Abstract
Soybean farming has faced several losses in productivity due to drought events in the last few decades. However, plants have molecular mechanisms to prevent and protect against water deficit injuries, and transcription factors play an important role in triggering different defense mechanisms. Understanding the expression patterns of transcription factors in response to water deficit and to environmental diurnal changes is very important for unveiling water deficit stress tolerance mechanisms. Here, we analyzed the expression patterns of ten APETALA2/Ethylene Responsive Element Binding-like (AP2/EREB-like) transcription factors in two soybean genotypes (BR16: drought-sensitive; and Embrapa 48: drought-tolerant). According to phylogenetic and domain analyses, these genes can be included in the DREB and ERF subfamilies. We also analyzed a GmDRIP-like gene that encodes a DREB negative regulator. We detected the up-regulation of 9 GmAP2/EREB-like genes and identified transcriptional differences that were dependent on the levels of the stress applied and the tissue type analyzed (the expression of the GmDREB1F-like gene, for example, was four times higher in roots than in leaves). The GmDRIP-like gene was not induced by water deficit in BR16 during the longest periods of stress, but was significantly induced in Embrapa 48; this suggests a possible genetic/molecular difference between the responses of these cultivars to water deficit stress. Additionally, RNAseq gene expression analysis over a 24-h time course indicates that the expression patterns of several GmDREB-like genes are subject to oscillation over the course of the day, indicating a possible circadian regulation.
Introduction
Soybeans (Glycine max L. Merrill) are one of the most important cultivated oil crops due to their use in human and animal feed and their potential as a biofuel. Despite the increasing improvements in productivity that have been obtained in the last few years, soybean production shows significant losses during drought events. Classified as “sensitive to drought,” especially during the emergence period, soybean crop productivity decreases drastically under water deficit conditions, which may be amplified by the impacts of global warming in the near future [1].
Different mechanisms are used by plants to protect themselves against water deficit; these include changes in stomatal conductance mediated by the hormone abscisic acid (ABA) [2], osmotic adjustment [3], the accumulation of osmoprotectant molecules in the cytosol, which protects cell structures [4], and the activity of antioxidant proteins [5]. The interaction between different physiological mechanisms, triggered by the up- and down-regulation of many genes, demonstrates that water deficit tolerance is a multigenic process. Precise control of this complex network of metabolic pathways allows plants to tolerate periods of water deficit. In this context, transcription factors (TFs) play an important role in this process, from stress-signal perception to transmission via signal transduction pathways and the triggering of different defense mechanisms.
Molecular responses to water deficit can be divided into ABA-dependent and ABA-independent pathways [6]. In the ABA-independent pathway, transcription factors from the AP2/EREBP (APETALA2/Ethylene Responsive Element Binding Protein) superfamily, also known as AP2/ERF (APETALA2/Ethylene Responsive Factor), activate the cis-elements that are present in the promoters of stress-induced genes [7]. The AP2/EREBP superfamily is composed of the AP2, ERF, and RAV families. The ERF family includes the ERF and CBF/DREB subfamilies, which are involved in plant responses to abiotic stress, such as water deficit [6], [8].
Of the CBF/DREB subfamilies, the most well-studied transcription factors are DREB1 and DREB2 [9]. The CBF/DREB transcription factors have an ERF domain, which consists of 58–60 amino acids that recognize and bind to GCC-box and C-repeat CRT/Dehydration Responsive Element (DRE) motifs in the target genes [10]. In spite of these common domains, AtDREB1 has been implicated in cold stress responses [11], whereas the functions of the AtDREB2 genes have been mainly described in response to water deficit and osmotic stress [12], [13]. Using an in silico analysis strategy, Wang and colleagues [14] searched for Arabidopsis thaliana genes with DRE motifs and identified 474 target genes to which the DREB transcription factors might bind. Of these genes, 160 were responsive to abiotic stresses, 27 of which were specifically regulated in response to water deficit. In addition, another genome-wide analysis of DREB-like transcription factors led to the identification of 36 CBF/DREB-like genes in Vitis vinifera [15], 57 genes in Arabidopsis thaliana, 52 genes in Oryza sativa [16], 77 genes in Populos trichocarpa [17], and 36 genes in Glycine max [18].
Overexpression of the DREB genes in many crop plants increased abiotic stress tolerance to high temperatures, low temperatures, salt stress, and water deficit [12], [19], [20]. However, studies have shown that the DRIP (DREB-Interacting Protein) [21] and PIF7 (Phytochrome-Interacting Factor 7) [22] proteins negatively regulate the DREB genes. Although the exact mechanisms of activation of the DREB transcription factors remain unclear, there is significant evidence indicating that the stability of the DREB proteins in the nucleus plays an important role in their activation [22]. The DRIP proteins contain a C3HC4 RING domain and can act as E3 ubiquitin ligases that mediate DREB ubiquitination and degradation. This indicates that DRIP proteins are important negative regulators of the DREB transcription factors and, therefore, of the DREB-mediated responses to abiotic stresses.
We have previously conducted studies on water deficit responses in soybean focusing on the tolerance that is conferred by the overexpression of the DREB transcription factors. In one of these studies, a drought-sensitive soybean cultivar, BR16, was transformed with the AtDREB1A gene to generate the novel soybean line P58. These genetically modified plants showed enhanced water deficit tolerance compared with the wild [23]. Therefore, it is important to identify and characterize the expression patterns of soybean orthologs/paralogs of the DREB genes, which could be used to improve water deficit tolerance through genetic engineering approaches similar to those used for A. thaliana genes. Using quantitative PCR, we characterized the expression of ten differentially expressed genes from the AP2/EREB and DRIP family identified using subtractive libraries constructed from two contrasting soybean genotypes (BR 16 and Embrapa 48) subjected to water deficit. We also evaluated the influence of the time of day in the expression patterns of these genes using RNAseq to analyze gene expression over the course of the day.
Materials and Methods
1. Plant Materials and Experimental Design
1.1. For subtractive library and qPCR assays
The experiments were performed as described by Rodrigues et al. [24]. Briefly, leaves and roots from control and stressed plants were obtained from Embrapa 48 and BR 16 soybean cultivars that had been grown hydroponically, as described by Martins et al. [25]. When the plants reached the V4 stage, they were subjected to progressive water deficit treatments. Leaves and roots from both cultivars were harvested after 25, 50, 75, 100, 125, and 150 min of exposure to dehydration conditions. To construct the subtractive libraries, the samples were grouped, and the L1 (25 and 50 min of dehydration), L2 (75 and 100 min of dehydration), and L3 (125 and 150 min of dehydration) leaf samples and the corresponding R1, R2, and R3 root samples were formed. The differentially expressed transcripts that were obtained were sequenced by Next Generation Sequencing, and the data were deposited in the Genosoja Soybean Database (http://lge.ibi.unicamp.br/soybean) [26], which was created by a Brazilian Consortium for the Soybean Genome (Genosoja project) and used to search for differentially expressed genes. For the qPCR analysis, we used the same experimental design, but each period of exposure to dehydration (25, 50, 75, 100, 125, and 150 min) was evaluated individually.
