Abstract
Purpose
The glucocorticoid receptor (GR) gene produces GRα and GRβ isoforms by alternative splicing of a C-terminal exon. GRα binds glucocorticoids, modulates transcription in a glucocorticoid-dependent manner, and plays a growth inhibitory role in prostate cells. Due to this role, glucocorticoids are often used to treat androgen-independent prostate cancer. By contrast, GRβ possesses intrinsic transcriptional activity and binds mifepristone (RU486), but not glucocorticoids, to control gene expression. The role of GRβ in prostate cell proliferation is unknown.
Materials and Methods
We determined the levels of GRβ in various prostate cancer cell lines by RT-PCR and western blotting. The effect of GRβ on the kinetics of prostate cancer cell growth was determined by cell counting and flow cytometry upon mifepristone and dexamethasone treatment. Cell proliferation was also examined following siRNA-mediated knock-down and overexpression of GRβ.
Results
GRβ mRNA and protein were upregulated in LNCaP cells overexpressing the androgen receptor co-factor ARA70β. Treatment of LNCaP-ARA70β with mifepristone or siRNA targeting GRβ inhibited proliferation, compared to parental LNCaP cells. An immortal but non-tumorigenic (RC165) prostate cell line, as well as a tumorigenic (DU145) prostate cell line with endogenous GRβ also showed partial growth reduction upon depletion of GRβ, albeit to a lesser extent than LNCaP-ARA70β cells. The growth-stimulatory effect of ARA70β on LNCaP cells is, in part, GRβ-dependent, as is the proliferation of RC165 and, to a lesser extent, DU145 cells.
Conclusions
These results suggest that patients whose primary tumors express GRβ and ARA70β may benefit from mifepristone treatment.
Keywords: glucocorticoid receptor beta, mifepristone, prostatic neoplasms
INTRODUCTION
The major obstacle in the treatment of prostate cancer is the development of androgen-independent disease resulting in metastasis-related mortality 1. One method currently used to treat androgen-independent prostate cancer is treatment with glucocorticoids, although the mechanism of glucocorticoid action in prostate cancer is not well-understood 2, 3. Mechanisms proposed for glucocorticoid action in prostate cancer include: formation of heterodimers between androgen (AR) and glucocorticoid (GR) receptors, leading to the inhibition of androgen receptor-dependent transcription 4, interference with cytokine and growth factor secretion and activity 5, 6, and inhibition of angiogenesis 7. In contrast, some reports suggest that glucocorticoids may promote androgen-independent prostate cancer in some contexts 8, 9. One of the reasons for these conflicting views may be the focus, until relatively recently, on the major form of glucocorticoid receptor, GRα, without consideration of the role of other isoforms.
GRs are nuclear receptors that bind glucocorticoids, such as cortisol or dexamethasone. Upon hormone binding, they translocate into the nucleus, where they bind to glucocorticoid response elements and up-regulate the expression of anti-inflammatory proteins, or repress the expression of pro-inflammatory proteins 10. Multiple GR isoforms result from the alternative splicing of GR pre-mRNA and alternative translation initiation 11. The most abundant of these isoforms are GRα and GRβ, which are identical from amino acid 1 to 727. At their C-termini, GRα and GRβ have an additional 50 or 15 amino acids, respectively, with the extra amino acids of GRβ, distinct from those of GRα 12. The alternative splicing of GRβ results in changes in its ligand-binding domain, causing inability to bind glucocorticoids. Previously, the physiological effect exerted by GRβ was thought to occur via its action as a dominant-negative regulator of GRα 13. However, recently it was shown that GRβ binds ligand, the glucocorticoid antagonist mifepristone (RU486), followed by translocation to the nucleus, where it can regulate gene expression 14.
We previously observed that GRβ mRNA expression is markedly elevated in LNCaP prostate cancer cells overexpressing the AR cofactor ARA70β 15. AR is a steroid receptor, which upon binding to androgens (such as testosterone or dihydrotestosterone) in the cytoplasm, forms homodimers and translocates to the nucleus. There it functions as a transcription factor which binds to regulatory sequences of target genes 16. As a transcription factor, AR interacts with a number of cofactors which modulate its effect on gene expression 17. One of these cofactors is ARA70, with two isoforms having distinct functions 18. The full-length 70 kDa ARA70α inhibits cell growth and invasion when overexpressed 19, while the alternatively-spliced 35 kDa ARA70β promotes cell growth, invasion, and transformation in androgen-dependent manner 15. Consistent with its growth-promoting properties, ARA70β expression is increased in prostate cancer 15.
In dissecting the role of GRβ and its interplay with AR in the growth of prostate cells overexpressing the AR co-activator ARA70β, our findings suggest that glucocorticoid signaling may be exploited to treat advanced prostate cancer.
