Abstract
Memories of learned associations between the rewarding properties of drugs of abuse and environmental cues contribute to craving and relapse in humans. Disruption of reconsolidation dampens or even erases previous memories. Dopamine (DA) mediates acquisition of reward memory and drugs of abuse can pathologically change related neuronal circuits in the mesolimbic DA system. Previous studies showed that DA D3 receptors are involved in cocaine-conditioned place preference (CPP) and reinstatement of cocaine-seeking behavior. However, the role of D3 receptors in reconsolidation of cocaine-induced reward memory remains unclear. In the present study, we combined genetic and pharmacological approaches to investigate the role of D3 receptors in reconsolidation of cocaine-induced CPP. We found that the mutation of the D3 receptor gene weakened reconsolidation of cocaine-induced CPP in mice triggered by a 3-minute (min) retrieval. Furthermore, treatment of a selective D3 receptor antagonist PG01037 immediately following the 3-min retrieval disrupted reconsolidation of cocaine-induced CPP in wild-type mice and such disruption remained at least one week after the 3-min retrieval. These results suggest that D3 receptors play a key role in reconsolidation of cocaine-induced CPP in mice, and that pharmacological blockade of these receptors may be therapeutic for the treatment of cocaine craving and relapse in clinical settings.
Keywords: dopamine D3 receptor, reconsolidation, reward learning, cocaine, mutant mice, antagonist PG01037
INTRODUCTION
Drug addiction is a brain disorder characterized by compulsively taking and persistently seeking drugs despite negative consequences, and by a high likelihood of relapse by exposure to drugs or drug-associated cues, even long after abstention (Dackis and O’Brien, 2005; Kalivas and Volkow, 2005; Everitt and Robbins, 2005). In clinical settings, the major challenge in treating drug addiction is prevention of drug-seeking and relapsing behavior in addicts (Kalivas and Volkow, 2005). Repeated exposure to addictive drugs leads to long-term changes in neuronal circuits, cell signaling cascades and gene expression in brain reward circuits and pathologically changes neuronal processes including those for learning and memory (Nestler, 2005; Hyman et al. 2006; Luscher and Malenka, 2011).
Memories of learned associations between the rewarding properties of drugs and environmental cues contribute to craving and relapse in humans (Hyman et al. 2006; Milton and Everitt, 2012). Reconsolidation is a process in which memory undergoes a transiently labile stage after its retrieval and needs to be consolidated again in order to be maintained (Nader et al., 2000; Miller and Sweatt, 2006; Tronson and Tayler, 2007; Alberini, 2011; Sorg, 2012). Disruption of reconsolidation has been shown to dampen or even erase previous memories (Lee et al., 2005; 2006a; Miller and Marshall, 2005; Valjent et al., 2006; Tayler et al., 2009). Pharmacological or molecular manipulations of reconsolidation of acquired drug memory disrupt drug-seeking and relapsing behavior as measured by cocaine-induced conditioned place preference (CPP), morphine-induced CPP, intravenous cocaine self-administration and reinstatement (Lee et al., 2005; 2006a; Miller and Marshall, 2005; Valjent et al., 2006; Sanchez et al., 2010; Xue et al., 2012), suggesting that understanding the molecular basis of reconsolidation of reward memory may help to develop new medications for the treatment of drug addiction (Milton and Everitt, 2012; Spanagel and Vengeliene, 2013).
Emerging studies suggest that drug-induced changes in mesolimbic dopaminergic circuits mediate acquisition of reward memory (Nestler, 2005; Hyman et al. 2006; Wise, 2008; Volkow et al., 2009; Milton and Everitt, 2012). Abused drugs increase synaptic levels of dopamine (DA) that is required for reward and reinforcement (Di Chiara and Imperato, 1988; Ito et al., 2000; Stuber et al., 2005; Schultz, 2010). DA binds to DA receptors to trigger many molecular, physiological and behavioral changes. Five DA receptors have been identified and classified into two subfamilies (Neve et al., 2004). The D1-like family includes D1 and D5 receptors that interact with Gs proteins. The D2-like family includes D2, D3 and D4 receptors that interact with Gi or G0 proteins (Neve et al., 2004). DA D3 receptors are preferentially expressed in mesocorticolimbic DA projection areas (Choi et al., 2010) that have been found to be critically involved in reward-related learning induced by drugs of abuse (Di Chiara and Imperato, 1988; Nestler, 2005; Stuber et al., 2005; Hyman et al. 2006; Schultz, 2010). This expression pattern has sparked numerous studies on the role of this receptor in drug-induced behaviors and motivated intense efforts in drug discovery (Parsons et al., 1996; Pilla et al., 1999; Heidbreder et al., 2005; Micheli and Heidbreder, 2008; Heidbreder and Newman, 2010; Spanagel and Vengelience, 2013).
Previous studies have shown that DA D3 receptors contribute to locomotor-stimulant effects of cocaine, acquisition and extinction of cocaine-induced reward memory, and reinstatement of cocaine-seeking behavior (Parsons et al., 1996; Xu et al, 1997; Vorel et al., 2002; Di Ciano et al., 2003; Neisewander et al., 2004; Xi et al., 2004, 2005; 2006; Karasinska et al., 2005; Cervo et al., 2007; Martelle et al., 2007; Di Ciano, 2008; Peng et al., 2009; Achat-Mendes, et al., 2010; Thiel et al., 2010; Chen and Xu, 2010; Kong et al, 2011; Song et al., 2012a and b). However, the role of D3 receptors in reconsolidation of cocaine-induced reward memory has not been explored. In this study, we used the CPP paradigm to investigate effects of either a genetic mutation or pharmacological blockade of D3 receptors on reconsolidation of cocaine-induced reward memory. We found that D3 receptors play a key role in retrieval-induced reconsolidation of cocaine-induced CPP in mice.