1.2. For RNAseq assays
The seeds from the BR16 genotype were cultivated in peat pots (Jiffy) with Supersoil® (Scotts Miracle-Gro Company, Marysville, Ohio, USA). The plants were grown in growth chambers set to 14 h light/10 h night cycles, with 500 µmol m-2s-1 of white light provided by cool white fluorescent bulbs. The temperatures in the growth chamber were set to 28°C during the light period and 20°C during the dark period. Fifteen days after germination, when the plants reached the V2 developmental stage (according to Fehr and colleagues [27]), water was withheld in the stress treatments to induce a water deficit. The soil moisture was calculated by the gravimetric humidity (GH), which corresponds to the percentage of water in the soil in relation to the dry weight of the soil. The volume of irrigation was adjusted to 70% (GH) (near field capacity) for the unstressed treatment, 30% GH for the water deficit stress treatment. Fully expanded V1 leaves were collected from the six plants in each treatment at 4-h intervals from the time the lights came on and were immediately frozen in liquid N2 and stored at −80°C until further use. The samples obtained in the dark were collected with the aid of a small green LED light (PhotonLight.com).
2. Gene Identification, Domain Analysis, and Phylogeny
Using the Genosoja Soybean database, 11 target genes that were up-regulated by water deficit were selected based on their similarity to genes from the AP2/EREBP superfamily and the DRIP proteins. Phylogenetic relationships between the AP2/EREB-like genes and the AP2/EREB genes from Fabaceae were considered. For this purpose, the amino-acid-deduced sequences of the genes were subjected to global alignment, and a phylogenetic tree was constructed with the ClustalW tool of the Molecular Evolutionary Genetics Analysis version 5.0 (MEGA 5) software package [28] using the Neighbor-Joining (NJ) method with the following parameters: Poisson correction, pairwise deletion, and bootstrap (1000 replicates; random seed). Given that the AP2 domain is important for the function and classification of transcription factors from the AP2/EREBP subfamilies, the AP2 domains of the proteins encoded by the selected genes were identified by screening using the ScanProsite online tool (http://prosite.expasy.org/scanprosite/). The amino acid sequences were also aligned using MEGA 5 software [28] through the ClustalW algorithm to assess the pattern of conservation and the differences between the sequences.
3. Gene Expression Analysis by qPCR
3.1. Primer design and efficiency analysis
Primers for the target genes were designed based on the GeneModels of the selected genes using the program Primer Express 3.0 (Applied Biosystems/Life Technologies, Grand Island, NY, USA) (Table S1). Primer sequences were determined for the 3' end of each gene, and the amplicons spanned up to 150 base pairs (bp). Primer sequences were BLASTed against the soybean genome (Phytozome database v1.0, http://www.phytozome.net/search.php) to verify the specificity of each primer, and standard curves were produced from serial dilutions of a cDNA pool to estimate the efficiency of the PCR amplification reactions. The primer concentrations were adjusted to obtain efficiency rates higher than 85%, as detailed in Table S1.
3.2. Selection of endogenous genes
To measure relative gene expression, it is essential to normalize the raw data using endogenous genes. However, endogenous gene expression can vary depending on the experimental treatment or time point or the plant developmental stage [29]. The endogenous genes β-tubulin (Glyma20g27280) [30], α-tubulin (Glyma08g12140) [30], Elongation factor 1-β (Glyma13g04050) [30], β-actin (Glyma15g05570) [31], rRNA 18S (Glyma13g12030) [31], and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Glyma06g01850) [31] (Table S1) were assayed to determine the most stably expressed gene in the water deficit-stressed soybean plants. The expression stability of the endogenous genes was evaluated in the leaves and roots of the drought-sensitive BR16 cultivar [32] and was measured using the GeNorm [33] and NormFinder [34] programs. Ct values were transformed into relative quantities using standard curves, and the data were transformed according to the Δ-Ct formula described by Vandesompele et al. [33]. The most stable endogenous genes were chosen.
3.3. Expression analysis
elative expression levels of the target genes GmAP2/EREB-like and GmDRIP-like were measured in root and leaf samples from Embrapa 48 and BR16 plants. For each time point (0, 25, 50, 75, 100, 125, and 150 min under water deficit), three biological replicates, each with three technical replicates, were analyzed. After DNAse treatment (Invitrogen/Life Technologies, Grand Island, NY, USA), high quality total RNA was used to synthesize cDNA strands (Superscript II First Strand Synthesis, Invitrogen/Life Technologies, Grand Island, NY, USA), and cDNA quality was verified using a standard PCR reaction with an actin primer that spanned an intronic region. After carrying out the amplification efficiency analysis, the genes were amplified by qPCR using a 7500 RT-qPCR Thermocycler (Applied Biosystems/Life Technologies, Grand Island, NY, USA) with the following cycling parameters: 50°C for 2 min, 95°C for 10 min, 45 cycles at 95°C for 2 min, 60°C for 30 seconds and 72°C for 30 seconds. Data were collected during the extension phase, and dissociation curves were performed by heating each amplicon from 60 to 95°C and taking readings at one-degree intervals to verify the specificity of the primers.
The Rest2009 software package [35] was used to evaluate the data because this program provided a more robust statistical analysis. The normalization of the real-time quantitative RT-PCR was performed by taking the geometric average of the selected endogenous genes (Elongation factor 1-β and β-actin), and the control plants (0 min under stress) were used to normalize the relative expression. Hypothesis testing was used to determine whether the differences between the control and treatment conditions were significant [35].
4. Gene Expression Analysis by RNAseq
The soybean transcriptome was analyzed in leaf samples from BR16 plants. After DNase treatment (Life TechnologiesGrand Island, NY, USA), high-quality total RNA was used to analyze the transcripts for each time point: 8, 12, 16, 20, 24, and 4 h. Bulks of leaves from two plants were used in the RNA extraction to compose one replication. Three replications for each time point/treatment were sequenced. The RNAseq libraries were built using the Nugen-Ovation® kit according to the manufacturer’s instructions (NuGEN Technologies Inc., San Carlos, CA, USA). The libraries obtained were subjected to sequencing by Illumina HiSeq2000 (Illumina, San Diego, CA, USA). Mapping of the reads was performed with the Soybean genome (Phytosome Glycine max v1.1) using the GeneSifter platform (http://www.geospiza.com/Products/AnalysisEdition.shtml). To compare gene expression between different times and conditions, we log2-transformed the normalized reads per mapped million (RPM) value. We then ran a variance test (t-test for two group comparisons or ANOVA equal variance for multiple group comparisons).
