upon the addition of ATP, suggesting that Pmc1p activity is augmented and that changes in activity might be due to reduced interactions with Nyv1p.
There are several possible mechanisms by which the efflux of Ca2+ might contribute to the regulation of fusion. We have found that vacuole fusion strictly depends upon the formation of membrane microdomains that are enriched in the proteins and lipids that regulate fusion [6–8]. Thus any perturbation to the assembly pathway needed for the formation of vertices would undoubtedly alter fusion. Others have found that Ca2+ at sub-micromolar concentrations induces clustering of plasma membrane SNAREs [45]. This is thought to occur through the neutralization of negatively charged side chains. It is also likely that Ca2+ relieves the electrostatic repulsion between negatively charged phospholipids [46,47]. Interestingly, Zilly et al. [45] also found that elevated Ca2+ concentrations (≥10 μM) inhibited SNARE pairing. Although the bulk measurement of Ca2+ efflux only measures sub-micromolar concentrations, it is possible that local concentrations of Ca2+ at the site of efflux may reach micromolar levels. Thus a delay in Ca2+ efflux might allow more trans-SNARE pair formation and enhanced fusion.
Acknowledgments
We thank Dr William Wickner (Dartmouth Medical School, Hanover, NH, U.S.A.) and Dr Daniel Klionsky (Department of Molecular, Cellular and Developmental Biology, Department of Biological Chemistry, University of Michigan, Ann Arbor, MI, U.S.A.) for gifts of antisera. We also thank members of the Fratti laboratory for critical reading of the paper prior to submission.
FUNDING
This research was supported by the University of Illinois Research Board (to R.A.F.), the National Institutes of Health [grant number GM101132 (to R.A.F.)], and by start-up funds provided to R.A.F. by the University of Illinois at Urbana-Champaign.
Abbreviations used
- ABC transporter
ATP-binding cassette transporter
- CBP
calmodulin-binding peptide
- Cy5
indodicarbocyanine
- GDI
guanine-nucleotide-dissociation inhibitor
- GFP
green fluorescent protein
- GST
glutathione transferase
- HOPS
homotypic fusion and vacuole protein sorting
- MRP
multidrug resistance-associated protein
- MTM1
myotubularin 1
- NSF
N-ethylmaleimide-sensitive factor
- SB
solubilization buffer
- SNAP
soluble NSF-attachment protein
- SNARE
SNAP receptor
- WT
wild-type
Footnotes
AUTHOR CONTRIBUTION
All three authors designed the research. Terry Sasser and Mark Padolina performed the experiments. Terry Sasser and Rutilio Fratti analysed the data and wrote the paper.
References
- 1.Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- 2.Wickner W. Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a rab, all dancing in a ring on yeast vacuoles. Annu Rev Cell Dev Biol. 2010;26:115–136. doi: 10.1146/annurev-cellbio-100109-104131. [DOI] [PubMed] [Google Scholar]
- 3.Ungermann C, Sato K, Wickner W. Defining the functions of trans-SNARE pairs. Nature. 1998;396:543–558. doi: 10.1038/25069. [DOI] [PubMed] [Google Scholar]
- 4.Mayer A, Wickner W. Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF) J Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA. 2000;97:9402–9947. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fratti RA, Jun Y, Merz AJ, Margolis N, Wickner W. Interdependent assembly of specific regulatory lipids and membrane fusion proteins into the vertex ring domain of docked vacuoles. J Cell Biol. 2004;167:1087–1098. doi: 10.1083/jcb.200409068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Seeley ES, Wickner W, Merz AJ. Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell. 2002;108:357–369. doi: 10.1016/s0092-8674(02)00632-3. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Merz AJ, Collins KM, Wickner W. Hierarchy of protein assembly at the vertex ring domain for yeast vacuole docking and fusion. J Cell Biol. 2003;160:365–374. doi: 10.1083/jcb.200209095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabrera M, Ostrowicz CW, Mari M, LaGrassa TJ, Reggiori F, Ungermann C. Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites. Mol Biol Cell. 2009;20:1937–1948. doi: 10.1091/mbc.E08-09-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasser T, Qiu QS, Karunakaran S, Padolina M, Reyes A, Flood B, Smith S, Gonzales C, Fratti RA. Yeast lipin 1 orthologue pah1p regulates vacuole homeostasis and membrane fusion. J Biol Chem. 2012;287:2221–2236. doi: 10.1074/jbc.M111.317420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- 12.Qiu QS, Fratti RA. The Na+/H+ exchanger Nhx1p regulates the initiation of Saccharomyces cerevisiae vacuole fusion. J Cell Sci. 2010;123:3266–3275. doi: 10.1242/jcs.067637. [DOI] [PubMed] [Google Scholar]
- 13.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulshan K, Moye-Rowley WS. Vacuolar import of phosphatidylcholine requires the ATP-binding cassette transporter Ybt1. Traffic. 2011;12:1257–1268. doi: 10.1111/j.1600-0854.2011.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paumi CM, Chuk M, Snider J, Stagljar I, Michaelis S. ABC transporters in Saccharomyces cerevisiae and their interactors: new technology advances the biology of the ABCC (MRP) subfamily. Microbiol Mol Biol Rev. 2009;73:577–593. doi: 10.1128/MMBR.00020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szczypka MS, Wemmie JA, Moye-Rowley WS, Thiele DJ. A yeast metal resistance protein similar to human cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance-associated protein. J Biol Chem. 1994;269:22853–22857. [PubMed] [Google Scholar]
- 17.Wemmie JA, Moye-Rowley WS. Mutational analysis of the Saccharomyces cerevisiae ATP-binding cassette transporter protein Ycf1p. Mol Microbiol. 1997;25:683–694. doi: 10.1046/j.1365-2958.1997.5061868.x. [DOI] [PubMed] [Google Scholar]
- 18.Petrovic S, Pascolo L, Gallo R, Cupelli F, Ostrow JD, Goffeau A, Tiribelli C, Bruschi CV. The products of YCF1 and YLL015w (BPT1) cooperate for the ATP-dependent vacuolar transport of unconjugated bilirubin in Saccharomyces cerevisiae. Yeast. 2000;16:561–571. doi: 10.1002/(SICI)1097-0061(200004)16:6<561::AID-YEA551>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz DF, St Pierre MV, Abdulmessih A, Arias IM. A yeast ATP-binding cassette-type protein mediating ATP-dependent bile acid transport. J Biol Chem. 1997;272:15358–15365. doi: 10.1074/jbc.272.24.15358. [DOI] [PubMed] [Google Scholar]
- 20.Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 21.Sharma KG, Kaur R, Bachhawat AK. The glutathione-mediated detoxification pathway in yeast: an analysis using the red pigment that accumulates in certain adenine biosynthetic mutants of yeasts reveals the involvement of novel genes. Arch Microbiol. 2003;180:108–117. doi: 10.1007/s00203-003-0566-z. [DOI] [PubMed] [Google Scholar]
- 22.Nichols BJ, Ungermann C, Pelham HR, Wickner WT, Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- 23.Haas A, Wickner W. Homotypic vacuole fusion requires Sec17p (yeast α-SNAP) and Sec18p (yeast NSF) EMBO J. 1996;15:3296–3305. [PMC free article] [PubMed] [Google Scholar]
- 24.Ungermann C, Nichols BJ, Pelham HR, Wickner W. A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J Cell Biol. 1998;140:61–69. doi: 10.1083/jcb.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 26.Starai VJ, Jun Y, Wickner W. Excess vacuolar SNAREs drive lysis and Rab bypass fusion. Proc Natl Acad Sci USA. 2007;104:13551–13558. doi: 10.1073/pnas.0704741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier JM, Parton RG, Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19:4577–4488. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor GS, Maehama T, Dixon JE. Inaugural article: myotubularin, a protein tyrosine phosphatase mutated in myotubular myopathy, dephosphorylates the lipid second messenger, phosphatidylinositol 3-phosphate. Proc Natl Acad Sci USA. 2000;97:8910–8915. doi: 10.1073/pnas.160255697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fratti RA, Collins KM, Hickey CM, Wickner W. Stringent 3Q: 1R composition of the SNARE 0-layer can be bypassed for fusion by compensatory SNARE mutation or by lipid bilayer modification. J Biol Chem. 2007;282:14861–14867. doi: 10.1074/jbc.M700971200. [DOI] [PubMed] [Google Scholar]