MATERIALS AND METHODS
Cell culture, cell proliferation and flow cytometry analysis
LNCaP, LNCaP-AI 20, DU145 and PC3 cells were cultivated in RPMI1640 media (Gibco) supplemented with 10% fetal bovine serum (Gibco), 1 U/ml penicillin, 1 μg/ml streptomycin, and 2 μg/ml puromycin where required. RC165 and RC170 cells were cultured in the Keratinocyte SFM medium (Gibco), supplemented with 1 U/ml penicillin and 1 μg/ml streptomycin. To measure the proliferation rate, 2×104 cells were seeded into 6-well plates and counted using hemocytometer (Reichert). Cells were prepared for flow cytometry as described previously 19. The cell cycle analysis was performed on FACSCalibur flow cytometer (BD Biosciences)
RNA interference
The siRNA-mediated knock-downs were performed using annealed RNA nucleotides (Table 1) For GRβ, oligonucleotides correspond to the C-terminal sequence in exon 9β (Sigma). siRNAs were transfected into cells using HiPerFect transfection reagent (Qiagen).
Table 1.
Nucleotide sequences used.
| RNA nucleotides | |
| GRβ 38 | 5′-GGCUUUUCAUUAAAUGGGAtt-3′ 5′-UCCCAUUUAAUGAAAAGCCtc-3′ |
| AR | 5′-UGCAUUACGUUAAAGCAAAtt-3′ 5′-UGCAAUGGUAAAUUCCGUGtt-3′ |
| ARA70β | 5′-UAUUGCAAUUCUUGGCUUUtt-3′ 5′-UAUUGCGUUAUGCCUUGGUtt-3′ |
| DNA nucleotides | |
| GRα | 5′-gaactggcagcggttttatc-3′ 5′-tggtatctgattggtgatgatttc-3′ |
| GRβ | 5′-gaactggcagcggttttatc-3′ 5′-aaagggcacagcttcttttc-3′ |
| GRγ | 5′-cttcaaaagagcagtggaaggta-3′ 5′-ctcctgtagtggcctgctg-3′ |
| PRα+β 39 | 5′-acagaattcatgagccggtccgggtgcaag-3′ 5′-acaagatctccacccagagcccgaggttt-3′ |
| PRβ 39 | 5′-acagaattcatgactgagctgaaggcaaagggt-3′ 5′-acaagatctcaaacaggcaccaagagctgctga-3′ |
| ARA70β | 5′-accttggagaacagtcagca-3′ 5′-tcacatctgtagaggagttcgat-3′ |
| AR | 5′-cctcctgtagtttcagattac-3′ 5′-tttccaccccagaagacctgc-3′ |
| 18S rRNA | 5′-aggaattgacggaagggcac-3′ 5′-gtgcagccccggacatctaag-3′ |
Western blot
Whole cell extracts were subjected to electrophoresis on SDS-PAGE and then transferred to a nitrocellulose membrane. Blots were incubated with antibodies raised against GRα (Abcam, ab3580) and GRβ (Abcam, ab3581), ARA70β 21, AR and β-actin (Sigma) and with the appropriate secondary antibody (Amersham Biosciences). The protein bands were detected using the SuperSignal West Dura kit (Thermo Scientific).
RT-PCR analysis
For RT-PCR, total RNA was extracted using the RNAqueous-4PCR kit (Ambion). cDNA was synthesized using the RETROscript kit (Ambion) and used as template in PCR reactions to detect GRβ, GRα, GRγ, PRα, PRβ, ARA70β, AR, and 18S rRNA with the help of corresponding DNA primers (Table 1). The oligonucleotides for specific detection of ARA70β were selected so that the left primer had 3′ sequence cagCA, which is unique to ARA70β splice junction (ARA70α has cagAC); the right primer starts from stop codon and anneals to both ARA70β and ARA70α.
Statistical analysis
Flow cytometry and cell growth assays were performed in triplicates, and standard deviation was calculated for each data point. The RT-PCR and western blotting experiments were repeated at least three times each, with similar results; a representative blot for each experiment is presented. To test whether differences between samples were statistically significant, the corresponding p-values were determined based on samples’ Student’s t-distribution, with t-values determined by one-sample t-test.