EXPERIMENTAL PROCEDURES
Mice
The generation of DA D3 receptor mutant mice, which results in a complete loss of D3 receptors, has been described in a previous report (Xu et al., 1997). Homozygous D3 receptor mutant mice and wild-type (WT) littermates were obtained by crossing D3 receptor heterozygous mice. The genotype of D3 receptor mutant and WT mice was determined by the Southern blotting method (Xu et al., 1997). D3 receptor mutant and WT mice were group housed under controlled temperature and humidity conditions with a 12-h light/dark cycle. Water and food were available ad libitum. Roughly equal numbers of male and female mice, 10 to 15 weeks old, were used and weighed around 30 g at the beginning of the experiments. All procedures followed National Institutes of Health Guide for the Care and Use of Laboratory Animal and were approved by the University of Chicago Institutional Animal Care and Use Committee.
Drugs
Cocaine hydrochloride was purchased from Sigma Chemical Co. (St. Louis, MO) and dissolved in sterile 0.9% saline. PG01037 (N-{4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-trans-but-2-enyl}-4-pyridine-2-yl-benzamide) was synthesized by J. Cao in the Medicinal Chemistry Section (National Institute on Drug Abuse-Intramural Research Program, Baltimore, MD) using previously published methods (Grundt et al., 2005; 2007). PG01037 was first dissolved in DMSO and then diluted with sterile saline to 2% DMSO in saline. All injections were administered intraperitoneally (i.p.) in a volume of 10 ml/kg body weight. All behavioral testing was performed during the light phase of the light/dark cycle (6 am–6 pm).
CPP
Eight three-compartment chambers for CPP (MedAssociates, E. Fairfield, VT) were used in the present study. Each CPP chamber consisted of two large compartments (16.8×12.7×12.7 cm) and one small compartment (7.2×12.7×12.7 cm) which separated the two large compartments. The three compartments had different visual and tactile cues. One large compartment was black with a stainless steel grid rod floor. The other large compartment was white with a stainless steel mesh floor. The small compartment was gray with a smooth polyvinyl chloride floor. Each chamber had a clear Plexiglas top with a light on it.
We used a biased CPP procedure similar to that described before (Zhang et al., 2006; Chen and Xu, 2010; Kong et al., 2011). On days 1–2 (preconditioning phase), mice were placed in the small compartment and were allowed to freely explore the three compartments for 20 min daily. The time spent in each compartment was recorded. Mice spending over 500 seconds in the small compartment or over 800 seconds in either large compartment were excluded. Days 3–10 were cocaine conditioning phase with one session per day. During this phase, mice alternatively received an i.p. injection of cocaine (20 mg/kg) or saline (10 ml/kg). Mice were confined in the white compartment for 30 min when receiving an administration of cocaine, and were confined in the black compartment for 30 min when receiving an injection of saline. On day 11 (expression phase), all mice were allowed to freely explore the three compartments for 20 min without injections, and the time spent in each compartment was recorded.
Effects of the DA D3 receptor gene mutation on reconsolidation of cocaine-induced CPP
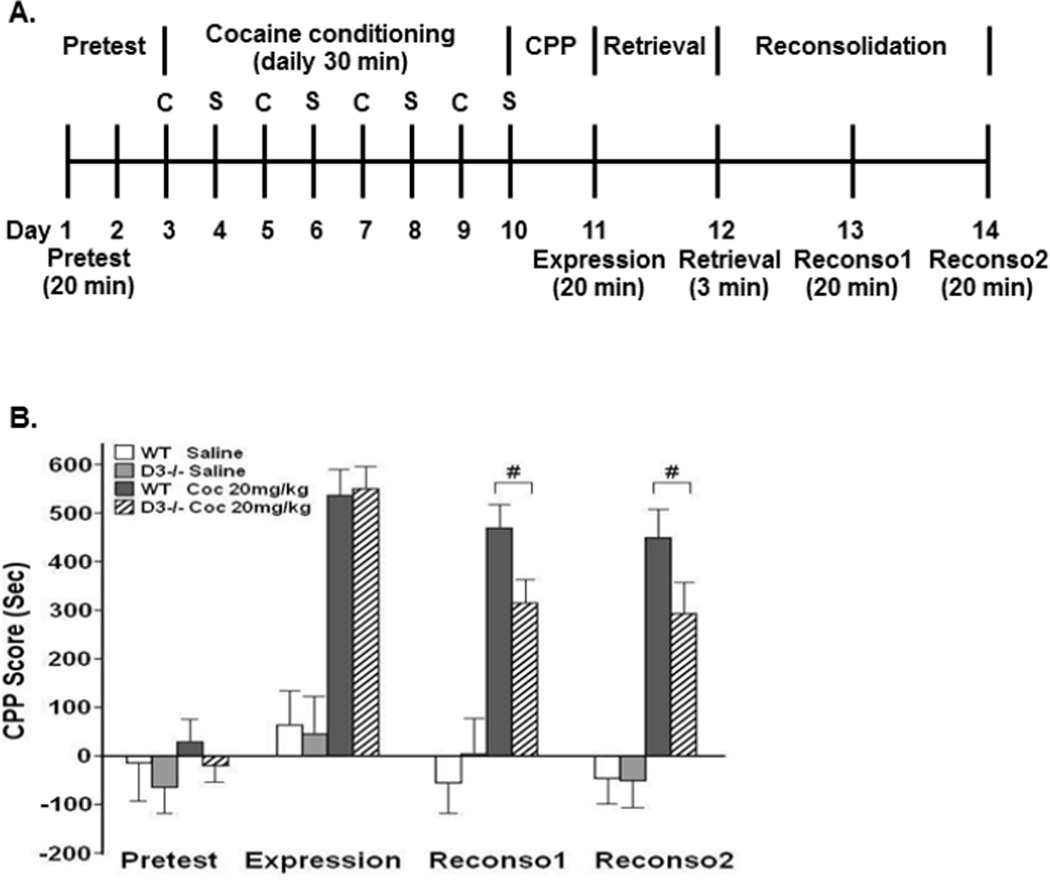
For the cocaine-conditioning groups, the timeline for the experiment is shown in Fig 1A. Once D3 receptor mutant mice and WT littermates showed cocaine-induced CPP on day 11, all mice were placed in the cocaine-conditioned compartment for 3 min (3-min retrieval) on day 12. Afterwards, all mice were returned to their home cages. On days 13 and 14, the mice were allowed to freely explore the three compartments for 20 min without injections, and the time spent in each compartment was recorded. For saline-conditioning groups, only saline (10 ml/kg, i.p.) was used for the place conditioning and the experimental timeline was the same as the timeline shown in Fig. 1A.
