Abstract
Introduction
Certain chemotherapeutic agents commonly used for advanced non-small cell lung cancer (NSCLC) require minimum threshold renal function for administration. To determine how such requirements affect treatment options, we evaluated renal function patterns in this population.
Methods
We performed a single-center retrospective analysis of patients treated for stage IV NSCLC from 2000 to 2007. Associations between patient characteristics, calculated creatinine clearance (CrCl), and clinical outcomes were determined using univariate and multivariate analyses, Cox proportional hazard models, and mixed model analysis.
Results
298 patients (3,930 Cr measurements) were included in the analysis. Patients had a median of 5 (interquartile range [IQR] 4-18) Cr measurements. Median baseline CrCl was 96 mL/min (IQR 74-123 mL/min); median nadir CrCl was 78 mL/min (IQR 56-100 mL/min). Renal function was associated with age (P<0.001), race (P=0.009), and gender (P=0.001). 23% of patients had a recorded CrCl < 60 mL/min (threshold for cisplatin), with median onset 83 days after diagnosis and median time to recover to ≥ 60 mL/min of 27 (IQR 3-85) days; 11% of patients had a recorded CrCl < 45 mL/min (threshold for pemetrexed), with median onset 122 days after diagnosis and median recovery time of 36 (IQR 3-73) days. For both thresholds, approximately 35% of patients had no documented recovery.
Conclusions
In this cohort of patients treated for stage IV NSCLC, renal function falls below commonly used thresholds for cisplatin and for pemetrexed in fewer than a quarter of patients. However, these declines may preclude administration of these drugs for prolonged periods.
Keywords: Non-small cell lung cancer, chemotherapy, cisplatin, pemetrexed, renal function, creatinine clearance
Introduction
A number of chemotherapeutic agents commonly employed for the treatment of non-small cell lung cancer (NSCLC) require minimum threshold renal function for administration. For pemetrexed (Eli Lilly, Indianapolis, USA), an anti-folate approved for first-line, second-line, and maintenance therapy for advanced NSCLC, the recommended creatinine clearance (CrCl) cut-off is 45 mL/min.1 Below that threshold, the potential for diminished pemetrexed clearance may place patients at heightened risk for toxicities such as myelosuppression and gastrointestinal effects.2,3 For cisplatin (Bristol-Myers Squibb, New York City, USA), a platinum analog with potential renal toxicity employed in adjuvant, locally advanced, and advanced disease settings, a CrCl threshold of 60 mL/min is commonly used.4
For several reasons, patients with lung cancer may be at risk for diminished renal function. The median age at diagnosis is approximately 70 years.5 On average, CrCl decreases by 1% per year from a peak in early adulthood, with elderly individuals having substantially lower renal function than younger populations.6 Additionally, certain chemotherapeutic agents, concomitant medications, or iodinated contrast dye may have nephrotoxic effects. Finally, chemotherapy-induced anorexia, nausea, and vomiting can lead to volume depletion, resulting in impaired renal function.
Renal function has been characterized in a number of cancers. In gynecologic malignancies, the impact of pelvic radiation and chemotherapy on renal function has been evaluated.7 In urothelial cancer, where disease-related anatomic changes may compromise kidney function and cisplatin is a mainstay of medical therapy, the assessment and impact of renal function has been explored extensively.8,9 Similar analyses have been conducted in patients with lymphoma. 10,11 However, to date, renal function has not been systematically characterized in patients with advanced NSCLC. Because this clinical parameter has substantial implications for therapy, we performed an analysis of renal function over time in a contemporary, real-world cohort of patients with advanced NSCLC treated with chemotherapy.
Materials and Methods
Data Extraction
Approval for this research was obtained from the University of Texas Southwestern (UT Southwestern) Institutional Review Board (IRB). The IRB waived the requirement for informed consent for the following reasons: (1) the research involved only minimal risk to subjects (compilation of data and subsequent risk of loss of confidentiality); (2) the waiver did not adversely affect the rights and welfare of the subjects (no treatment or invasive procedures); (3) the research could not practicably be carried out without the waiver (a retrospective medical records review of a large volume of cases).
We identified consecutive patients diagnosed with advanced (stage IIIB malignant effusion and stage IV by American Joint Committee on Cancer [AJCC] 6th edition staging) NSCLC diagnosed between 1/1/2000, and 12/31/2007, who received chemotherapy at clinical facilities associated with UT Southwestern.
We obtained demographic, disease, treatment, and outcome data from local tumor registries. We obtained patient weight and serum creatinine (Cr) values from individual medical records. Overall survival was defined as the interval between date of diagnosis and date of death. Weight was recorded from the most recent oncology visit preceding the initiation of chemotherapy and carried forward as a constant for subsequent creatinine clearance (CrCl) calculations. All available Cr values starting the date of diagnosis were recorded.