- 30.Fratti RA, Wickner W. Distinct targeting and fusion functions of the PX and SNARE domains of yeast vacuolar Vam7p. J Biol Chem. 2007;282:13133–13138. doi: 10.1074/jbc.M700584200. [DOI] [PubMed] [Google Scholar]
- 31.Haas A, Scheglmann D, Lazar T, Gallwitz D, Wickner W. The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO J. 1995;14:5258–5570. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins KM, Wickner WT. Trans-SNARE complex assembly and yeast vacuole membrane fusion. Proc Natl Acad Sci USA. 2007;104:8755–8760. doi: 10.1073/pnas.0702290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longtine MS, McKenzie A, 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein AL, Pan X, McCusker JH. Heterologous URA3MX cassettes for gene replacement in Saccharomyces cerevisiae. Yeast. 1999;15:507–511. doi: 10.1002/(SICI)1097-0061(199904)15:6<507::AID-YEA369>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 35.Jun Y, Wickner W. Assays of vacuole fusion resolve the stages of docking, lipid mixing, and content mixing. Proc Natl Acad Sci USA. 2007;104:13010–13015. doi: 10.1073/pnas.0700970104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson FL, Dixon JE. Myotubularin phosphatases: policing 3-phosphoinositides. Trends Cell Biol. 2006;16:403–412. doi: 10.1016/j.tcb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reese C, Heise F, Mayer A. trans-SNARE pairing can precede a hemifusion intermediate in intracellular membrane fusion. Nature. 2005;436:410–414. doi: 10.1038/nature03722. [DOI] [PubMed] [Google Scholar]
- 39.Reese C, Mayer A. Transition from hemifusion to pore opening is rate limiting for vacuole membrane fusion. J Cell Biol. 2005;171:981–990. doi: 10.1083/jcb.200510018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merz AJ, Wickner W. trans-SNARE interactions elicit Ca2+ efflux from the yeast vacuole lumen. J Cell Biol. 2004;164:195–206. doi: 10.1083/jcb.200310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- 42.Tarassov K, Messier V, Landry CR, Radinovic S, Serna Molina MM, Shames I, Malitskaya Y, Vogel J, Bussey H, Michnick SW. An in vivo map of the yeast protein interactome. Science. 2008;320:1465–1470. doi: 10.1126/science.1153878. [DOI] [PubMed] [Google Scholar]
- 43.Miseta A, Kellermayer R, Aiello DP, Fu L, Bedwell DM. The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 1999;451:132–136. doi: 10.1016/s0014-5793(99)00519-0. [DOI] [PubMed] [Google Scholar]
- 44.Takita Y, Engstrom L, Ungermann C, Cunningham KW. Inhibition of the Ca2+ -ATPase Pmc1p by the v-SNARE protein Nyv1p. J Biol Chem. 2001;276:6200–6206. doi: 10.1074/jbc.M009191200. [DOI] [PubMed] [Google Scholar]
- 45.Zilly FE, Halemani ND, Walrafen D, Spitta L, Schreiber A, Jahn R, Lang T. Ca2+ induces clustering of membrane proteins in the plasma membrane via electrostatic interactions. EMBO J. 2011;30:1209–1220. doi: 10.1038/emboj.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boettcher JM, Davis-Harrison RL, Clay MC, Nieuwkoop AJ, Ohkubo YZ, Tajkhorshid E, Morrissey JH, Rienstra CM. Atomic view of calcium-induced clustering of phosphatidylserine in mixed lipid bilayers. Biochemistry. 2011;50:2264–2273. doi: 10.1021/bi1013694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellenbroek WG, Wang YH, Christian DA, Discher DE, Janmey PA, Liu AJ. Divalent cation-dependent formation of electrostatic PIP2 clusters in lipid monolayers. Biophys J. 2011;101:2178–2184. doi: 10.1016/j.bpj.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones EW, Zubenko GS, Parker RR. PEP4 gene function is required for expression of several vacuolar hydrolases in Saccharomyces cerevisiae. Genetics. 1982;102:665–677. doi: 10.1093/genetics/102.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haas A, Conradt B, Wickner W. G-protein ligands inhibit in vitro reactions of vacuole inheritance. J Cell Biol. 1994;126:87–97. doi: 10.1083/jcb.126.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]