Fig. 1.
A genetic mutation of the D3 receptor gene weakened reconsolidation of cocaine-induced CPP in mice. A indicates the experimental timeline. B indicates effects of the mutation of D3 receptors on reconsolidation of cocaine-induced CPP in mice. Two groups of either D3 receptor mutant (D3−/−) or wild-type (WT) mice received either cocaine (Coc at 20 mg/kg) (n=27 for D3−/− and 26 for WT) or saline (n=6 for D3−/− and 5 for WT) for place conditioning. #p<0.05 compared between the two genotypes at a dose of 20 mg/kg of cocaine on day 13 or 14. A subset of D3−/− Coc 20 mg/kg and WT Coc 20 mg/kg groups (N=10 each) were tested once again for the reconsolidation on day 14 (reconsolidation test 2), Reconso1: reconsolidation test 1; reconso2: reconsolidation test 2.
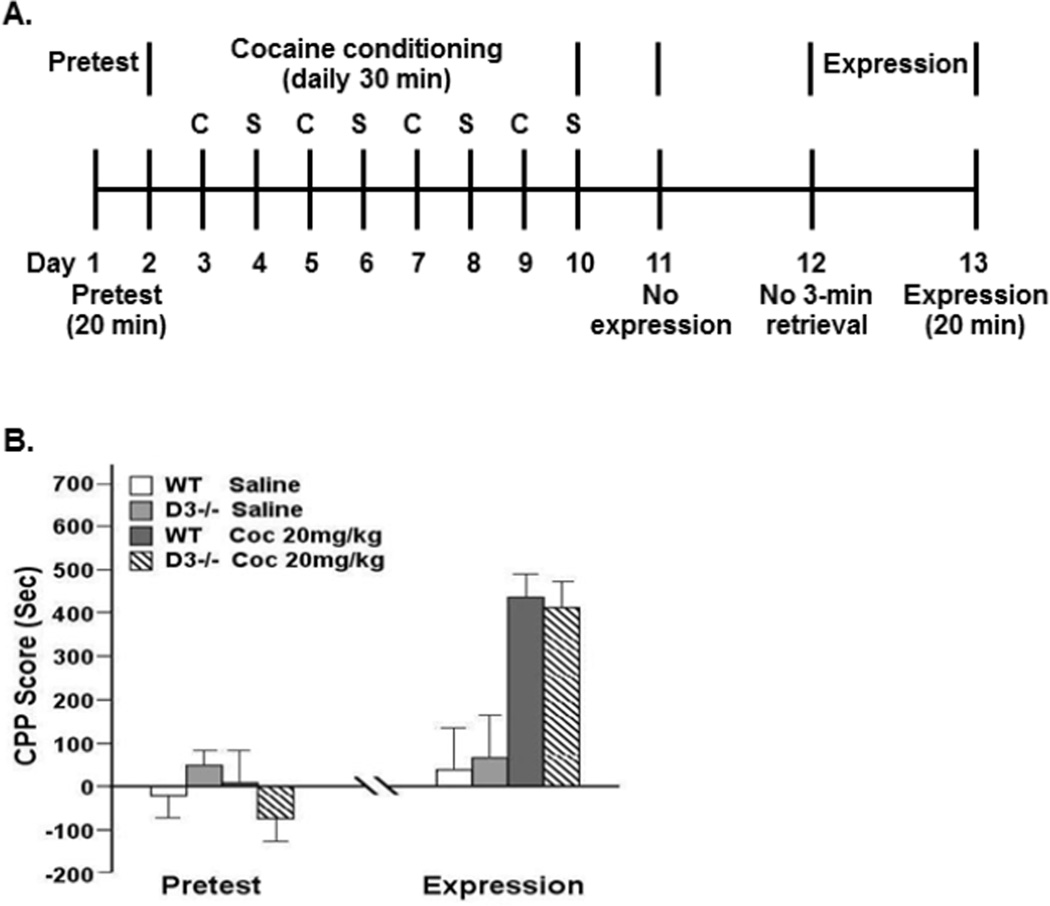
As an essential control for studying the effects of the D3 receptor mutation on reconsolidation, we included no retrieval control groups. The experimental timeline for the control groups without the 3-min retrieval is shown in Fig 2A. D3 receptor mutant mice and WT littermates were subjected to preconditioning (days 1–2) and cocaine (20 mg/kg, i.p.) conditioning (days 3–10 alternatively), but there was neither post-testing on day 11 nor a 3-min retrieval on day 12. On day 13, mice were allowed to freely explore the three compartments for 20 min without injections, and the time spent in each compartment was recorded. An additional group of D3 receptor mutant mice or WT littermates was subjected to saline-conditioning following the same experimental timeline shown in Fig. 2A as a control.
Fig. 2.
The genetic mutation of the D3 receptor gene had no effects on reconsolidation of cocaine-induced CPP without the 3-min retrieval. A indicates the experimental timeline. B indicates that there was no significant difference in expression of cocaine-induced CPP betweenD3 receptor mutant mice (D3−/−) and WT littermates on day 13 without the 3-min retrieval on day 12. Two groups of D3−/− or WT mice received either cocaine (Coc at 20 mg/kg) or saline for place conditioning. N=6 for each group.