CrCl was determined using three formulae. Cockcroft-Gault: CrCl = {[(140-age in years) × (weight in kg)] / [(serum Cr in mg/dL) × (72)]} × 0.85 if female.12 Jelliffe: CrCl (male) = 98 – 0.8 × (age in years – 20) / (serum Cr in mg/dL). CrCl (female) = 88 – 0.7 (age in ears – 20) / (serum Cr in mg/dL).13 Modification of Diet in Renal Disease (MDRD): CrCl = 175 × (serum Cr in mg/dL)−1.154 × (age in years)−0.203 × 1.212 (if black) × 0.742 (if female).14 Baseline and nadir CrCl were calculated using all three formulae. Because it is the most commonly used formula in the clinical care of cancer patients and has been shown to correlate with measured CrCl in multiple disease settings,15-18 we report only Cockcroft-Gault calculations in subsequent analyses.
Statistical analysis
Descriptive statistics were generated for baseline demographic and clinical characteristics. Baseline and nadir calculated CrCl were compared among demographic subgroups. Fluctuation of CrCl was investigated by calculating the following parameters: duration from diagnosis to CrCl falling below given threshold values (60 or 45 mL/min); duration from falling below given threshold values to recovering to those thresholds; and nadir CrCl.
We evaluated the association between baseline variables and calculated CrCl. We dichotomized these variables as follows: age (<65 years and ≥65 years), race/ethnicity (white and non-white), and gender (male and female). We analyzed the association between CrCl and overall survival using univariate and multivariate Cox regression.
All statistical analyses were performed using 64-bit R 2.13 (R Development Core team, Auckland, New Zealand).
Results
Study Population
A total of 298 patients (with a total of 3,930 Cr measurements) were included in the analysis. Among these patients, mean age at diagnosis was 58 years, 57% were men, and 47% were white. Patients had a median of 5 (IQR 4-18) and a mean of 12.3 (SD 14.35) Cr measurements. Additional baseline characteristics of the study population are listed in Table 1. Treatment administered was as follows: platinum doublet (86%), single-agent cytotoxic chemotherapy (7%), tyrosine kinase inhibitor (6%), and non-platinum doublet (1%). While data on specific agents is not available, similar to other centers in the United States,19 at our institution carboplatin is more commonly employed than cisplatin in platinum doublets for the treatment of advanced NSCLC. Total number of cycles of first-line chemotherapy received was as follows: one (20%), two (21%), three (13%), four (19%), five or more (24%), and unknown (3%). Data on second-line treatments were not available.
Table 1.
Baseline patient, disease, and treatment characteristics
| Characteristics | Number (%) or mean (SD) |
|---|---|
| Number of patients | 298 |
| Age (y) | 58.0 (11.0) |
| Weight (kg) | 72.0 (17.6) |
| Gender | |
| Male | 169 (56.7) |
| Female | 129 (43.3) |
| Race/ethnicity | |
| White (non-Hispanic) | 141 (47.3) |
| African American | 113 (37.9) |
| Hispanic | 32 (10.7) |
| Asian | 11 (3.7) |
| Unknown | 1 (0.3) |
| Histology | |
| Adenocarcinoma | 140 (47.0) |
| Squamous cell | 59 (19.8) |
| Large cell | 7 (2.3) |
| Other | 92 (30.9) |
Overall, baseline, and nadir renal function
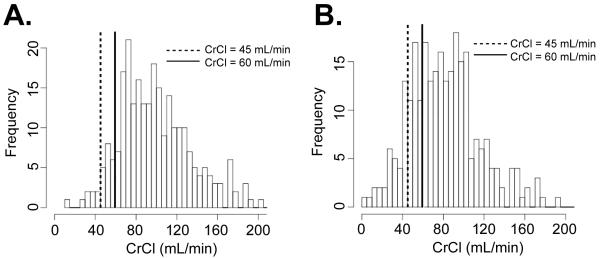
Among all patients and across all time points, the median calculated CrCl was 92.0 mL/min (IQR 68-124) by Cockcroft-Gault, 85.0 mL/min (IQR 64-113) by Jelliffe, and 109.2 mL/min (IQR 82-138) by MDRD. Median pre-treatment baseline calculated CrCl were 95.9 mL/min (IQR 74-123) (Cockcroft-Gault), 84.1 mL/min (IQR 68-102) (Jelliffe), and 104.3 mL/min (IQR 86-131) (MDRD) (Table 2). The distribution of baseline calculated CrCl is shown in Figure 1A. 9% of patients had a baseline CrCl less than 60 mL/min; 3% had a CrCl less than 45 mL/min.