Results
1. Gene Identification and Analysis of Domains and Phylogeny
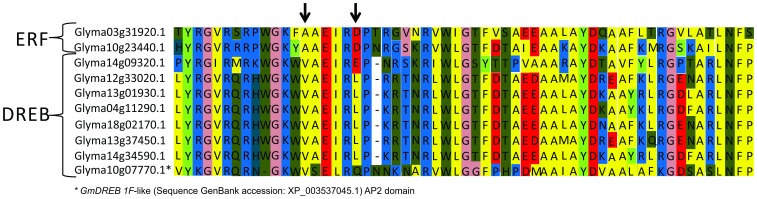
After evaluating differentially expressed genes in soybean plants that had been subjected to short-term water deficit, we selected ten genes with similarity to the AP2/EREB family. We also found one gene encoding a GmDRIP-like protein that, although a negative regulator of DREB genes in A. thaliana, was induced in the same water deficit conditions in soybean. The identification of the selected genes, their BlastX similarities and their ontologies are shown in Table 1. By analyzing the AP2 domain of the proteins using the ClustalW algorithm (MEGA 5), we classified ten genes as members of the ERF and DREB subfamilies (Fig. 1). Because the Glyma10g07770.1 annotation in the Phytozome v1.0 database lacked the AP2 domain, we used the predicted protein sequence of XGmDREB 1F-like gene (Sequence GenBank accession: XP_003537045.1), which was very similar to Glyma10g07770.1 (Table 1) and contained an AP2 domain.
Table 1. Selected DRIP and ERF superfamily target genes.
| BLASTx NCBI | BLAST Gene Ontology | ||||
| Gene Model | Description | e-value | GenBankaccession | Biological process | Molecular function |
| Glyma03g31920.1 | Uncharacterized proteinLOC100818907[Glycine max] | 2.00E-148 | ACU20075.1 | GO:0006355: regulation of transcription,DNA-dependent, | GO:0003700: sequence-specificDNA-binding transcriptionfactor activity |
| Glyma10g23440.1 | PREDICTED: ethylene-responsive transcriptionfactor ERF105-like | 4.00E-91 | XP_003535939 | GO:0006355: regulation of transcription,DNA-dependent | GO:0003700: sequence-specificDNA-binding transcriptionfactor activity |
| Glyma10g07770.1 | PREDICTED: dehydration-responsive element-bindingprotein 1F-like [Glycine max] | 1.00E-106 | XP_003537045.1 | GO:0045893: positive regulation of transcription,DNA-dependent, GO:0009409: response to cold,GO:0009414: response to water deprivation | GO:0003700: sequence-specificDNA-binding transcriptionfactor activity |
| Glyma14g09320.1 | Dehydration responsiveelement binding proteinDREB1 [Glycine max] | 1.00E-124 | AAP47161.1 | GO:0045893: positive regulation of transcription,DNA-dependent, GO:0009409: response to cold,GO:0009414: response to water deprivation | GO:0003700: sequence-specificDNA-binding transcriptionfactor activity |
| Glyma18g02170.1 | PREDICTED: ethylene-responsive transcriptionfactor ERF060-like | 3.00E-180 | XP_003552083.1 | GO:0009873: ethylene mediated signaling pathway,GO:0006355: regulation of transcription, DNA-dependent, GO:0009409: response to cold,GO:0009416: response to light stimulus,GO:0006970: response to osmotic stress,GO:0009414: response to water deprivation | GO:0003700: sequence-specificDNA-binding transcriptionfactor activity, O:0005515:protein-binding |
| Glyma04g11290.1 | Dehydration responsiveelement-binding protein 3[Glycine max] | 1.00E-136 | AAZ03388.1 | GO:0009873: ethylene mediated signaling pathway,GO:0006355: regulation of transcription, DNA-dependent, GO:0009409: response to cold,GO:0009416: response to light stimulus,GO:0006970: response to osmotic stress,GO:0009414: response to water deprivation | GO:0003700: sequence-specificDNA-binding transcriptionfactor activity, O:0005515:protein-binding |
| Glyma13g01930.1 | Dehydration responsiveelement-binding protein 3[Glycine max] | 6.00E-65 | AAZ03388.1 | GO:0006355: regulation of transcription, DNA-dependent, GO:0009409: response to cold,GO:0006970: response to osmotic stress,GO:0009414: response to water deprivation, | GO:0003700: sequence-specificDNA-binding transcriptionfactor activity, GO:0005515:protein-binding |
| Glyma14g34590.1 | Dehydration responsiveelement-binding protein 3[Glycine max] | 1.00E-66 | AAZ03388.1 | GO:0006355: regulation of transcription, DNA-dependent, GO:0009409: response to cold,GO:0006970: response to osmotic stress,GO:0009414: response to water deprivation, | GO:0003700: sequence-specificDNA-binding transcriptionfactor activity, GO:0005515:protein-binding |
| Glyma12g33020.1 | Drought responsiveelement binding protein 5[Glycine max] | 2.00E-178 | CCF23313.1 | GO:0006355: regulation of transcription, DNA-dependent, GO:0009409: response to cold,GO:0006970: response to osmotic stress,GO:0009414: response to water deprivation, | GO:0003700: sequence-specificDNA-binding transcriptionfactor activity |
| Glyma13g37450.1 | Drought responsiveelement binding protein 5[Glycine max] | 7.00E-103 | CBZ41765.1 | GO:0006355: regulation of transcription, DNA-dependent, GO:0009409: response to cold,GO:0006970: response to osmotic stress,GO:0009414: response to water deprivation, | GO:0003700: sequence-specificDNA-binding transcriptionfactor activity |
| Glyma19g34440.1 | PREDICTED: E3 ubiquitinprotein ligase DRIP2-like[Glycine max] | 0.0 | XP_003553481.1 | GO:0016567: protein ubiquitination, GO:0009414:response to water deprivation, | GO:0005515: protein binding,GO:0004842: ubiquitin-proteinligase activity |
The BLAST description and Gene Ontology are presented for each gene, and the sequences with greater similarity were used (GenBank access #). The BLAST results are from Aug. 2012 and the GO terms for Biological Process and Molecular Function are listed in the Gene Ontology annotation.
The BLAST description and Gene Ontology are presented for each gene; the sequences with greater similarity were used (GenBank accession #). The BLAST results are from Aug. 2012, and the GO terms for Biological Processes and Molecular Function are listed in the Gene Ontology annotation.
Figure 1. Amino-acid sequence alignment of the AP2 domains.
Regions of amino-acid conservation are shown. Letters represent the amino acids of the protein sequences, and dashes delimit the specific 14th and 19th positions for each DREB or ERF subfamily member.