Effects of the D3 receptor antagonist PG01037 on reconsolidation of cocaine-induced CPP
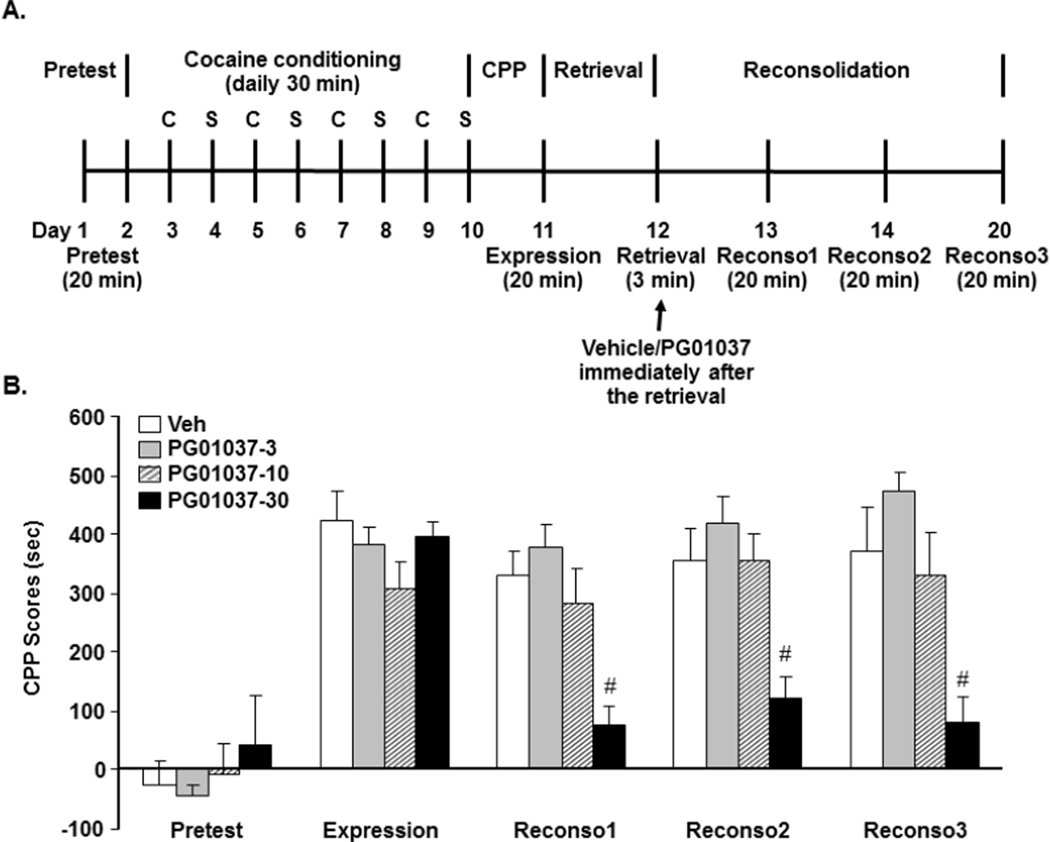
The timeline and treatment for the experiment are shown in Fig 3A. Once WT mice showed cocaine-induced CPP on day 11, mice were placed in cocaine-conditioned compartment for 3 min (3-min retrieval) immediately followed by an i.p. injection of PG01037 (0–30 mg/kg) on day 12. The selection of PG01037 dose range was based on its reported behavioral effects in rats (Orio et al., 2010; Higley et al., 2011). All mice returned to their home cages afterwards. On days 13, 14 and 20, mice were allowed to freely explore the three compartments for 20 min without injections, and the time spent in each compartment was recorded.
Fig. 3.
The D3 receptor antagonist PG01037 attenuated reconsolidation of cocaine-induced CPP in wild-type mice. A indicates the experimental timeline and PG01037 treatment for studying effects of pharmacological blockade of D3 receptors on reconsolidation of cocaine-induced CPP in WT mice. B indicates effects of the D3 receptor antagonist PG01037 on 3-min retrieval-triggered reconsolidation of cocaine-induced CPP in WT mice. Four different groups of WT mice were trained to acquire CPP. On day 12, three groups received PG01037 injections at different doses and one group received vehicle injections. PG01037 or vehicle were administered i.p. immediately after the 3-min retrieval [N=12 for vehicle (Veh), N=8 for 3 (PG01037-3), N=10 for 10 (PG01037-10), and N=11 for 30 mg/kg (PG01037-30)]. Reconsolidation testing was performed on either day 13 (24 h after), day 14 (48 h after) or day 20 (one week after either vehicle or PG01037 treatment). #p<0.05 compared with vehicle group during the same reconsolidation testing. C: cocaine; S: saline; Reconso1: reconsolidation test 1; reconso2: reconsolidation test 2; reconso3: reconsolidation test 3.
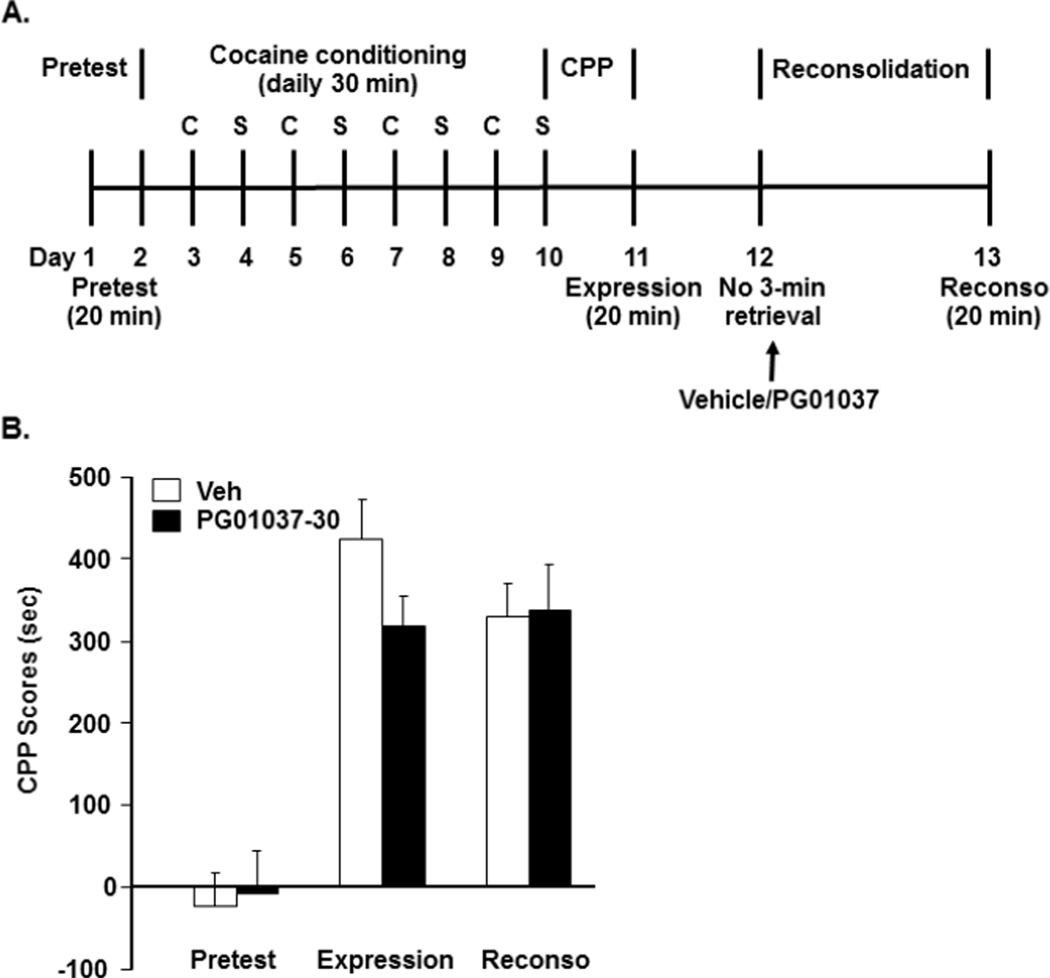
We also included control groups without the 3-min retrieval. The timeline and treatment for the experiment are shown in Fig 4A. Once WT mice showed cocaine-induced CPP on day 11, mice were given an i.p. injection of either vehicle or PG01037 (30 mg/kg) in the behavioral testing room, but were not placed in cocaine-conditioned compartment after the injection on day 12. On days 13, 14 and 20, mice were allowed to freely explore the three compartments for 20 min without injections, and the time spent in each compartment was recorded.