Table 2.
Baseline CrCl according to population characteristics
| Cohort | Baseline Serum Cr |
Baseline CrCl (mL/min) | |||||
|---|---|---|---|---|---|---|---|
| Cockcroft- Gault |
P value | Jeliffe |
P value |
MDRD |
P value |
||
|
All
patients |
0.8 | 95.9 | 84.1 | 104.3 | |||
| Age | <0.001 | <0.001 | <0.001 | ||||
| <65 y | 0.7 | 103.2 | 91.5 | 110 | |||
| ≥65 y | 0.8 | 72.6 | 66.2 | 91.5 | |||
| Sex | 0.06 | 0.07 | 0.06 | ||||
| Male | 0.8 | 100.5 | 80.4 | 106.2 | |||
| Female | 0.7 | 92.6 | 87.9 | 102.2 | |||
| Race | 0.05 | 0.59 | 0.04 | ||||
| White | 0.8 | 101.4 | 87.4 | 98.6 | |||
| African- American |
0.8 | 91.3 | 83.1 | 114.0 | |||
| Hispanic | 0.7 | 97.2 | 86.9 | 106.3 | |||
| Asian | 0.8 | 75.7 | 78.2 | 86.4 | |||
| Race | 0.02 | 0.48 | 0.02 | ||||
| White | 0.8 | 103.4 | 87.4 | 98.6 | |||
| Non- white |
0.8 | 91.4 | 83.1 | 110 | |||
Figure 1.
CrCl distribution histograms. (A) Baseline CrCl distribution as calculated by the Cockcroft-Gault formula. Vertical lines indicate thresholds for chemotherapy administration. (B) Distribution of the lowest (nadir) CrCl achieved in the patient population at any time, as calculated by the Cockcroft-Gault formula. Vertical lines indicate thresholds for chemotherapy administration. (solid: 60 mL/min, dashed: 45 mL/min)
Median baseline pre-treatment calculated CrCl in population subgroups is listed in Table 2. CrCl (by Cockcroft-Gault) was significantly associated with age and race, and there was a non-significant trend toward association with sex, with lower CrCl among older patients, non-white (especially Asian) patients, and women. The three CrCl formulas we evaluated had varying effects on baseline calculated CrCl in different subpopulations. For instance, compared to Cockcroft-Gault calculations, MDRD raised median baseline calculated CrCl by 7.0 mL/min among patients age < 65 years, but by almost 20.0 mL/min among patients age ≥ 65 years. With the MDRD formula, median baseline calculated CrCl for white patients decreased by approximately 3.0 mL/min, but increased by approximately 9.0 mL/min for Hispanic patients and by almost 23.0 mL/min for African-American patients.
Median nadir calculated CrCl values were 78.3 mL/min (IQR 56-100) (Cockcroft-Gault), 68.0 mL/min (IQR 50-88) (Jelliffe), and 81.6 mL/min (IQR 59-106) (MDRD) (Supplementary Table 1). Similar to baseline calculated CrCl, nadir CrCl (by Cockcroft-Gault) was associated with age and sex, and displayed a trend toward association with race/ethnicity. Again, lower nadir CrCl was observed among older, female, and Asian patients. 23% of patients had a nadir calculated CrCl < 60 mL/min; 11% had a nadir CrCl < 45 mL/min ( Figure 1B).
Incidence and timing of CrCl decline and recovery
We used commonly employed CrCl thresholds (60 mL/min and 45 mL/min) to determine the incidence and timing of CrCl decline and recovery. Table 3 displays the proportion of patients by subgroup that experienced declines to and recovery above these thresholds, as well as the median time to these events. The Cockcroft-Gault formula was used for these analyses. Overall, 23% of patients experienced CrCl < 60 mL/min, occurring a median 83 days after diagnosis, of whom 66% recovered to CrCl ≥ 60 mL/min after a median 27 (IQR 3-85) days.
Table 3.