RESULTS
Expression of GR and PR isoforms in prostate cancer cell lines
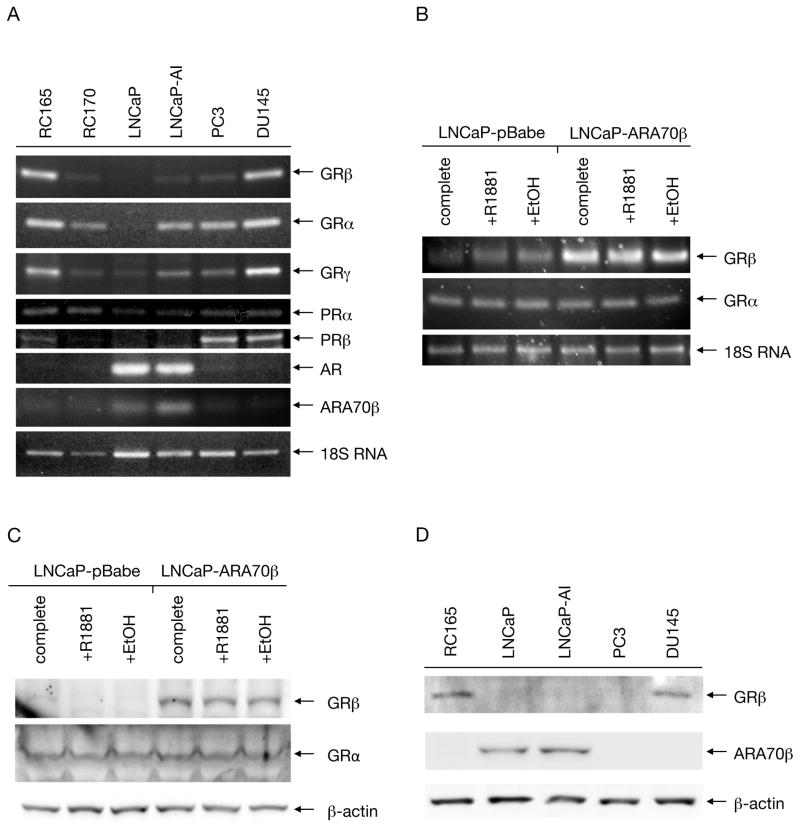
We determined the expression of GR isoforms in a number of benign and malignant prostate cell lines using reverse transcriptase PCR (Fig. 1.A). We detected GRα transcripts in all the cell lines tested, with comparably highest levels observed in benign RC165 and malignant, androgen-independent DU145, and barely detectable levels in LNCaP cells. RC165 and DU145 cell lines also expressed the highest levels of GRβ mRNA, however, only very low levels of this transcript were detected in benign RC170 and cancerous LNCaP-AI and PC3. No GRβ transcripts were detected in LNCaP cells. The expression profile of GRγ was similar to that of GRα, except that a low level of the transcript was detected in LNCaP cells as well. GRγ represents a splice variant with an extra amino acid in the DNA binding domain 22.
Figure 1. Expression of GRs in prostate cell lines.
A: The levels of transcripts of GR and PR isoforms, as well as AR and ARA70β were analyzed using RT-PCR in benign prostate cell lines RC165 and RC170, as well as malignant prostate cell lines LNCaP, LNCaP-AI, PC3, and DU145. B: Cells overexpressing ARA70β as well as control cells were grown in complete media and in hormone-free media in the presence or absence of R1881. The levels of GRα and GRβ transcripts were analyzed by RT-PCR, with 18S RNA as a loading control. C: The protein levels of samples prepared as in B were detected using GRα and GRβ-specific monoclonal antibodies. D: Protein levels of GRβ and ARA70β were detected using isoform-specific antibodies in the benign cell line RC165 and cancer cell lines LNCaP, LNCaP-AI, PC3, and DU145.
Since GR shares overlapping ligand-binding specificities with progesterone receptor (PR), we tested the distribution of transcripts of two major PR isoforms as well. While PRα transcripts were present in all the tested cell lines, PRβ messages were detected only in PC3 and DU145, and in low levels in RC165. Additionally, we also examined the expression profile of AR and ARA70β. High amounts of AR were present only in LNCaP and LNCaP-AI, but contrary to previous reports 23, no or very low levels of AR were detected in RC165. ARA70β was present at highest levels in LNCaP and LNCaP-AI cells as well, while in the rest of the surveyed cell lines its levels were low or undetectable (Figure 1.A).
GRβ mRNA and protein expression in LNCaP cells as a function of ARA70β
Our previous study revealed an increase in GRβ expression in LNCaP cells overexpressing ARA70β 15. To confirm and extend this result we analyzed GRβ, as well as GRα, mRNA expression by RT-PCR in LNCaP cells stably overexpressing ARA70β (LNCaP-ARA70β) or control parental LNCaP cells transfected with empty vector (LNCaP-pBabe) (Figure 1.B). The mRNA levels of GRα were uniform in both LNCaP-vector and LNCaP-ARA70β cells grown in complete medium, as well as hormone-free medium and hormone-free medium supplemented with synthetic androgen R1881. Whereas GRα mRNA expression remained unchanged, GRβ showed higher expression in LNCaP-ARA70β relative to control cells. Moreover, androgen treatment had virtually no impact on GRα or GRβ mRNA expression in LNCaP-ARA70β cells.