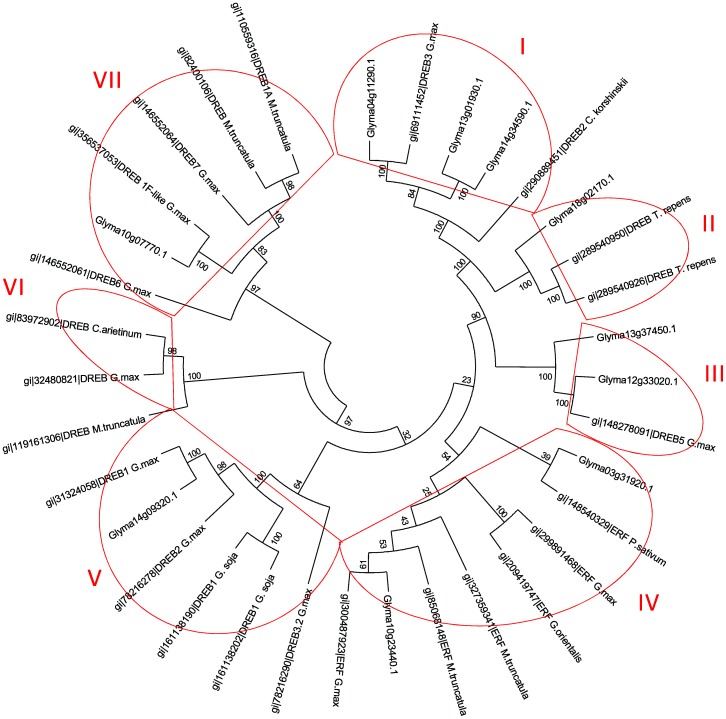
The translated amino-acid sequences for these genes were also globally aligned, and a phylogenetic tree was constructed. This analysis shows the phylogenetic relationships between the soybean genes from the DREB and ERF subfamilies and other plants of the Fabaceae family that are found in the NCBI database (Fig. 2). This analysis identified seven main groups of genes: (I) soybean DREB3 and Caragana korshinskii DREB2; (II) Trifolium repens DREB; (III) soybean DREB5; (IV) ERF subfamily; (V) soybean DREB1 and DREB2; (VI) C. Arietinum, soybean, and M. truncatula DREB; (VII) soybean DREB6, DREB7 and DREB 1F and two M. truncatula DREB.
Figure 2. Phylogenetic tree. Proteins encoded by the candidate genes and the DREB/ERF protein that was described in the NCBI database were used to construct the tree using the ClustalW algorithm with the MEGA 5 program.
The Neighbor-Joining (NJ) method was used with the following parameters: Poisson correction, pairwise deletion, and bootstrap (1000 replicates; random seed). Candidate genes are represented by the GeneModels, and the homologous DREB/ERF sequences from Fabaceae (Glycine max, Medicago truncatula, Cypripedium arietinum, Trifolium repens, Glycine soja, Caragana korshinskii, Pisum sativum, and Galega orientalis) are represented by GI.
2. Analysis of Endogenous Genes
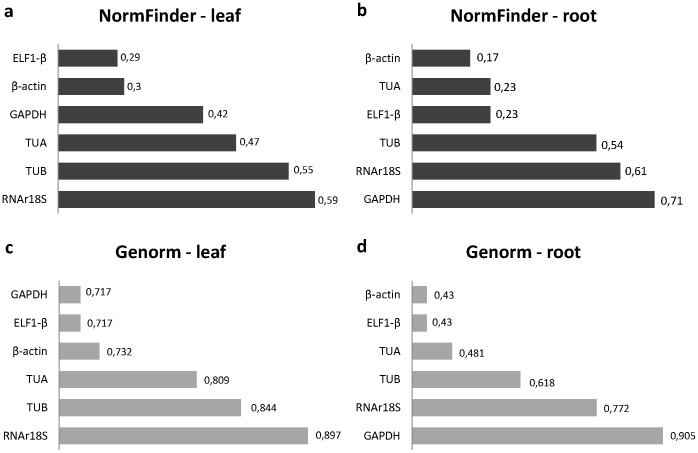
Based on results obtained using the NormFinder software package, the Elongation Factor 1β (ELF-1β) and β-actin genes were the most stably expressed in the leaf samples, whereas in the roots, the α-tubulin, ELF-1β and β-actin genes were also significantly stable (Figs. 3A and B).
Figure 3. Stability analyses of endogenous genes.
In total, six candidate genes were evaluated using the NormFinder and GeNorm programs to select the most stable genes. The Y axis represents the Expression Stability Measure (M) from the GeNorm program and the Stability value from the NormFinder program. Genes are ranked from less stable (higher values) to most stable (genes with lower values).
Based on the analysis performed using the GeNorm program, we found that the ELF-1β and β-actin genes were also the most stably expressed in the roots (Fig. 3D). However, in the leaves, the gene expression of ELF-1β and GAPDH was most stable (Fig. 3C), and β-actin was classified as being the third-most stably expressed gene (Fig. 3C).
3. Relative Expression of the AP2/EREB and DRIP-like Genes in Response to Drought
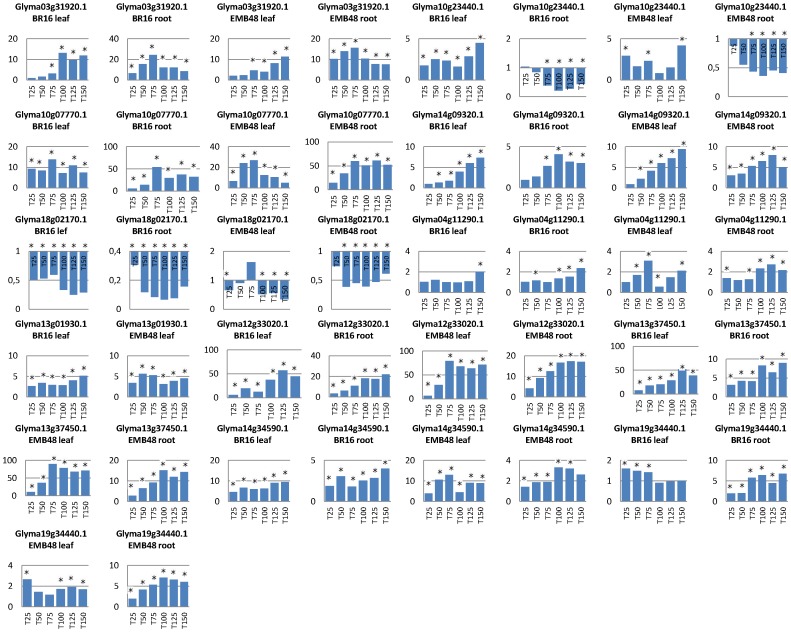
We evaluated the expression patterns of ten of the GmAP2/EREB-like genes and detected nine genes that were up-regulated when both Embrapa 48 and BR16 soybean plants were exposed to water-deficit conditions (Fig. 4). We also detected the up-regulation of a GmDRIP-like gene in both cultivars in response to water deficit. The Rest2009 software package allowed for the determination of statistical significance, as detailed in Table S2. The genes showed different transcriptional patterns throughout the water-deficit treatment (25 to 150 min under dehydration) and within the analyzed tissues. Of the up-regulated genes, the GmDREB1F-like gene (Glyma10g07770.1) and the GmDREB5-like genes (Glyma12g33020.1 and Glyma13g37450.1) showed the highest stress-induced expression in both cultivars (Fig. 4). In contrast, Glyma18g02170.1 was repressed or non-differentially expressed in response to water deficit during the evaluated stress periods.
Figure 4. Quantitative PCR of the AP2/EREB genes.
Gene expression was measured in root and leaf tissues of BR 16 and Embrapa 48 soybean cultivars that were subjected to different periods of water deficit (25 to 150 min). The raw data were normalized to the expression of the ELF1-β and the β-actin endogenous genes, and the relative expression was determined and compared with the control sample (T0 min).