Proportion of patients by subgroup that experienced declines to and recovery above given CrCl thresholds and the median time to these events A: CrCl threshold of 60 mL/min B: CrCl threshold of 45 mL/min
| A. | ||||
|---|---|---|---|---|
| Cohort | Patients with recorded CrCl < 60 mL/min (%) |
Median time to onset of CrCl < 60 mL/min (days) |
Patients recovering to CrCl ≥ 60 mL/min (%)* |
Median time to recovery to CrCl ≥ 60 mL/min (days) |
|
All
patients |
23 | 83 | 66 | 27 |
| Age | ||||
| <65 | 17 | 92 | 56 | 21 |
| ≥65 | 40 | 2 | 78 | 36 |
| Sex | ||||
| Male | 18 | 77 | 68 | 21 |
| Female | 29 | 94 | 65 | 31 |
| Race | ||||
| White | 22 | 41 | 68 | 30 |
| Afr-Amer | 23 | 117 | 69 | 21 |
| Hispanic | 19 | 95 | 67 | 1 |
| Asian | 36 | 0 | 25 | 121 |
| Race | ||||
| White | 18 | 41 | 68 | 30 |
| Non- white |
24 | 89 | 65 | 21 |
| B. | ||||
|---|---|---|---|---|
| Cohort | Patients with recorded CrCl < 45 mL/min (%) |
Median time to onset of CrCl < 45 mL/min (days) |
Patients recovering to CrCl ≥ 45 mL/min (%)* |
Median time to recovery to CrCl ≥ 45 mL/min (days) |
|
All
patients |
11 | 122 | 61 | 36 |
| Age | ||||
| <65 | 18 | 108 | 67 | 6 |
| ≥65 | 19 | 232 | 53 | 72 |
| Sex | ||||
| Male | 8 | 36 | 62 | 17 |
| Female | 16 | 260 | 60 | 57 |
| Race | ||||
| White | 9 | 175 | 58 | 68 |
| Afr- Amer |
15 | 254 | 59 | 33 |
| Hispanic | 3 | 94 | 100 | 1 |
| Asian | 18 | 55 | 50 | 8 |
| Race | ||||
| White | 9 | 175 | 58 | 68 |
| Non- white |
13 | 94 | 62 | 8 |
Of those patients who had recorded CrCl < 60 mL/min
Of those patients who had recorded CrCl < 45 mL/min
11% of patients experienced CrCl < 45 mL/min, occurring a median 122 days after diagnosis, of whom 61% recovered to CrCl ≥ 45 mL/min after a median 36 (IQR 3-73) days.
CrCl trends over time
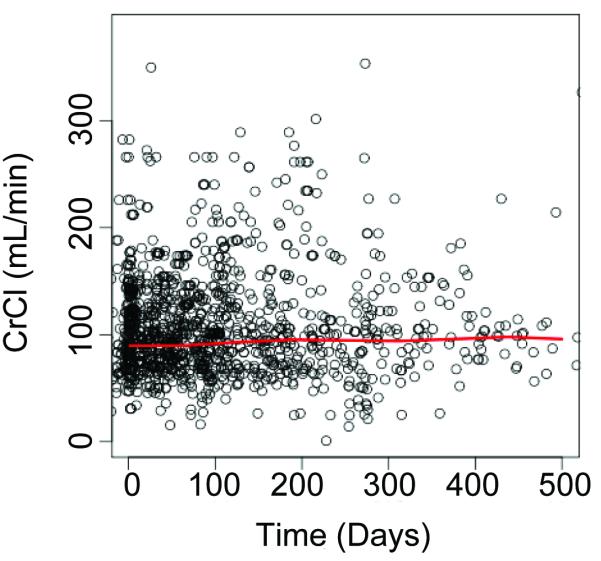
Figure 2 depicts CrCl over time in the overall study population and in subgroups according to age, gender, and race/ethnicity. There was no significant change over time in CrCl in any of these cohorts.
Figure 2.
CrCl trend of study population over time.
Association between CrCl and survival
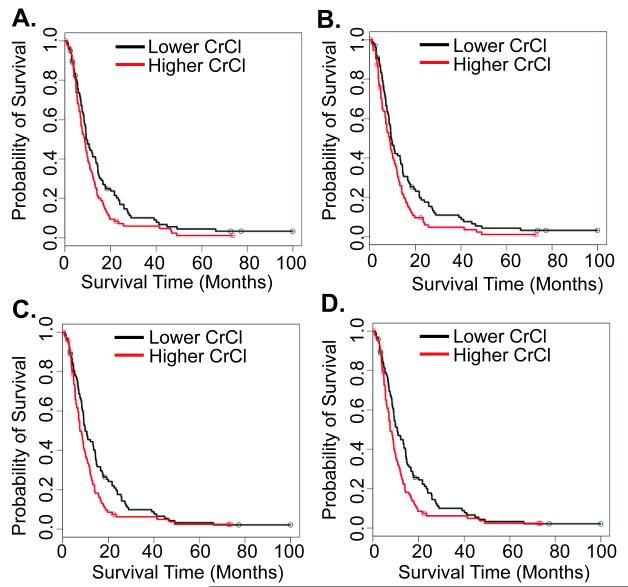
The association between baseline, nadir, median and proportional change (from baseline to nadir) in CrCl calculated using the Cockcroft-Gault formula and overall survival is shown in Figure 3. For these analyses, CrCl was dichotomized at median values. In univariate analyses, higher baseline (HR 1.36; 95% CI, 1.02-1.83; P=0.04), nadir (HR 1.41; 95% CI, 1.05-1.89; P=0.02), and median (HR 1.44; 95% CI, 1.08-1.94; P=0.01) CrCl were significantly associated with worse overall survival. These associations were not maintained in a multivariable model adjusting for age, gender, and race/ethnicity (data not shown). Proportion change from baseline to nadir CrCl was not associated with survival (HR 0.98; 95% CI, 0.73-1.31; P=0.91).