Next, we performed western blotting of extracts from control LNCaP-pBabe and LNCaP-ARA70β cells to determine whether the differences in GRα and GRβ transcript levels also translated to the corresponding protein levels. Blotting with antibody specific to the GRβ isoform confirmed high levels in LNCaP-ARA70β, and virtually no expression in LNCaP-vector cells. The presence or absence of androgen had little effect on the GRβ levels. Western blotting with a GRα-specific antibody showed low, uniform levels of this isoform in both LNCaP-vector and LNCaP-ARA70β in both the presence and absence of androgen (Figure 1.C).
For GRβ and ARA70β, we also tested whether the differences in transcript levels translated to the levels of the corresponding protein products. Western blotting with a GRβ-specific antibody demonstrated that GRβ protein was expressed in RC165 and DU145 cells, but was not detected in LNCaP, LNCaP-AI, or PC3 cells, mimicking the distribution of the transcript levels in the cell lines. Blotting of the cell extracts with an ARA70β-specific antibody confirmed that, similar to the mRNA levels, ARA70β protein was present only in LNCaP and LNCaP-AI cell lines, but not in RC165, PC3, or DU145 (Figure 1.D). This suggests that the relationship between ARA70β and GRβ expression is cell type specific.
Inhibition of GRβ, either by siRNA or mifepristone, reduced prostate cancer cell growth
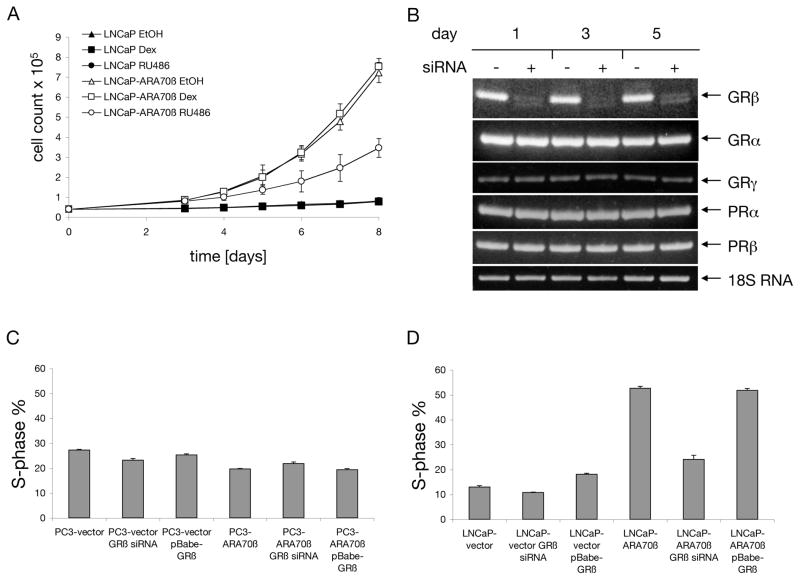
To test whether high levels of GRβ in LNCaP-ARA70β cells were responsible for the increased growth rate of this cell line, we grew the cells in androgen media supplemented either with mifepristone (a GR antagonist), or dexamethasone (a GR agonist), and followed the growth rate by cell counting. In control LNCaP cells, both dexamethasone and mifepristone had no effect on proliferation (p=0.81 and p=0.84, respectively). Interestingly, in LNCaP-ARA70β cells, mifepristone treatment led to a 50% reduction (p=6.9×10−4), while dexamethasone had no significant effect (p=0.46) on cell growth after eight days, (Figure 2.A).
Figure 2. Effect of GRβ on growth of prostate cell lines.
A: LNCaP cells, stably overexpressing ARA70β or transfected with empty vector, were seeded in 6-well plates, treated with 1 μM dexamethasone or 1 μM mifepristone (with repeated additions every other day) and number of cells was counted. B: Cells were collected on days 1, 3, and 5 after transfection, and the transcript levels of GR and PR isoforms were determined using RT-PCR with 18S RNA as internal control. C, D: PC3 and LNCaP cells either stably overexpressing ARA70β or transfected with control vector were transiently transfected with GRβ-expressing plasmid. Cells were processed as above and fraction of cells in S-phase of cell cycle was plotted.