4. Influence of Time of the Day on GmDREB-like Genes Responses to Water Deficit
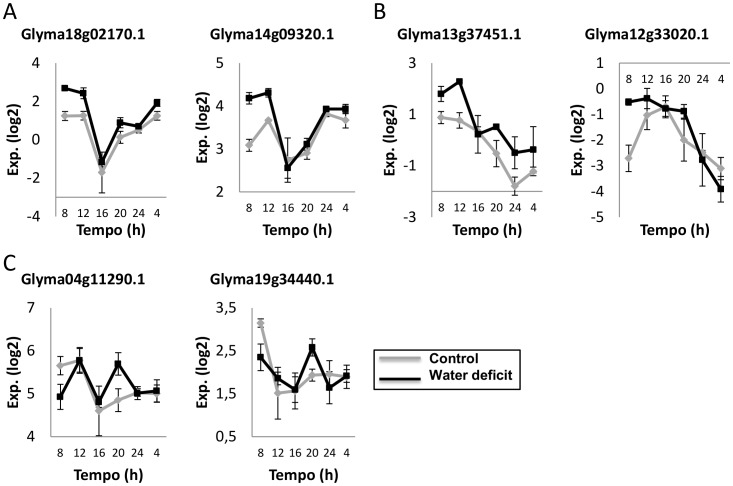
To observe if the target genes oscillated in their expression patterns over the course of the day, we used the RNAseq reads from the BR16 genotype, which were mapped in the soybean genome. Under control conditions, the expression of the DREB-related genes generally increased just before dawn and reached their peak expression between 8 and 12 h (Fig. 5). Interestingly, the stress induction of these genes also displayed a strong oscillation over the course of the day. Based on the gene expression patterns in response to water deficit, the genes we analyzed could be divided into three groups: group A was characterized by high expression levels during the end of the night and the beginning of the day, with lowest expression levels in the afternoon (16 h); group B corresponded to genes with higher expression levels in the beginning of the day and low expression levels at the end of the day; group C was represented by genes significantly induced by water deficit stress approximately 2 h before lights went out, at 20 h.
Figure 5. RNAseq data of the AP2/EREB and DRIP2-like genes.
Gene expression was measured in leaf tissues of BR 16 soybeans subjected to water deficit (drought) and normal hydration conditions (control). Gene expression (Exp. Log. [log2]) was evaluated over a 24-h time course from the time the light came on (ZT0) in 4-h intervals. Error bars represent the standard error (SEM).
Discussion
1. Identification of Target Genes
The large AP2/EREB family includes transcription factors that share one or two domains (AP2/EREB) and function in plant development and physiological processes that are related to abiotic stress responses [36]. The DRIP transcription factors act as negative regulators of the AP2/EREB family in A. thaliana, and these proteins specifically regulate the DREB subfamily. Therefore, studies aiming to evaluate the transcriptional profiles of genes from these families are important for understanding the relationship between the antagonistic transcription factors AP2/EREB and DRIP.
Bioinformatics has been a powerful tool for in silico analyses because databases have grown rapidly over the last several years due to the large amount of data that has been generated by genomics and transcriptomics techniques. The gene expression data that was stored in the soybean database [24] was useful for identifying AP2/EREB- and DRIP-family transcripts that were differentially expressed under water-deficit conditions. The differentially expressed transcripts were present in the soybean genome in the Phytozome database v1.0 (http://www.phytozome.net/search.php), which allowed us to examine correlations between the expressed transcripts and the soybean gene models (Glymas). Gene anchorage provided information about the size of the sequence, coding sequence regions, and the 3' and 5' untranslated regions (UTRs), which was essential for phylogenetic analyses and primer design. The gene models are putative genes that are predicted through in silico analyses of the soybean genome. Our study provides experimental proof of the functionality of these genes and describes the predicted genes as in vivo-expressed genes. Additionally, the BlastX tool [37] has allowed for the identification of probable proteins that are coded by those transcripts and their attributed ontologies through searches in the GenBank [http://www.ncbi.nlm.nih.gov/genbank/] and Gene Ontology [http://www.geneontology.org/] databases (Table 1). Importantly, the majority of the genes that were evaluated in our study were similar to predicted and uncharacterized proteins, showing, once again, the importance of this study to the description of these genes as expressed genes.
Amino acids that are located at the 14th and 19th positions of their protein sequence are highly conserved in AP2/EREB subfamily members. The transcription factors from the DREB subfamily contain a conserved valine (V14) and glutamic acid (E19) at these positions, whereas the ERF subfamily has a conserved alanine and aspartic acid at these positions [38]. Of the AP2/EREB-selected target genes, two belong to the ERF subfamily, and seven belong to the DREB subfamily (Fig. 1). However, of the DREB members, we noticed a higher degree of conservation at the 14th position than at the 19th position. This subfamily classification was confirmed by a phylogenetic analysis, which showed the separation of the DREB and ERF subgroups into distinct segments (Fig. 2). We also observed the formation of some smaller groups within the DREB and ERF subfamilies that were related to soybean and other Fabaceae genes (Fig. 2). The close phylogenetic relationship between the target genes and genes from the AP2/EREB family corroborates the similarity that was found by BLASTing the sequences; this can indicate homology of function for sequences that present high phylogenetic relatedness, as suggested by Oh et al. [39].
2. Endogenous Genes
The endogenous stability analysis performed using the GeNorm and NormFinder programs generally indicated that ELF-1β and β-actin were the most stable endogenous genes (Fig. 3). However, in leaf tissues, the GeNorm program identified the GAPDH gene as one of the two most stable endogenous genes instead of β-actin (Fig. 3C and D). However, the M value (expression stability measure) for the GAPDH gene differed by only 0.015 units from that of the β-actin gene (the third-best endogenous gene) (Fig. 3C), and the GAPDH gene showed a low stability in evaluations of root tissues (Fig. 3A and C). Based on these results, we selected the ELF-1β and β-actin endogenous genes as expression controls for the qPCR analysis.
3. Transcriptional Profiles of the Target Genes
Studies have previously been conducted to evaluate the expression of the DREB and ERF soybean genes in response to stresses such as cold and water deficit [13], [18], [40]. However, the genes that were evaluated in our study were predicted to be candidate genes in soybean via in silico analysis. To our knowledge, our work using subtractive libraries and qPCR analysis of expression are the first experimental reports of the expression of these genes in response to water deficit in soybeans. We have quantified the expressions of the genes after different periods of time under stress – which correspond to different levels of stress – in both root and leaf tissues. The results provide an abundant set of information regarding the expression of these genes in response to water deficit in the BR16 and Embrapa 48 soybean cultivars.
We observed an increase in the expression of the GmDREB1-like gene (Glyma14g09320.1) in leaf tissues that was proportional to the increase in the severity of the stress (from 25 min to 150 min of dehydration) (Fig. 4). In addition to the differences in expression over time, we also observed differences in the gene expression profiles between the root and leaf tissues. For example, the GmERF-like gene (Glyma10g23440.1) was only induced in response to water deficit in the leaves, whereas, in the root tissues, its expression was repressed (Fig. 4). This gene was previously identified in forwarded subtractive libraries as being expressed in the leaf tissues of BR 16 plants [24], which corroborated the findings of this study. This contrasting behavior in different tissues should be highlighted because it might indicate that the promoter acts in a tissue-specific manner. Candidate genes with tissue-specific promoters are interesting due to their potential for use in biotechnology applications [41].