Figure 3.
Overall survival of patients according to CrCl values. A: baseline CrCl. B: nadir CrCl. C: mean CrCl. D: median CrCl.
Discussion
Although renal function has been characterized in patients with multiple malignancies, including ovarian cancer,7 urothelial cancer,8,9 and hematologic malignancies,11 this parameter has been little described in lung cancer. In contrast to these other diagnoses, lung cancer is unlikely to impact renal function through direct anatomic or metabolic effects. Nevertheless, patients’ advanced age, medical comorbidities, and treatment toxicities may impair renal function. A previously published series found that patients with lung cancer and chronic kidney disease (defined as CrCl < 90 mL/min calculated by Cockcroft-Gault formula for > 6 months preceding the diagnosis of lung cancer) reported that presenting symptoms, histologic distribution, and overall survival were comparable to historical data in patients with lung cancer but without chronic kidney disease.20 However, this study did not describe trends related to clinically relevant thresholds that impact disease management. Accordingly, to evaluate the impact of renal function on treatment options and clinical outcomes, we characterized CrCl over time in patients with advanced NSCLC treated with chemotherapy.
Despite the advanced age, anticipated comorbidities, and treatment toxicities in our cohort, renal function was generally adequate. Across all time points, the median calculated CrCl was 92 mL/min. As has been described previously in other populations,21-23 CrCl was lower in older patients, women, and non-white patients. Yet even in these higher risk subgroups, the likelihood of CrCl falling below thresholds required for cisplatin and pemetrexed remained low. Median nadir calculated CrCl was 60 mL/min in patients age ≥ 65 years, 75 mL/min in women, and 76 mL/min in non-white patients.
The timing and duration of CrCl decline provides insight into the potential impact of this clinical event. For the 23% of patients who developed CrCl < 60 mL/min (threshold for cisplatin) during the course of their disease, median time to onset was 83 days or just under 3 months. Assuming systemic chemotherapy generally starts within one month of diagnosis of stage IV NSCLC, this time-point approximates the interval between the third and fifth cycles of first-line therapy. As the majority of patients treated with first-line chemotherapy receive at least four treatment cycles,24 it is conceivable that this decline in renal function could hinder the ability to administer cisplatin. It is noteworthy that the median time to CrCl < 60 mL/min among patients age ≥ 65 years was two days, suggesting that a substantial proportion of these patients have a calculated CrCl < 60 mL/min at the time of diagnosis. For all patients who developed CrCl < 60 mL/min, the median time to recovery was 27 days, a period corresponding to 1.3 21-day treatment cycles. The timing and duration of renal dysfunction was particularly evident within the small cohort of Asian patients. Approximately 35% had CrCl < 60 mL/min, with a median onset at the time of diagnosis and a median time to CrCl ≥ 60 mL/min of 121 days (which exceeds the duration of four cycles of a typical first-line chemotherapy regimen. Notably, for 34% of patients developing CrCl < 60 mL/min, there was no documented recovery to this threshold.
In contrast to cisplatin, which is generally restricted to first-line treatment of advanced NSCLC, pemetrexed is employed in first-line, maintenance, and second-line settings. Accordingly, declines in CrCl to < 45 mL/min at any point after diagnosis could potentially impact administration of this agent. Among the 11% of patients who developed CrCl < 45 mL/min after diagnosis, median time to occurrence was 122 days, which falls after a sixth cycle of chemotherapy would be administered to patients who initiate treatment within two weeks after diagnosis. For these cases, median time to recovery was 36 days, corresponding to two treatment cycles. For 39% of cases, no documented recovery occurred.
The impact of renal dysfunction on treatment options in advanced NSCLC may be mitigated by the availability and efficacy of agents that do not require a minimum CrCl. In testicular cancer and bladder cancer, cisplatin has demonstrated clear superiority to carboplatin, a platinum analog with less gastrointestinal, renal, neurologic, and auditory toxicities and no minimum CrCl requirement.25,26 By contrast, carboplatin is widely used in the treatment of advanced NSCLC because the small survival benefit of cisplatin over carboplatin described in meta-analyses has generally been considered of limited clinical consequence.27,28 Among non-platinum cytotoxic agents commonly employed in the treatment of advanced NSCLC, only pemetrexed requires a threshold CrCl. Gemcitabine and taxanes do not. Nor do molecularly targeted therapies such as bevacizumab and erlotinib.