We next used an siRNA designed to specifically silence the GRβ isoform and tested whether selectively reducing GRβ affected LNCaP-ARA70β cell proliferation. Prior to embarking on the functional studies, we determined that the GRβ siRNA did not affect the transcript levels of GRα, GRγ, PRα, and PRβ. For this test we employed the RC165 cell line, since these cells have high or detectable levels of both GR and PR isoforms. While the amount of GRβ mRNA was reduced by more than 90% in the presence of the siRNA, the levels of the remaining transcripts were not affected (Figure 2.B).
We further examined the functional consequences of the loss or overexpression of GRβ as a function of AR signaling, using LNCaP cells, which express AR, and PC3 cells which do not contain detectable levels of AR. We transiently overexpressed GRβ in LNCaP and PC3 cells and confirmed the increased levels of GRβ by western blotting (data not shown). Using flow cytometry, we then analyzed the effect of GRβ overexpression and siRNA-mediated knock-down on cell proliferation, manifested here as the percentage of cells in the S phase of the cell cycle. In LNCaP cells transfected with an empty vector, the treatment with GRβ siRNA led only to a negligible decrease in the number of S phase cells (p=0.02), and the transient overexpression of GRβ in these cells led to ~5% increase in the S-phase cell population (p=5.1×10−3). However, in the LNCaP-ARA70β cell line, treatment with GRβ siRNA led to a 53% reduction of S-phase cells (p=1.7×10−3). Further overexpression of GRβ in the LNCaP-ARA70β line did not result in any significant change (p=0.33) in the S phase cell population (Figure 2.D). Thus the proliferative advantage conferred by ARA70β expression in LNCaP cells is in part mediated by GRβ, and is consistent with the growth inhibitory effects of mifepristone treatment in these same cells.
In PC3-vector cells, the treatment with GRβ siRNA led only to a minimal 3% decrease in the S phase cell population (p=6.6×10−3), and overexpression of GRβ led to 1.5% S phase decrease (p=9.9×10−3). In contrast to LNCaP cells, PC3 cells stably overexpressing ARA70β do not show any significant increase in their growth rate 15. In fact, PC3-ARA70β cells showed ~7% decrease in the number of S phase cells compared to PC3-vector cells (p=1.4×10−4). Treatment of PC3-ARA70β cells with GRβ siRNA resulted in small increase in proliferative status (2% more cells in S phase, p=3.4×10−2), and transient overexpression of GRβ in these cells had no effect (p=0.35) on their growth (Figure 2.C). It therefore appears that PC3 cells show little change in proliferative response as a function of GRβ.
Simultaneous silencing of AR and GRβ
To examine the possible cross talk between AR and GR signaling pathways and to determine whether these receptors function in synergy, we simultaneously knocked down both AR and GRβ and examined the effect of this treatment on cell growth (Figure 3). To ensure the efficient knock-down of both AR and GRβ the cells were treated with the corresponding siRNAs sequentially and the GRβ and AR protein levels were monitored by western blotting for the duration of the experiments.
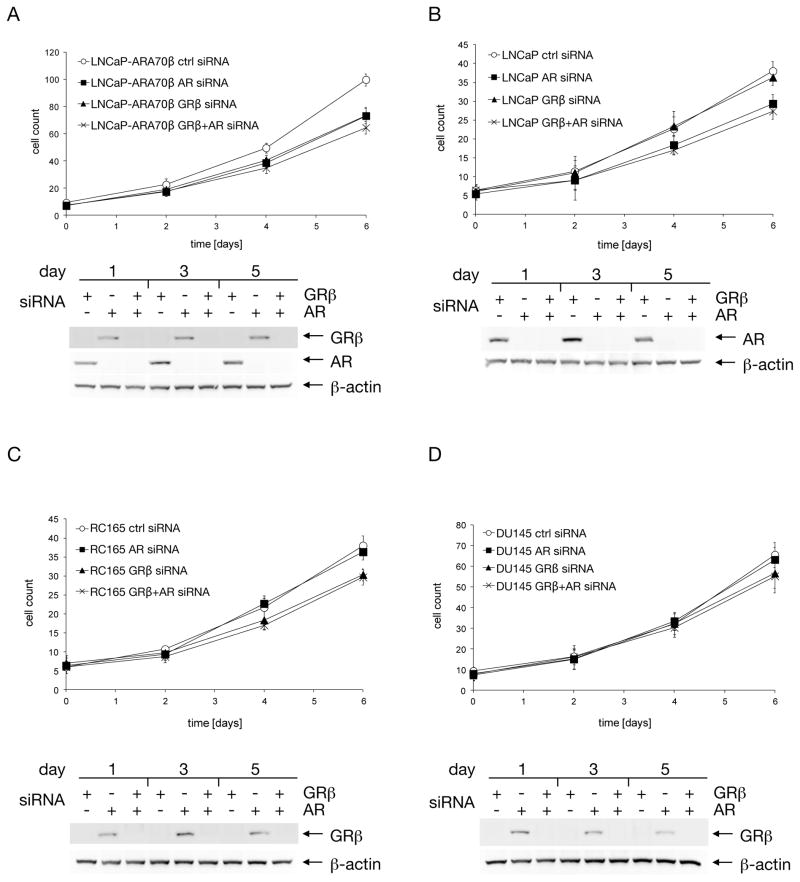
Figure 3. Simultaneous knock-down of GRβ and AR.