The GmDREB5-like genes (Glyma12g33020.1 and Glyma13g37450.1) and the GmDREB1F-like gene (Glyma10g07770.1) also showed differential expression patterns in root and leaf tissues (Fig. 4). For example, Glyma10g07770.1 was expressed two-fold higher in the roots than in the leaves of Embrapa 48 plants that had been exposed to 75 min of water deficit, and this gene was expressed more than four-fold more in the roots than in the leaves of the BR16 cultivar at the same time point (75 min). This gene (Glyma10g07770.1) is highly similar to a predicted soybean DREB1F-like sequence (Table 1) and has a close phylogenetic relationship to the DREB subfamily (Fig. 2). However, it was not possible to identify an AP2 domain, which is typical of this type of gene, in the Glyma10g07770.1 sequence. Our global alignment analysis shows that the Glyma10g07770.1 sequence is very similar to the in silico-predicted soybean DREB1F-like sequence that was deposited at the NCBI, but the Glyma protein lacks the initial 52 amino acids that encode a major portion of the AP2 domain, likely due an incorrect annotation of the protein sequence in Phytozome v1.0. Based on our data, we propose, for the first time, the inclusion of Glyma10g07770.1 in the AP2/EREB family and report that this gene is water deficit-inducible. In contrast, the expression of the GmDREB5-like genes (Glyma12g33020.1 and Glyma13g37450.1) was higher in the leaves than in the roots of both cultivars (Fig. 4), which indicates differences between the responses of each tissue to stress. This finding is consistent with those of Wang et al. [42], who reported increased expression of a DREB subfamily gene (CkDBF) in the leaves of C. korshinskii after 4 h of dehydration, whereas the expression level of this gene in the roots was only moderate [42]. These genes are similar to the DREB5 sequence in soybean and comprise group III of the phylogenetic tree that was presented in Fig. 2. The GmDREB5 gene was identified in soybean; however, currently, there is no published information on the expression of the sequence that is deposited at NCBI (GenBank: ABQ53928.1).
The Glyma04g11290.1, Glyma13g01930.1, and Glyma14g34590.1 genes were similar to the soybean DREB3 gene (Table 1) and were placed in phylogenetic Group I (Fig. 2), which justifies the denomination of these genes as GmDREB3-like genes. The GmDREB3 gene was recently identified in the soybean genome, and its expression was associated with plant responses to cold [40]. Although it was first identified in cold stress responses, the authors reported that superexpression of the GmDREB3 gene in transgenic plants increased their tolerance to water deficit; however, gene expression analyses in non-transgenic plants were unable to confirm the responsiveness of the GmDREB3 gene to water deficit. Our results show that GmDREB3-like genes (Glyma04g11290.1, Glyma13g01930.1 and Glyma14g34590.1) are up-regulated in response to water deficit in the BR 16 and Embrapa 48 genotypes (Fig. 4). Additionally, the global alignment and phylogenetic analyses highlight the close correlation between the GmDREB3-like genes and DREB2 of Caragana korshinskii (CkDBF), which comprise group I of the phylogenetic tree (Fig. 2). C. korshinskii is a plant that is adapted to areas with limited water availability, and it is typically found in desert areas of China [42]. Superexpression of CkDREB2 in tobacco triggered various stress-related genes and enhanced the response of transgenic plants to water-deficit stress. Hence, this finding highlights the importance of the orthologous soybean DREB3-like genes (Glyma04g11290.1, Glyma13g01930.1 and Glyma14g34590.1) that were studied here.
To understand the molecular mechanisms of the response to water deficit, we also analyzed the expression of a GmDRIP2-like gene (Glyma19g34440.1), which is differentially expressed in soybean BR16 leaf subtractive libraries and encodes the protein DRIP2-like, which ortholog is a negative regulator of DREB factors in A. thaliana. Plant responses to water stress involve several genes that are involved in signaling via a complex metabolic network. Transcription factors play a key role in this signaling network, and plants are able to respond to stress efficiently only when this regulation is precisely controlled. Dong and Liu [43] evaluated the action of the DREB repressor molecule RAP2.1 and concluded that repressors are important for maintaining tight control of the stress responses and preventing metabolic damage and wear caused by a “runaway” stress response [43]. Here, we found that the repressor GmDRIP2-like gene was up-regulated at different time-points after the induction of water deficit in the Embrapa 48 and BR 16 cultivars (Fig. 4). Considering that this higher level of mRNA could potentially result in higher levels of the GmDRIP2-like protein, this may be an important mechanism for maintaining tight control of the water deficit response, as has been proposed for other DREB repressors [43]. However, a difference in the gene expression of the GmDRIP-like gene was evident in the leaves and roots; specifically, higher expression of this gene was observed in the roots compared with the leaves (Fig. 4) for both cultivars. Additionally, in the roots, expression of this gene increased over the time-course as the severity of the water deficit stress increased. This evidence suggests that different mechanisms control the water deficit response in different soybean tissues. Furthermore, some differences in the expression of this gene were observed between the cultivars. For example, in the BR16 leaves, the GmDRIP2-like gene was not induced during the longest exposures to stress (100, 125, and 150 min), whereas in Embrapa 48, an increase in gene expression was observed during these exposures (Fig. 4). Therefore, this variation could be a genetic/molecular difference between the responses of these cultivars to water deficit stress.
4. Influence of Time of the Day on the Expression Pattern
According to the gene expression patterns in response to water deficit, the genes identified in the RNAseq analysis were divided into groups A, B, and C. Group A is composed of Glyma18g02170.1 and Glyma14g09320.1 genes, which had the highest expression levels during the end of the night and the beginning of the day and the lowest expression levels at 16 h. The Glyma18g02170.1 gene, as previously mentioned, is phylogenetically related to DREB3 from T. repens (group II), a gene identified through structural genomics studies [44]. Until recently, there has been no public data on the transcriptional patterns of this gene. Despite the induction of Glyma18g02170.1 could not be detected by qPCR analyses, in the RNAseq analysis this gene was induced and displayed day oscillation in response to water deficit stress, indicating that the circadian clock might influence the water deficit responses in soybeans. The difference in the transcription patterns between the qPCR and RNAseq data can be explained by disparity in plant developmental stages (V4 and V2 for qPCR and RNAseq, respectively), as demonstrated in previous studies in which changes in gene expression were detected between the developmental stages of Arabidopsis [45] and soybean [46].
According to the BLAST and Phylogenetic analyses (Table 1 and Fig. 2), the genes Glyma12g33020.1 and Glyma13g37450.1 are related to GmDREB5. Interestingly, both genes exhibited similar expression patterns in response to water deficit over the course of the day: the highest expression levels at the beginning of the day and the lowest expression levels at the end of the day. These genes comprise group B (Fig. 5). To date, our results are the first showing the influence of the time of the day on GmDREB5 gene expression. This suggests that the circadian clock seems to be acting to modulate the expression profiles of genes with related functions so as to be coordinately expressed in the best period of the day, contributing to an efficient water deficit response. The circadian control of related genes was previously described in soybean studies in which transcripts encoding proteins with distinct roles in seed metabolism and biochemistry were segregated by phase/time of day [47].