The finding that calculated CrCl differed substantially according to formula echoes observations from a number of earlier studies.9,29 Indeed, the reliability of these methods has been question ed.9,30 However, other approaches are unlikely to provide better determinations. Measured CrCl depends on accurate 12- or 24-hour urine collections, which are difficult to achieve in ambulatory and even hospital settings.10,17 Direct assessment of glomerular filtration rate, as with inulin or 99mTc-DTPA, is not practical in most clinical settings.
Although unexpected, our observation that CrCl is inversely related to overall survival in univariate analyses may be attributed to well-documented disease patterns. Women, who have been shown in multiple settings to have better survival in NSCLC than do men,31-34 may have lower calculated CrCl due to differences in size and the Cockcroft-Gault correction factor of 0.85 for female gender. Asians, who had a median baseline CrCl 25 mL/min less than that of white patients in this cohort, also have superior clinical outcomes.35-37
There are a number of limitations of the current analysis. While our single-center setting provides a racially and socioeconomically diverse cohort,38,39 the average age of 59 years is a full decade lower than the national average at the time of lung cancer diagnosis. Accordingly, renal function may appear better in this population than that generally seen in patients with lung cancer. The study sample includes only those patients treated with chemotherapy, due to inadequate data availability for the estimated 50 percent of patients with advanced NSCLC at our center who never receive systemic therapy.31 It is not known how renal function differs between these populations, nor to what extent renal dysfunction may have prevented chemotherapy administration. Importantly, because only baseline patient weight was reliably available, this value was carried forward as a constant for subsequent Cockcroft-Gault CrCl calculations. Because patients with lung cancer experience clinically significant weight loss over the course of the disease,40,41 our post-baseline CrCl determinations may be overestimated, resulting in an under-reporting of patients with CrCl falling below specific thresholds. However, with median weight loss during lung cancer treatment ranging 10-12% of pre-treatment body weight (correlating to a 10-12% decline in CrCl), it seems like that a relatively small number of additional patients would have experienced CrCl decline below clinically relevant cut-offs.42,43 Finally, the frequency and duration of CrCl assessment was not standardized in this population. This variability may affect the incidence and duration of declines in renal function.
In conclusion, among patients with advanced NSCLC treated with chemotherapy in a real-world setting, renal function rarely decreases below thresholds required for administration of drugs such as cisplatin and pemetrexed. However, these events occur relatively frequently in certain populations, including older patients, women, and Asians. Given the timing of these events, when such decreases do occur they are likely to impact chemotherapy delivery—approximately one third of patients never recover to these thresholds, and recovery generally requires a number of weeks in those who do. As the use of cisplatin- and pemetrexed-containing regimens rises in various lung cancer indications, renal function is likely to become an increasingly relevant factor in the care of these patients.
Supplementary Material
Funding/Acknowledgements
Funding provided Accepted Manuscript by the National Institutes of Health CTSA Grant KL2RR024983 (North and Central Texas Clinical and Translational Science Initiative) (to D.E.G.) and National Cancer Institute Clinical Investigator Team Leadership Award (1P30 CA142543-01 supplement) (to D.E.G.).
The authors thank Joan Cox and Alejandra Madrigales for providing data from the Parkland Health and Hospital System and UT Southwestern Tumor Registries. The authors thank Drew W. Rasco, MD, for assistance gathering patient data.