LNCaP-ARA70β (A), LNCaP (B), RC165 (C), and DU145 (D) cells were first transfected with AR siRNA and the following day with GRβ siRNA. Cells grown in 6-well plates were collected every other day and counted. In addition, total protein was extracted and GRβ and AR knockdown was confirmed by western blot.
In LNCaP cells overexpressing ARA70β, targeting AR or GRβ by siRNA inhibited growth to a similar extent, by approximately 30% (p=2.2×10−3, p=5.1×10−3, respectively). When the siRNAs were administrated sequentially, leading to diminished levels of both AR and GRβ, the growth rate was only marginally lower (p=7.3×10−2, p=8.3×10−2, respectively) compared to the both single knock-downs (Figure 3.A). When the same experiment was performed in control LNCaP cells, knock-down of AR resulted in growth inhibition of about 20% (p=1.5×10−2). However, treatment with GRβ-specific siRNA had no effect on the growth rate (p=0.44), and the sequential application of the siRNAs resulted in growth rate similar (p=0.35) to that of the cells subjected to single AR knock-down (Figure 3.B).
Unlike LNCaP cells, which express AR but lack GRβ, RC165 and DU145 cells lack AR and express GRβ. In the sequential double knock-down of AR and GRβ, these cell lines behaved in a similar fashion: single knock-down of AR had no effect on the growth rate (p=0.44), while both single knock-down of GRβ (p=1.2×10−2) and double GRβ-AR knock-downs (p=1.3×10−2) led to growth inhibition, which was more pronounced in RC165 cells as compared to DU145 cells (p=0.35, p=3.0×10−2, p=2.4×10−2, respectively) (Figure 3.C, D). Our findings suggest significant communication between the AR and GR β pathways in modulating the cell proliferative response of prostate cancer cells.
DISCUSSION
Despite the widespread use of glucocorticoids in treating patients with hormone-resistant prostate cancer, the exact mechanism of their action and specific properties that determine cell sensitivity are not known. In this paper we report that GRβ, a relatively understudied isoform of the major glucocorticoid receptor GRα, is required for the increased growth rate of LNCaP cells overexpressing ARA70β.
In LNCaP cells overexpressing ARA70β, we observed increased levels of GRβ. However, the levels of GRα remained unchanged, indicating possible distinct functions of these isoforms. Interestingly, in the survey of various benign and cancerous prostate cell lines, we found that the levels of GRβ and ARA70β did not correlate, at either the transcript or protein levels. In the cell lines where ARA70β was detected (LNCaP, LNCaP-AI), this discrepancy may possibly be due to relatively low endogenous levels of ARA70β as compared to cells artificially overexpressing it. In the cell lines with high levels of GRβ, but absent ARA70β, this may point to an upregulation mechanism of GRβ distinct from the one dependent on ARA70β. The expression of GRα in the cell lines tested corresponded to the results obtained previously by other groups 24, 25.
When LNCaP-ARA70β cells were treated with GRβ antagonist mifepristone, the resulting decrease of growth rate was up to 50%. However, the treatment with dexamethasone, a GRα agonist, did not result in any increase in growth, demonstrating that the observed effect was GRβ-dependent. This was corroborated by reducing GRβ by siRNA in LNCaP-ARA70β, which also resulted in growth suppression. LNCaP control cells did not show any change in growth rate after the treatment with mifepristone and dexamethasone, as reported previously 24.
Consistent with the antagonist studies, when the GRβ levels were reduced by siRNA, we observed a shift in cell cycle distribution away from the S phase, indicating slower growth. Blocking the activity of PR also led to a slight decrease of the fraction of proliferating cells, however, compared to GRβ this effect was only minor (data not shown). When we knocked down GRβ and AR individually in LNCaP-ARA70β cells, the growth rate was reduced as expected. However, simultaneous knock down did not produce any additive effect. Similarly, in LNCaP cells only AR depletion led to slower growth, consistent with the absence of GRβ, and in RC165 and DU145 only depletion of GRβ caused growth inhibition, consistent with the absence of AR in these cell lines. In agreement with our knock-down experiments, the GRβ inhibitor mifepristone was reported to inhibit the growth rate of DU145 cells 24. Although the effect of GRβ overexpression on growth was not as dramatic as its knock-down, overexpression still resulted in 1.4-fold increase in the population of LNCaP cells in S-phase (compare the first and third columns in Figure 2.D). For PC3 cells (Figure 2.C) there is no observable effect of overexpression, possibly due to the absence of androgen receptor in this cell line.