Group C is composed of the genes Glyma04g11290.1 and Glyma19g34440.1. Glyma04g11290.1 is similar to a GmDREB3 gene (Fig. 2, Table 1), and Glyma19g34440.1 encodes a DRIP2-like protein, which, in Arabidopsis, regulates the expression of DREB factors. Although the interaction between DREB and DRIP has been investigated at the protein level, there is a lack of information regarding these genes’ relationship at the transcript level. Based on qPCR expression data, we previously proposed that GmDRIP2-like expression might be part of an important mechanism for maintaining tight control of the water deficit response. Supporting this idea, our RNAseq data indicates that the GmDRIP2-like gene is significantly induced by water deficit coordinately with a soybean DREB gene (DREB3-like) (Fig. 5).
Conclusion
Here, we provide an abundant set of information concerning the expression of AP2/EREB transcription factors in different tissues of the Embrapa 48 and BR 16 Brazilian soybean cultivars in response to varying water deficit levels. We detected differences in gene expression that depended on (1) the level of stress that was applied and (2) the tissue that was evaluated, where contrasting behavior within different tissues might indicate promoters that act in a tissue-specific manner.
The genes that were evaluated here are predicted gene models in soybean (identified through in silico analyses), and most of the genes were similar to predicted or uncharacterized proteins. To our knowledge, our results using Subtractive libraries and qPCR analyses are the first evidence of the expression of these genes in response to water deficit in soybeans. We also experimentally identified a new AP2/EREB-like gene (Glyma10g07770.1) and showed its up-regulation by water-deficit stress conditions. Furthermore, we believe that the present study is the first report on the up-regulation of GmDREB3-like and GmDREB5-like genes in response to water deficit in soybeans. The differential expression patterns of the GmDRIP-like gene in the BR16 and Embrapa 48 cultivars is the first reported genetic/molecular difference between these cultivars in response to water deficit.
Additionally, our results show that several DREB-like and the DRIP2-like genes have daily oscillation patterns in their expression profiles. This suggests a circadian clock control over these genes, even under water deficit conditions. The circadian clock is known to confer adaptive advantages to organisms by synchronizing the best time of the day for biochemical reactions to occur to optimize development or to endure stressful situations. Thus, improving our understanding of the oscillation patterns of important water deficit stress effectors, such as DREB genes, becomes of great interest.
Supporting Information
Primers for the target genes. Nucleotide sequences and the denaturation temperatures (Tm) of the Forward (F) and Reverse (R) primers are shown. The amplification efficiency was calculated using a standard curve.
(XLSX)
Statistical analysis. The Rest 2009 software package was used to calculate iterations of gene expression among all experimental treatment periods and their statistical significances. P(H1) is the probability of observing the difference between the control plants and those exposed to water deficit by chance. The Result columns indicate the direction of the change in expression when p<0.05 (UP = up-regulated; DOWN = down-regulated; ND = not differentially expressed).
(XLSX)
Acknowledgments
We thank Embrapa Soybean for use of the greenhouse and laboratory facilities.
Funding Statement
This study was supported by the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq [http://www.cnpq.br/] Genosoja grant 552735/2007-8) and Coordenação de Pessoal de Nível Superior (CAPES [http://www.capes.gov.br/]). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bates B, Kundzewicz ZW, Wu S, Palutikof JP (2008) Introduction to climate change and water. IPCC Secretariat, editor Geneva.
- 2. Melcher K, Ng L-M, Zhou XE, Soon F-F, Xu Y, et al. (2009) A gate-latch-lock mechanism for hormone signalling by abscisic acid receptors. Nature 462: 602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silva EN, Ferreira-Silva SL, Viégas RA, Silveira JAG (2010) The role of organic and inorganic solutes in the osmotic adjustment of drought-stressed Jatropha curcas plants. Environmental and Experimental Botany 69: 279–285. [Google Scholar]
- 4. Almeida AM, Cardoso LA, Santos DM, Torné JM, Fevereiro PS (2007) Trehalose and its applications in plant biotechnology. In Vitro Cellular & Developmental Biology - Plant 43: 167–177. [Google Scholar]
- 5. Uzilday B, Turkan I, Sekmen AH, Ozgur R, Karakaya HC (2012) Comparison of ROS formation and antioxidant enzymes in Cleome gynandra and Cleome spinosa under drought stress. Plant science: an international journal of experimental plant biology 182: 59–70. [DOI] [PubMed] [Google Scholar]
- 6. Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. Journal of experimental botany 58: 221–227. [DOI] [PubMed] [Google Scholar]
- 7. Lin R, Zhao W, Meng X, Peng Y-L (2007) Molecular cloning and characterization of a rice gene encoding AP2/EREBP-type transcription factor and its expression in response to infection with blast fungus and abiotic stresses. Physiological and Molecular Plant Pathology 70: 60–68. [Google Scholar]
- 8. Zhang G, Chen M, Li L, Xu Z, Chen X, et al. (2009) Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. Journal of experimental botany 60: 3781–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends in plant science 10: 88–94. [DOI] [PubMed] [Google Scholar]
- 10. Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, et al. (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. The Plant cell 10: 1391–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee YP, Fleming AJ, Körner C, Meins F (2009) Differential expression of the CBF pathway and cell cycle-related genes in Arabidopsis accessions in response to chronic low-temperature exposure. Plant biology (Stuttgart, Germany) 11: 273–283. [DOI] [PubMed] [Google Scholar]
- 12. Chen M, Wang Q-Y, Cheng X-G, Xu Z-S, Li L-C, et al. (2007) GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochemical and biophysical research communications 353: 299–305. [DOI] [PubMed] [Google Scholar]
- 13. Sakuma Y, Maruyama K, Osakabe Y, Qin F, Seki M, et al. (2006) Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 18: 1292–1309 doi:10.1105/tpc.105.035881.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang S, Yang S, Yin Y, Guo X, Wang S, et al. (2009) An in silico strategy identified the target gene candidates regulated by dehydration responsive element binding proteins (DREBs) in Arabidopsis genome. Plant molecular biology 69: 167–178. [DOI] [PubMed] [Google Scholar]
- 15. Zhuang J, Peng R-H, Cheng Z-M, Zhang J, Cai B, et al. (2009) Genome-wide analysis of the putative AP2/ERF family genes in Vitis vinifera . Scientia Horticulturae 123: 73–81. [Google Scholar]
- 16. Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-Wide Analysis of the ERF Gene Family. Plant Physiology 140: 411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhuang J, Cai B, Peng R-H, Zhu B, Jin X-F, et al. (2008) Genome-wide analysis of the AP2/ERF gene family in Populus trichocarpa . Biochemical and biophysical research communications 371: 468–474. [DOI] [PubMed] [Google Scholar]