Fig. 4.
The D3 receptor antagonist PG01037 did not affect reconsolidation of cocaine-induced CPP in wild-type mice without the 3-min retrieval. A indicates the experimental timeline. B indicates effects of PG01037 treatment at 30 mg/kg on reconsolidation of cocaine-induced CPP in WT mice without the 3-min retrieval. Two different groups of WT mice were trained to acquire CPP. On day 12, one group received PG01037 injections and the other group received vehicle injections. PG01037 or vehicle were administered i.p. and mice were not subjected to the 3-min retrieval [N=12 for vehicle (Veh) and N=10 for 30 mg/kg (PG01037-30)]. Reconsolidation testing (Reconso) was performed on day 13, which was 24 h after either vehicle or PG01037 treatment. C: cocaine; S: saline.
Data analysis
The behavioral data were analyzed as time spent on the saline-paired side subtracted from time spent on the drug-paired side and were presented as mean ± SEM (Zhang et al, 2006; Chen and Xu, 2010; Kong et al., 2011). For experiments using D3 receptor mutant and WT mice, a two-way repeated measure ANOVA was used, with CPP testing (repeated test: pretest, expression, reconsolidation 1 and reconsolidation 2) as a within-subjects factor and group (WT with saline, D3 receptor mutant with saline, WT with cocaine, and D3 receptor mutant with cocaine) as a between-subjects factor, followed by one-way ANOVA test with post-hoc LSD. For experiments using WT mice and the pharmacological method, a two-way repeated measure ANOVA was used, with CPP testing (repeated test: pretest, expression, reconsolidation 1, reconsolidation 2 and reconsolidation 3) as a within-subjects factor and treatment (vehicle and three different doses of PG01037) as a between-subjects factor, followed by one-way ANOVA test with post-hoc LSD or Student t tests. The statistically significant level was set at p<0.05.
RESULTS
Reconsolidation of cocaine-induced CPP is attenuated in D3 receptor mutant mice as compared with that in wild-type littermates
To determine the role of D3 receptors in reconsolidation of reward-related learning and memory, D3 receptor mutant mice and WT littermates were used to establish cocaine-induced CPP following the timeline shown in Fig 1A. Two-way ANOVA analysis with CPP testing and group as fixed factors revealed that the main effects of CPP testing [F (2, 222) = 20.32] and groups [F (3, 222) = 45.84] were significant (Fig 1B). The LSD post-hoc test showed that there was a significant difference in reconsolidation of cocaine-induced CPP between D3 receptor mutant mice (D3−/−) and WT littermates [test 1 p = 0.007; test 2: p = 0.033]. During the pretesting, there was no significant difference among the four groups of mice [one-way ANOVA: F (3, 63) = 0.61, p = 0.61]. During expression testing, both D3 receptor mutant and WT mice developed cocaine-induced CPP on day 11 at a dose of 20 mg/kg [one-way ANOVA: F (3, 63) = 31.42, p = 0.74]. When only saline was used for the place conditioning, both D3 receptor mutant and WT animals failed to acquire CPP on day 11 (Fig 1B). During reconsolidation test 1, which was 24 h after the 3-min exposure to cocaine-paired compartment on day 12, D3 receptor mutant mice showed significantly attenuated reconsolidation of cocaine-induced CPP on day 13 as compared with their WT littermates [F (3, 63) = 17.49, p < 0.05]. A subset of the D3 receptor mutant and WT mice were tested once again on day 14 (reconsolidation test 2: 48 h after the 3-min exposure to cocaine-paired compartment on day 12). As shown in Fig 1B, the attenuated reconsolidation of cocaine-induced CPP in D3 receptor mutant mice persisted as compared with that in their WT littermates [F (3, 30) = 17.23, p < 0.05] on day 14. For saline control groups, there was no significant difference in reconsolidation testing on days 13 or 14 between D3 receptor mutants and WT littermates (Fig 1B).
To further support the role of D3 receptors in reconsolidation of cocaine memory, it is necessary to show that, in the absence of retrieval, the memory remains unchanged by our experimental manipulations. To confirm that the reduced reconsolidation of cocaine-induced CPP exhibited by the D3 receptor mutant mice was dependent on the 3-min retrieval on day 12, another two control groups of each of the D3 receptor mutant and WT mice were subjected to either saline or cocaine-conditioning but were not subjected to the 3-min retrieval on day 12 following the timeline shown in Fig 2A. Without the 3-min retrieval on day 12, as shown in Fig 2B, there was no significant difference in CPP testing between D3 receptor mutant mice and WT littermates on day 13 [F (3, 23) = 11.20, p = 0.79]. As expected, both D3 receptor mutants and WT littermates showed clear cocaine-induced CPP but did not show saline-induced CPP. These results suggest that the genetic mutation of the D3 receptor gene dampens reconsolidation of cocaine-induced CPP triggered by a 3-min retrieval in mice.