CONCLUSIONS
Although prostate tumors can be initially treated by androgen ablation, advanced-stage prostate cancer becomes refractory to hormone inhibition, leading to metastases and death 1. Glucocorticoids (such as dexamethasone) were shown to be beneficial in treatment of androgen-independent prostate cancer and are commonly prescribed together with paclitaxel 3. Clinical studies have shown that glucocorticoid treatment led to significant reduction of the prostate-specific antigen in patients 26, 27. The main target in glucocorticoid therapy of hormone-resistant prostate cancer is assumed to be GRα. However, little attention has been paid to GRβ, a GR isoform thought to competitively inhibit GRα 28 and to have an intrinsic transcriptional activity14. The level of GRβ is increased in several disease states, among them acute lymphoblastic leukemia 29. Therefore, inhibition of GRβ could potentially increase the effectiveness of glucocorticoid therapy. However, the GRβ inhibitor mifepristone not only binds GRβ, but it binds and inhibits GRα as well 14. In certain cancers, where GRα expression plays a small role compared to the oncogenic effect of GRβ, such as when the malignancy is caused by overexpression of ARA70β, inhibition of GRβ by mifepristone might prove beneficial. Mifepristone has been tested in a clinical trial for treatment of castration-resistant prostate cancer, with focus on its role as an inhibitor of AR, with negative results 30. We suggest testing mifepristone in the subset of prostate cancer where GRβ plays the role of an oncogene.
Acknowledgments
Financial support: This work was funded by NYU Cancer Institute translational pilot grant from NIH P30 CA016087 and seed fund from NYUSOM Center of Excellence for Urologic Diseases.
Footnotes
Conflicts of interest: Authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Isaacs JT. The biology of hormone refractory prostate cancer. Why does it develop? Urol Clin North Am. 1999;26:263. doi: 10.1016/s0094-0143(05)70066-5. [DOI] [PubMed] [Google Scholar]
- 2.Kassi E, Moutsatsou P. Glucocorticoid receptor signaling and prostate cancer. Cancer Lett. 2011;302:1. doi: 10.1016/j.canlet.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Fakih M, Johnson CS, Trump DL. Glucocorticoids and treatment of prostate cancer: a preclinical and clinical review. Urology. 2002;60:553. doi: 10.1016/s0090-4295(02)01741-7. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Wang J, Yu G, et al. Androgen and glucocorticoid receptor heterodimer formation. A possible mechanism for mutual inhibition of transcriptional activity. J Biol Chem. 1997;272:14087. doi: 10.1074/jbc.272.22.14087. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura K, Nonomura N, Satoh E, et al. Potential mechanism for the effects of dexamethasone on growth of androgen-independent prostate cancer. J Natl Cancer Inst. 2001;93:1739. doi: 10.1093/jnci/93.22.1739. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Chen Y, Cao D, et al. Glucocorticoid up-regulates transforming growth factor-beta (TGFβ) type II receptor and enhances TGFβ signaling in human prostate cancer PC-3 cells. Endocrinology. 2006;147:5259. doi: 10.1210/en.2006-0540. [DOI] [PubMed] [Google Scholar]
- 7.Yano A, Fujii Y, Iwai A, et al. Glucocorticoids suppress tumor angiogenesis and in vivo growth of prostate cancer cells. Clin Cancer Res. 2006;12:3003. doi: 10.1158/1078-0432.CCR-05-2085. [DOI] [PubMed] [Google Scholar]
- 8.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 9.Zhao XY, Malloy PJ, Krishnan AV, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 10.Gross KL, Lu NZ, Cidlowski JA. Molecular mechanisms regulating glucocorticoid sensitivity and resistance. Mol Cell Endocrinol. 2009;300:7. doi: 10.1016/j.mce.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oakley RH, Cidlowski JA. Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem. 2011;286:3177. doi: 10.1074/jbc.R110.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J Biol Chem. 1996;271:9550. doi: 10.1074/jbc.271.16.9550. [DOI] [PubMed] [Google Scholar]