Footnotes
Conflict of Interest Statement and Financial Disclosures: B.K.C.: none declared H.S.: none declared D.E.G.: none declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 2.Brandes JC, Grossman SA, Ahmad H. Alteration of pemetrexed excretion in the presence of acute renal failure and effusions: presentation of a case and review of the literature. Cancer Invest. 2006;24:283–7. doi: 10.1080/07357900600629567. [DOI] [PubMed] [Google Scholar]
- 3.Rinaldi DA, Kuhn JG, Burris HA, et al. A phase I evaluation of multitargeted antifolate (MTA, LY231514), administered every 21 days, utilizing the modified continual reassessment method for dose escalation. Cancer Chemother Pharmacol. 1999;44:372–80. doi: 10.1007/s002800050992. [DOI] [PubMed] [Google Scholar]
- 4.Fischer DS, Knobf MT, Durivage HJ, editors. The cancer chemotherapy handbook. St Louis; Mosby: 2003. [Google Scholar]
- 5.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 6.Lindeman RD. Changes in renal function with aging. Implications for treatment. Drugs Aging. 1992;2:423–31. doi: 10.2165/00002512-199202050-00006. [DOI] [PubMed] [Google Scholar]
- 7.Schneider DP, Marti HP, Von Briel C, Frey FJ, Greiner RH. Long-term evolution of renal function in patients with ovarian cancer after whole abdominal irradiation with or without preceding cisplatin. Ann Oncol. 1999;10:677–83. doi: 10.1023/a:1007538917659. [DOI] [PubMed] [Google Scholar]
- 8.Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–13. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]
- 9.Raj GV, Iasonos A, Herr H, Donat SM. Formulas calculating creatinine clearance are inadequate for determining eligibility for Cisplatin-based chemotherapy in bladder cancer. J Clin Oncol. 2006;24:3095–100. doi: 10.1200/JCO.2005.04.3091. [DOI] [PubMed] [Google Scholar]
- 10.Gerber DE, Grossman SA, Batchelor T, Ye X. Calculated versus measured creatinine clearance for dosing methotrexate in the treatment of primary central nervous system lymphoma. Cancer Chemother Pharmacol. 2007;59:817–23. doi: 10.1007/s00280-006-0339-x. [DOI] [PubMed] [Google Scholar]
- 11.Cohen LF, Balow JE, Magrath IT, Poplack DG, Ziegler JL. Acute tumor lysis syndrome. A review of 37 patients with Burkitt’s lymphoma. Am J Med. 1980;68:486–91. doi: 10.1016/0002-9343(80)90286-7. [DOI] [PubMed] [Google Scholar]
- 12.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 13.Jelliffe RW. Estimation of creatinine clearance when urine cannot be collected. Lancet. 1971;1:975–6. doi: 10.1016/s0140-6736(71)91484-x. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Chambers JT, Chambers SK, Schwartz PE. Correlation between measured creatinine clearance and calculated creatinine clearance in ovarian cancer patients. Gynecol Oncol. 1990;36:66–8. doi: 10.1016/0090-8258(90)90110-7. [DOI] [PubMed] [Google Scholar]
- 16.Tsubaki T, Goodin S, Leader WG, Chandler MH. Estimation of creatinine clearance in patients with gynecologic cancer. Clin Pharm. 1993;12:685–90. [PubMed] [Google Scholar]
- 17.Davila E, Gardner LB. Clinical value of the creatinine clearance before the administration of chemotherapy with cisplatin. Cancer. 1987;60:161–4. doi: 10.1002/1097-0142(19870715)60:2<161::aid-cncr2820600206>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson P, West N, Hutchinson RJ. Predictive ability of creatinine clearance estimate models in pediatric bone marrow transplant patients. Bone Marrow Transplant. 1997;19:481–5. doi: 10.1038/sj.bmt.1700688. [DOI] [PubMed] [Google Scholar]
- 19.Azzoli CG, Kris MG, Pfister DG. Cisplatin versus carboplatin for patients with metastatic non-small-cell lung cancer --an old rivalry renewed. J Natl Cancer Inst. 2007;99:828–9. doi: 10.1093/jnci/djk222. [DOI] [PubMed] [Google Scholar]
- 20.Patel P, Henry LL, Ganti AK, Potti A. Clinical course of lung cancer in patients with chronic kidney disease. Lung Cancer. 2004;43:297–300. doi: 10.1016/j.lungcan.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 21.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–85. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 22.Kimmel PL, Lew SQ, Bosch JP. Nutrition, ageing and GFR: is age-associated decline inevitable? Nephrol Dial Transplant. 1996;11(Suppl 9):85–8. doi: 10.1093/ndt/11.supp9.85. [DOI] [PubMed] [Google Scholar]
- 23.Palmer Alves T, Lewis J. Racial differences in chronic kidney disease (CKD) and end-stage renal disease (ESRD) in the United States: a social and economic dilemma. Clin Nephrol. 2010;74(Suppl 1):S72–7. doi: 10.5414/cnp74s072. [DOI] [PubMed] [Google Scholar]
- 24.Gerber DE, Rasco DW, Le P, Yan J, Dowell JE, Xie Y. Predictors and impact of second-line chemotherapy for advanced non-small cell lung cancer in the United States: real-world considerations for maintenance therapy. J Thorac Oncol. 2011;6:365–71. doi: 10.1097/JTO.0b013e3181fff142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bajorin DF, Sarosdy MF, Pfister DG, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. J Clin Oncol. 1993;11:598–606. doi: 10.1200/JCO.1993.11.4.598. [DOI] [PubMed] [Google Scholar]