The selective D3 receptor antagonist PG01037 disrupted reconsolidation of cocaine-induced CPP in wild-type mice
To further confirm our findings on the role of D3 receptors in reconsolidation of cocaine memory, a similar retrieval-reconsolidation procedure of cocaine-induced CPP was used in WT mice to test pharmacological effects of a selective D3 receptor antagonist PG01037 (Fig 3A). Two-way ANOVA analysis with PG01037 treatment and CPP testing as fixed factors revealed that the main effects of PG01037 [F (3, 185) = 4.32, p = 0.0057] and CPP testing [F (4, 185) = 12.69, p <0.001] were significant (Fig 3B). In the pretesting phase, one-way ANOVA following with LSD post-hoc tests showed that there was no significant difference among four groups of mice [F (3, 37) = 0.13, p = 0.94]. In the expression testing phase, all groups of animals developed cocaine-induced CPP at a dose of 20 mg/kg, and there was no significant difference among the four groups [F (3, 37) = 0.61, p = 0.62]. In the reconsolidation testing phase on day 13, however, 24 h after the 3-min retrieval on day 12, D3 receptor antagonist PG01037 treatment at 30 mg/kg disrupted cocaine-induced CPP [F (3, 37) = 3.28, p = 0.0337], although vehicle or lower doses of PG01037 (3 or 10 mg/kg) did not have an effect. The disruption of reconsolidation of cocaine-induced CPP by PG01037 at 30 mg/kg remained 48 h [F (3, 37) = 2.70, p = 0.0494] and one week after the 3-min retrieval [F (3, 37) = 3.02, p = 0.0417].
We also investigated whether, in the absence of retrieval, the cocaine memory remained unchanged by experimental manipulations. For control groups without the 3-min retrieval on day 12, the experimental timeline is shown in Fig 4A. Two-way ANOVA analysis with PG01037 treatment and CPP testing as fixed factors revealed that the main effect of CPP testing was significant [F (4, 100) = 11.37, p <0.001], but that the main effect of PG01037 were not significant (Fig 3B, F (1, 100) = 0.42, p = 0.5175). LSD post-hoc tests showed that although two groups of WT mice acquired cocaine-induced CPP on day 11 (p < 0.001), there was no significant difference between the two groups mice (p = 0.22). With no 3-min retrieval on day 12, as shown in Fig 4B, the D3 receptor antagonist PG01037 treatment (30 mg/kg) did not affect cocaine-induced CPP during reconsolidation testing on day 13 [p = 0.92]. Together, these results indicate that a selective D3 receptor antagonist PG01037 disrupts retrieval-triggered reconsolidation of cocaine-induced CPP in wild-type mice and the disruption lasts for at least one week.
DISCUSSION
Memories of drug experience and drug-associated cues can elicit drug craving and relapse in humans. Recent studies showed that pharmacological or molecular manipulations of reconsolidation of drug-induced reward memory can reduce drug craving and seeking behavior (Lee et al., 2005; 2006a; Miller and Marshall, 2005; Valjent et al., 2006; Sanchez et al., 2010; Xue et al., 2012). These findings suggest that discovering novel molecular targets of reconsolidation may aid medication development for treating drug addiction (Taylor et al., 2009; Milton and Everitt, 2012; Spanagel and Vengeliene, 2013). The brain dopaminergic system mediates acquisition of reward memory and drugs of abuse can pathologically change related neuronal circuits in the mesolimbic DA system. We previously made a D3 receptor mutant mouse model and found that the genetic mutation of D3 receptor gene in mice potentiated the acquisition of cocaine-induced CPP at lower doses, but not higher doses of cocaine (Chen and Xu, 2010; Kong et al., 2011). The mutation of the D3 receptor gene in mice also delayed the extinction, but did not affect reinstatement of cocaine-induced CPP (Chen and Xu, 2010). Others have shown that D3 receptors contribute to reinstatement of cocaine-seeking behavior (Parsons et al., 1996; Vorel et al., 2002; Di Ciano et al., 2003; Neisewander et al., 2004; Xi et al., 2004, 2005 and 2006; Cervo et al., 2007; Martelle et al., 2007; Di Ciano, 2008; Peng et al., 2009; Achat-Mendes, et al., 2010; Thiel et al., 2010; Song et al., 2012a and b). In this study, we investigated the role of D3 receptors in reconsolidation of cocaine-induced CPP in mice using both a genetically engineered mouse model and pharmacological approaches. Our findings demonstrate for the first time that D3 receptors play a critical role in reconsolidation of cocaine-induced CPP in mice.
We found that the mutation of the D3 receptor gene in mice reduced reconsolidation of cocaine-induced CPP (Fig 1B) but not in the control group without the 3-min retrieval (Fig 2B). Such reduction in reconsolidation of cocaine-induced CPP lasted for at least two days. This suggests that D3 receptors may participate in mechanisms related to reconsolidation of cocaine-induced reward memory. Reconsolidation and extinction are both distinct and related, and depending on the reactivation interval and memory strength, one or both processes may occur (Pedreira and Maldonado, 2003; Suzuki et al., 2004; Lee et al., 2006b). A short reactivation protocol is thought to favor reconsolidation, whereas extinction requires multiple training sessions (Suzuki et al., 2004). We used a 3-min retrieval protocol that favors reconsolidation in the current study. We previously used D3 receptor mutant mice and showed that these mice exhibited a delayed extinction as measured in a cocaine CPP paradigm (Chen and Xu, 2011). In the current study, D3 receptor mutant mice showed a deficit in reconsolidation and a faster loss of CPP. Together, our current results are unlikely to be explained by a faster extinction of CPP in the D3 receptor mutant mice.