- 13.Bamberger CM, Bamberger AM, de Castro M, et al. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest. 1995;95:2435. doi: 10.1172/JCI117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis-Tuffin LJ, Jewell CM, Bienstock RJ, et al. Human glucocorticoid receptor beta binds RU-486 and is transcriptionally active. Mol Cell Biol. 2007;27:2266. doi: 10.1128/MCB.01439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Y, Li CX, Chen F, et al. Stimulation of prostate cancer cellular proliferation and invasion by the androgen receptor co-activator ARA70β. Am J Pathol. 2008;172:225. doi: 10.2353/ajpath.2008.070065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinkmann AO, Blok LJ, de Ruiter PE, et al. Mechanisms of androgen receptor activation and function. J Steroid Biochem Mol Biol. 1999;69:307. doi: 10.1016/s0960-0760(99)00049-7. [DOI] [PubMed] [Google Scholar]
- 17.McKenna NJ, Xu J, Nawaz Z, et al. Nuclear receptor coactivators: multiple enzymes, multiple complexes, multiple functions. J Steroid Biochem Mol Biol. 1999;69:3. doi: 10.1016/s0960-0760(98)00144-7. [DOI] [PubMed] [Google Scholar]
- 18.Alen P, Claessens F, Schoenmakers E, et al. Interaction of the putative androgen receptor-specific coactivator ARA70/ELE1a with multiple steroid receptors and identification of an internally deleted ELE1b isoform. Mol Endocrinol. 1999;13:117. doi: 10.1210/mend.13.1.0214. [DOI] [PubMed] [Google Scholar]
- 19.Ligr M, Li Y, Zou X, et al. Tumor suppressor function of androgen receptor coactivator ARA70α in prostate cancer. Am J Pathol. 2010;176:1891. doi: 10.2353/ajpath.2010.090293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu S, Tsai SY, Tsai MJ. Molecular mechanisms of androgen-independent growth of human prostate cancer LNCaP-AI cells. Endocrinology. 1999;140:5054. doi: 10.1210/endo.140.11.7086. [DOI] [PubMed] [Google Scholar]
- 21.Peng Y, Chiriboga L, Yee H, et al. Androgen receptor coactivator ARA70α and ARA70β isoform-specific antibodies: new tools for studies of expression and immunohistochemical localization. Appl Immunohistochem Mol Morphol. 2008;16:7. doi: 10.1097/PAI.0b013e31802e91ea. [DOI] [PubMed] [Google Scholar]
- 22.Rivers C, Levy A, Hancock J, et al. Insertion of an amino acid in the DNA-binding domain of the glucocorticoid receptor as a result of alternative splicing. J Clin Endocrinol Metab. 1999;84:4283. doi: 10.1210/jcem.84.11.6235. [DOI] [PubMed] [Google Scholar]
- 23.Gu Y, Li H, Miki J, et al. Phenotypic characterization of telomerase-immortalized primary non-malignant and malignant tumor-derived human prostate epithelial cell lines. Exp Cell Res. 2006;312:831. doi: 10.1016/j.yexcr.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 24.Yan TZ, Jin FS, Xie LP, et al. Relationship between glucocorticoid receptor signal pathway and androgen-independent prostate cancer. Urol Int. 2008;81:228. doi: 10.1159/000144067. [DOI] [PubMed] [Google Scholar]
- 25.Dovio A, Sartori ML, De Francia S, et al. Differential expression of determinants of glucocorticoid sensitivity in androgen-dependent and androgen-independent human prostate cancer cell lines. J Steroid Biochem Mol Biol. 2009;116:29. doi: 10.1016/j.jsbmb.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Sartor O, Weinberger M, Moore A, et al. Effect of prednisone on prostate-specific antigen in patients with hormone-refractory prostate cancer. Urology. 1998;52:252. doi: 10.1016/s0090-4295(98)00149-6. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura K, Nonomura N, Yasunaga Y, et al. Low doses of oral dexamethasone for hormone-refractory prostate carcinoma. Cancer. 2000;89:2570. doi: 10.1002/1097-0142(20001215)89:12<2570::aid-cncr9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi Y, Iwasaki Y, Tsugita M, et al. Glucocorticoid receptor-beta and receptor-gamma exert dominant negative effect on gene repression but not on gene induction. Endocrinology. 2010;151:3204. doi: 10.1210/en.2009-1254. [DOI] [PubMed] [Google Scholar]
- 29.Longui CA, Vottero A, Adamson PC, et al. Low glucocorticoid receptor alpha/beta ratio in T-cell lymphoblastic leukemia. Horm Metab Res. 2000;32:401. doi: 10.1055/s-2007-978661. [DOI] [PubMed] [Google Scholar]
- 30.Taplin ME, Manola J, Oh WK, et al. A phase II study of mifepristone (RU-486) in castration-resistant prostate cancer, with a correlative assessment of androgen-related hormones. BJU Int. 2008;101:1084. doi: 10.1111/j.1464-410X.2008.07509.x. [DOI] [PubMed] [Google Scholar]