- 18. Zhang G, Chen M, Chen X, Xu Z, Guan S, et al. (2008) Phylogeny, gene structures, and expression patterns of the ERF gene family in soybean (Glycine max L.). Journal of experimental botany 59: 4095–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qin F, Sakuma Y, Li J, Liu Q, Li Y-Q, et al. (2004) Cloning and functional analysis of a novel DREB1/CBF transcription factor involved in cold-responsive gene expression in Zea mays L. Plant & cell physiology. 45: 1042–1052. [DOI] [PubMed] [Google Scholar]
- 20. Almoguera C, Prieto-Dapena P, Díaz-Martín J, Espinosa JM, Carranco R, et al. (2009) The HaDREB2 transcription factor enhances basal thermotolerance and longevity of seeds through functional interaction with HaHSFA9. BMC plant biology 9: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qin F, Sakuma Y, Tran L-SP, Maruyama K, Kidokoro S, et al. (2008) Arabidopsis DREB2A-interacting proteins function as RING E3 ligases and negatively regulate plant drought stress-responsive gene expression. The Plant cell 20: 1693–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kidokoro S, Maruyama K, Nakashima K, Imura Y, Narusaka Y, et al. (2009) The phytochrome-interacting factor PIF7 negatively regulates DREB1 expression under circadian control in Arabidopsis. Plant physiology 151: 2046–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polizel AM, Medri ME, Nakashima K, Yamanaka N, Farias JRB, et al. (2011) Molecular, anatomical and physiological properties of a genetically modified soybean line transformed with rd29A:AtDREB1A for the improvement of drought tolerance. Genetics and molecular research: GMR 10: 3641–3656. [DOI] [PubMed] [Google Scholar]
- 24. Rodrigues FA, Marcolino-Gomes J, Fátima Corrêa Carvalho J, do Nascimento LC, Neumaier N, et al. (2012) Subtractive libraries for prospecting differentially expressed genes in the soybean under water deficit. Genetics and molecular biology 35: 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martins PK, Jordão BQ, Yamanaka N, Farias JRB, Beneventi MA, et al. (2008) Differential gene expression and mitotic cell analysis of the drought tolerant soybean (Glycine max L. Merrill Fabales, Fabaceae) cultivar MG/BR46 (Conquista) under two water deficit induction systems. Genetics and Molecular Biology 31: 512–521. [Google Scholar]
- 26. Nascimento LC, Costa GGL, Binneck E, Pereira GAG, Carazzolle MF (2012) A web-based bioinformatics interface applied to the GENOSOJA Project: Databases and pipelines. Genetics and molecular biology 35: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fehr WR, Caviness CE, Burmood DT, Pennington JS (1971) Stage of Development Descriptions for Soybeans, Glycine Max (L.) Merrill1. Crop Science 11: 929. [Google Scholar]
- 28. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular biology and evolution 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu R, Fan C, Li H, Zhang Q, Fu Y-F (2009) Evaluation of putative reference genes for gene expression normalization in soybean by quantitative real-time RT-PCR. BMC molecular biology 10: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jian B, Liu B, Bi Y, Hou W, Wu C, et al. (2008) Validation of internal control for gene expression study in soybean by quantitative real-time PCR. BMC molecular biology 9: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stolf-Moreira R, Lemos EG de M, Abdelnoor RV, Beneventi MA, Rolla AAP, et al. (2011) Identification of reference genes for expression analysis by real-time quantitative PCR in drought-stressed soybean. Pesquisa agropecuaria brasileira 46: 58–65. [Google Scholar]
- 32. Oya T, Nepomuceno AL, Neumaier N, Renato J, Farias B, et al. (2004) Drought tolerance characteristics of brazilian soybean cultivars: evaluation and characterization of drought tolerance of various brazilian soybean cultivars in the field. Plant Production Science 7: 129–137. [Google Scholar]
- 33. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome biology 3: RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andersen CL, Jensen JL, Ørntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer research 64: 5245–5250. [DOI] [PubMed] [Google Scholar]
- 35. Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic acids research 30: e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krishnaswamy S, Verma S, Rahman MH, Kav NNV (2011) Functional characterization of four APETALA2-family genes (RAP2.6, RAP2.6L, DREB19 and DREB26) in Arabidopsis. Plant molecular biology 75: 107–127. [DOI] [PubMed] [Google Scholar]
- 37. Altschul SF, Gish W, Pennsylvania T, Park U (1990) Basic Local Alignment Search Tool Department of Computer Science. Journal of molecular biology 215: 403–410. [DOI] [PubMed] [Google Scholar]
- 38. Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, et al. (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochemical and biophysical research communications 290: 998–1009. [DOI] [PubMed] [Google Scholar]
- 39. Oh S-J, Kim YS, Kwon C-W, Park HK, Jeong JS, et al. (2009) Overexpression of the Transcription Factor AP37 in Rice Improves Grain Yield under Drought Conditions. Plant Physiology 150: 1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen M, Xu Z, Xia L, Li L, Cheng X, et al. (2009) Cold-induced modulation and functional analyses of the DRE-binding transcription factor gene, GmDREB3, in soybean (Glycine max L.). Journal of experimental botany 60: 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuriakose B, Arun V, Gnanamanickam SS, Thomas G (2009) Tissue-specific expression in transgenic rice and Arabidopsis thaliana plants of GUS gene driven by the 5′ regulatory sequences of an anther specific rice gene YY2. Plant Science 177: 390–397. [Google Scholar]
- 42. Wang X, Dong J, Liu Y, Gao H (2010) A novel dehydration-responsive element-binding protein from Caragana korshinskii is involved in the response to multiple abiotic stresses and enhances stress tolerance in transgenic tobacco. Plant Molecular Biology Reporter 28: 664–675. [Google Scholar]
- 43. Dong C-J, Liu J-Y (2010) The Arabidopsis EAR-motif-containing protein RAP2.1 functions as an active transcriptional repressor to keep stress responses under tight control. BMC plant biology 10: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hand ML, Cogan NOI, Sawbridge TI, Spangenberg GC, Forster JW (2010) Comparison of homoeolocus organisation in paired BAC clones from white clover (Trifolium repens L.) and microcolinearity with model legume species. BMC plant biology 10: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baerenfaller K, Massonnet C, Walsh S, Baginsky S, Bühlmann P, et al. (2012) Systems-based analysis of Arabidopsis leaf growth reveals adaptation to water deficit. Molecular systems biology 8: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jiang Y, Han YZ, Zhang XM (2011) Expression profiles of a CONSTANS homologue GmCOL11 in Glycine max . Russian Journal of Plant Physiology 58: 928–935. [Google Scholar]
- 47. Hudson KA (2010) The Circadian Clock-controlled Transcriptome of Developing Soybean Seeds. The Plant Genome Journal 3: 3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers for the target genes. Nucleotide sequences and the denaturation temperatures (Tm) of the Forward (F) and Reverse (R) primers are shown. The amplification efficiency was calculated using a standard curve.
(XLSX)
Statistical analysis. The Rest 2009 software package was used to calculate iterations of gene expression among all experimental treatment periods and their statistical significances. P(H1) is the probability of observing the difference between the control plants and those exposed to water deficit by chance. The Result columns indicate the direction of the change in expression when p<0.05 (UP = up-regulated; DOWN = down-regulated; ND = not differentially expressed).
(XLSX)