Many D3 receptor-preferring antagonists have been developed and evaluated in vivo (Grundt et al., 2007; Micheli and Heidbreder, 2008; Heidbreder and Newman, 2010). Among these D3 receptor-preferring antagonists, PG01037 showed high affinity and selectivity for D3 receptors in vitro and in vivo (Grundt et al., 2005; 2007). Moreover, PG01037 rapidly penetrates the blood brain barrier and selectively localizes in D3 receptor-rich regions, such as the nucleus accumbens (NAc), Islets of Calleja and the hippocampus (Grunt et al., 2007). Indeed, studies characterizing the behavioral effects of PG01037 in blocking D3 agonist-induced yawning and in models of psychostimulant abuse have been carried out extensively in rats, mice and nonhuman primates (Collins et al., 2005, 2007; Xi et al., 2006; Martelle et al., 2007, Achat-Mendes et al., 2010; Higley et al. 2011.) Collectively, these studies showed that PG01037 selectively blocks D3-agonist induced yawning and attenuates reinstatement of drug-seeking via pharmacological antagonism of D3 receptors. To verify our findings in the D3 receptor mutant mice, we administered PG01037 immediately following the 3-min memory retrieval in WT mice. We found that PG01037 attenuated retrieval-triggered reconsolidation of cocaine-induced CPP (Fig 3B) but not in the control group without the 3-min retrieval (Fig 4B). Such attenuation in reconsolidation of cocaine-induced CPP remained at least one week after the retrieval. These findings suggest that inactivation of D3 receptors can affect the reconsolidation of cocaine-induced reward memory as measured in the CPP paradigm.
We note that the pharmacological inhibition of D3 receptors by PG01037 (30 mg/kg) was more effective in attenuating reconsolidation than the genetic mutation of D3 receptors (Fig 1B and Fig 3B). One possibility is that there may be compensatory developmental effects in D3 receptor mutant mice that made them more resistant to the disruption of CPP reconsolidation. Alternatively, we previously found that the genetic mutation of D3 receptors in mice potentiated the acquisition of cocaine-induced CPP at 17 lower, but not higher doses of cocaine (Chen and Xu, 2010; Kong et al., 2011). The apparently equal acquisition of cocaine-induced CPP at higher doses between D3 receptor mutant and WT mice might result from ceiling effects in expression of cocaine-induced CPP and there may be potentially higher expression of cocaine-induced CPP in D3 receptor mutant mice that has been masked by the ceiling effects of cocaine (20 mg/kg) (Fig. 1B). Consequently, the observed effects of D3 receptor gene mutation on reconsolidation of cocaine-induced CPP may actually be similar to those of the pharmacological blockade. Although PG01037 at a dose of 30 mg/kg has been typically used to demonstrate its antagonism on D3 receptor related behaviors (Collins et al., 2005; 2007, Achat-Mendes et al., 2010, Higley et al., 2011) across species, it is also possible that in addition to D3 receptors, higher doses of PG01037 may inhibit other molecular targets (Kumar et al., 2009). This may result in an apparently more complete inhibition of the reconsolidation process (Micheli and Heidbreder, 2008; Heidbreder and Newman, 2010).
Our current studies using both genetic and pharmacological approaches suggest that D3 receptors play a key role in reconsolidation of cocaine-induced reward learning. D3 receptors are mainly expressed in brain reward circuits including the basolateral amygdala (BLA), the prefrontal cortex (PFC), and NAc (Beaulieu and Gainetdinov, 2011). The BLA mediates learning of conditioned associations between the rewarding effects of drugs of abuse and cues. The PFC contributes to decision-making and execution of goal-directed actions. The NAc modulates motivation for drug seeking by integrating information from the BLA and PFC and relaying it to motor output structures, and it mediates reinforcement. These different brain structures coordinate to modulate reward-related reward learning induced by drugs of abuse. Furthermore, these brain regions play an important role in reconsolidation of drug memories (Théberge et al., 2010; Otis et al., 2013). D3 receptors expressed in the above brain regions may participate in the reconsolidation of cocaine-induced reward memory. Indeed, D3 receptors may be upregulated in these places in the brains of cocaine and methamphetamine abusers (Staley and Mash 1996; Boileau et al., 2012). Pharmacological blockade of D3 receptors may be therapeutic for the treatment of cocaine craving and relapse in clinical settings.
Genetic mutations in dopamine D3 receptors attenuate reconsolidation of cocaine-induced reward memory
Pharmacological blockade of D3 receptors disrupts reconsolidation of cocaine-induced reward memory
Dopamine D3 receptors regulate reconsolidation of cocaine memory
ACKNOWLEDGEMENTS
We greatly appreciate Dr. H. Jiao’s assistance for mouse genotyping, Dr. J. Katz for discussions and suggestions, and J. Cao for synthesizing PG01037. M.X. was supported by grants from NIDA (DA17323 and DA025088). A.H.N was supported by the NIDA Intramural Research Program.
ABBREVIATIONS
- C
cocaine
- CPP
conditioned place preference
- DMSO
dimethyl sulphoxide
- DA
dopamine
- D3−/−
dopamine D3 receptor mutant
- i.p.