- 26.Bellmunt J, Ribas A, Eres N, et al. Carboplatin-based versus cisplatin-based chemotherapy in the treatment of surgically incurable advanced bladder carcinoma. Cancer. 1997;80:1966–72. doi: 10.1002/(sici)1097-0142(19971115)80:10<1966::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 27.Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin-versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst. 2007;99:847–57. doi: 10.1093/jnci/djk196. [DOI] [PubMed] [Google Scholar]
- 28.Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Meta-analysis of randomized clinical trials comparing Cisplatin to Carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:3852–9. doi: 10.1200/JCO.2004.02.109. [DOI] [PubMed] [Google Scholar]
- 29.Spinler SA, Nawarskas JJ, Boyce EG, Connors JE, Charland SL, Goldfarb S. Predictive performance of ten equations for estimating creatinine clearance in cardiac patients. Iohexol Cooperative Study Group. Ann Pharmacother. 1998;32:1275–83. doi: 10.1345/aph.18122. [DOI] [PubMed] [Google Scholar]
- 30.Beck CL, Pucino F, Carlson JD, et al. Evaluation of creatinine clearance estimation in an elderly male population. Pharmacotherapy. 1988;8:183–8. doi: 10.1002/j.1875-9114.1988.tb04072.x. [DOI] [PubMed] [Google Scholar]
- 31.Rasco DW, Yan J, Xie Y, Dowell JE, Gerber DE. Looking beyond surveillance, epidemiology, and end results: patterns of chemotherapy administration for advanced non-small cell lung cancer in a contemporary, diverse population. J Thorac Oncol. 2010;5:1529–35. doi: 10.1097/JTO.0b013e3181e9a00f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel JD, Bach PB, Kris MG. Lung cancer in US women: a contemporary epidemic. JAMA. 2004;291:1763–8. doi: 10.1001/jama.291.14.1763. [DOI] [PubMed] [Google Scholar]
- 33.O’Connell JP, Kris MG, Gralla RJ, et al. Frequency and prognostic importance of pretreatment clinical characteristics in patients with advanced non-small-cell lung cancer treated with combination chemotherapy. J Clin Oncol. 1986;4:1604–14. doi: 10.1200/JCO.1986.4.11.1604. [DOI] [PubMed] [Google Scholar]
- 34.Radzikowska E, Glaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Annals of Oncology. 2002;13:1087–93. doi: 10.1093/annonc/mdf187. [DOI] [PubMed] [Google Scholar]
- 35.Ahn MJ, Lee J, Park YH, et al. Korean ethnicity as compared with white ethnicity is an independent favorable prognostic factor for overall survival in non-small cell lung cancer before and after the oral epidermal growth factor receptor tyrosine kinase inhibitor era. J Thorac Oncol. 2010;5:1185–96. doi: 10.1097/JTO.0b013e3181e2f624. [DOI] [PubMed] [Google Scholar]
- 36.Kawaguchi T, Matsumura A, Fukai S, et al. Japanese ethnicity compared with Caucasian ethnicity and never-smoking status are independent favorable prognostic factors for overall survival in non-small cell lung cancer: a collaborative epidemiologic study of the National Hospital Organization Study Group for Lung Cancer Southern California Regional Cancer Registry databases. J Thorac Oncol. 2010;5:1001. doi: 10.1097/JTO.0b013e3181e2f607. (NHSGLC) in Japan and a 10. [DOI] [PubMed] [Google Scholar]
- 37.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 38.Yorio JT, Xie Y, Yan J, Gerber DE. Lung cancer diagnostic and treatment intervals in the United States: a health care disparity? J Thorac Oncol. 2009;4:1322–30. doi: 10.1097/JTO.0b013e3181bbb130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yorio JT, Yan J, Xie Y, Gerber DE. Socioeconomic Disparities in Lung Cancer Treatment and Outcomes Persist Within a Single Academic Medical Center. Clin Lung Cancer. 2012;13:448–57. doi: 10.1016/j.cllc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott HR, McMillan DC, Forrest LM, Brown DJ, McArdle CS, Milroy R. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer. 2002;87:264–7. doi: 10.1038/sj.bjc.6600466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montazeri A, Milroy R, Hole D, McEwen J, Gillis CR. Quality of life in lung cancer patients: as an important prognostic factor. Lung Cancer. 2001;31:233–40. doi: 10.1016/s0169-5002(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 42.Hutton JL, Martin L, Field CJ, et al. Dietary patterns in patients with advanced cancer: implications for anorexia-cachexia therapy. Am J Clin Nutr. 2006;84:1163–70. doi: 10.1093/ajcn/84.5.1163. [DOI] [PubMed] [Google Scholar]
- 43.Lindsey AM, Piper BF. Anorexia and Weight Loss: Indicators of Cacexia in Small Cell Lung Cancer. Nutr Cancer. 1985;7:65–76. doi: 10.1080/01635588509513841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.