intraperitoneally
- S
saline
- Veh
vehicle
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Achat-Mendes C, Grundt P, Cao J, Platt DM, Newman AH, Spealman RD. Dopamine D3 and D2 receptor mechanisms in the abuse-related behavioral effects of cocaine: studies with preferential antagonists in squirrel monkeys. J Pharmacol Exp Ther. 2010;334:556–565. doi: 10.1124/jpet.110.167619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Front Behav Neurosci. 2011;5:12–21. doi: 10.3389/fnbeh.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, Wilkins D, Selby P, George TP, Zack M, Furukawa Y, McCluskey T, Wilson AA, Kish SJ. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci. 2012;32:1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Cocco A, Petrella C, Heidbreder CA. Selective antagonism at dopamine D3 receptors attenuates cocaine-seeking behaviour in the rat. Int J Neuropsychopharm. 2007;10:167–181. doi: 10.1017/S1461145705006449. [DOI] [PubMed] [Google Scholar]
- Chen LP, Xu M. Dopamine D1 and D3 receptors are differentially involved in cue-elicited cocaine seeking. J Neurochem. 2010;114:530–541. doi: 10.1111/j.1471-4159.2010.06775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JK, Mandeville JB, Chen YI, Grundt P, Sarkar SK, Newman AH, Jenkins BG. Imaging brain regional and cortical laminar effects of selective D3 agonists and antagonists. Psychopharmacology (Berl) 2010;212:59–72. doi: 10.1007/s00213-010-1924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology (Berl) 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Phar Exp Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis C, O’Brien C. Neurobiology of addiction: treatment and public policy ramifications. Nat Neurosci. 2005;8:1431–1436. doi: 10.1038/nn1105-1431. [DOI] [PubMed] [Google Scholar]
- Di Ciano P. Drug seeking under a second-order schedule of reinforcement depends on dopamine D3 receptors in the basolateral amygdala. Behav Neurosci. 2008;122:129–139. doi: 10.1037/0735-7044.122.1.129. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharm. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1488. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, Jenkins BG, Luedtke RR, Newman AH. Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents. J Med Chem. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann NY Acad Sci. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Spiller K, Grundt P, Newman AH, Kiefer SW, Xi ZX, Gardner EL. PG01037, a novel dopamine D3 receptor antagonist, inhibits the effects of methamphetamine in rats. J Psychopharmacol. 2011;25:263–273. doi: 10.1177/0269881109358201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Karasinska JM, George SR, Cheng R, O'Dowd BF. Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. Eur J Neurosci. 2005;22:1741–1750. doi: 10.1111/j.1460-9568.2005.04353.x. [DOI] [PubMed] [Google Scholar]
- Kong H, Kuang W, Li S, Xu M. Activation of dopamine D3 receptors inhibits reward-related learning induced by cocaine. Neurosci. 2011;176:152–161. doi: 10.1016/j.neuroscience.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Riddle L, Griffin SA, Grundt P, Newman AH, Luedtke RR. Evaluation of the D3 dopamine receptor selective antagonist PG01037 on L-dopa-dependent abnormal involuntary movements in rats. Neuropharm. 2009;56:944–955. doi: 10.1016/j.neuropharm.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006a;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006b;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA. Effects of two novel D3-selective compounds, NGB 2904 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide] and CJB 090 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide], on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Phar Exp Ther. 2007;321:573–582. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- Micheli F, Heidbreder C. Selective dopamine D3 receptor antagonists 1997–2007: a decade of progress. Expert Opin Ther Patents. 2008;18:821–840. [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Amnesia or retrieval deficit? Implications of a molecular approach to the question of reconsolidation. Learn Mem. 2006;5:498–505. doi: 10.1101/lm.304606. [DOI] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev. 2012;36:1119–1139. doi: 10.1016/j.neubiorev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RA, Tran-Nguyen LT, Weber SM, Coffey GP, Joyce JN. Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharm. 2004;29:1479–1487. doi: 10.1038/sj.npp.1300456. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat. Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Sorg BA. Reconsolidation of drug memories. Neurosci Biobehav Rev. 2012;36:1400–1417. doi: 10.1016/j.neubiorev.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J. Recept. Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Orio L, Wee S, Newman AH, Pulvirenti L, Koob GF. The dopamine D3 receptor partial agonist CJB090 and antagonist PG01037 decrease progressive ratio responding for methamphetamine in rats with extended-access. Addict Biol. 2010;15:312–323. doi: 10.1111/j.1369-1600.2010.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Dashew KB, Mueller D. Neurobiological dissociation of retrieval and reconsolidation of cocaine-associated memory. J Neurosci. 2013;33:1271–1281. doi: 10.1523/JNEUROSCI.3463-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Caine SB, Sokoloff P, Schwartz JC, Koob GF, Weiss F. Neurochemical evidence that postsynaptic nucleus accumbens D3 receptor stimulation enhances cocaine reinforcement. J Neurochem. 1996;67:1078–1089. doi: 10.1046/j.1471-4159.1996.67031078.x. [DOI] [PubMed] [Google Scholar]
- Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- Peng XQ, Ashby CR, Jr, Spiller K, Li X, Li J, Thomasson N, Millan MJ, Mocaër E, Muńoz C, Gardner EL, Xi ZX. The preferential dopamine D3 receptor antagonist S33138 inhibits cocaine reward and cocaine-triggered relapse to drug-seeking behavior in rats. Neuropharm. 2009;56:752–760. doi: 10.1016/j.neuropharm.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR. Reconsolidation of a cocaine-associated stimulus requires amygdalar protein kinase A. J Neurosci. 2010;30:4401–4407. doi: 10.1523/JNEUROSCI.3149-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: Basic and recent data. Behavioral and Brain Functions (BBF) 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, Li X, Gaál J, Xi ZX, Gardner EL. YQA14: a novel dopamine D(3) receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D(3) receptor-knockout mice. Addict Biol. 2012a;17:259–273. doi: 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Zhang HY, Li X, Bi GH, Gardner EL, Xi ZX. Increased vulnerability to cocaine in mice lacking dopamine D3 receptors. Proc Natl Acad Sci USA. 2012b;109:17675–17680. doi: 10.1073/pnas.1205297109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA. Reconsolidation of drug memories. Neurosci Biobehav Rev. 2012;36:1400–1417. doi: 10.1016/j.neubiorev.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Vengeliene V. New pharmacological treatment strategies for relapse prevention. Curr Top Behav Neurosci. 2013;13:583–609. doi: 10.1007/7854_2012_205. [DOI] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Wightman RM, Carelli RM. Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron. 2005;46:661–669. doi: 10.1016/j.neuron.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharm. 2009;56:186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théberge FR, Milton AL, Belin D, Lee JL, Everitt BJ. The basolateral amygdala and nucleus accumbens core mediate dissociable aspects of drug memory reconsolidation. Learn Mem. 2010;17:444–453. doi: 10.1101/lm.1757410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Wenzel JM, Pentkowski NS, Hobbs RJ, Alleweireldt AT, Neisewander JL. Stimulation of dopamine D2/D3 but not D1 receptors in the central amygdala decreases cocaine-seeking behavior. Behav Brain Res. 2010;214:386–394. doi: 10.1016/j.bbr.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corbillé AG, Bertran-Gonzalez J, Hervé D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci USA. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharm. 2009;56:3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR, Jr, Hagan JJ, Heidbreder CA, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharm. 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gitajn L, Gardner EL. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine's rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharm. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Tirado G, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, Tonegawa S. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron. 1997;19:837–848. doi: 10.1016/s0896-6273(00)80965-4. [DOI] [PubMed] [Google Scholar]
- Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, Zhu WL, Ding ZB, Bao YP, Shi J, Epstein DH, Shaham Y, Lu L. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lou D, Jiao H, Zhang D, Wang X, Ying X, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]