Abstract
Abnormal social behavior is a hallmark of several human neuropsychiatric and neurodevelopmental disorders for which appropriate treatment is lacking. The zebrafish has been proposed as a tool with which these disorders may be modeled and their mechanisms analyzed. A potential starting point of such analyses is the identification of genetic differences between distinct zebrafish strains. Here we compare AB and TU, two well established zebrafish strains, and characterize the developmental trajectories of their shoaling (social) behavior and of the levels of dopamine, serotonin as well as a metabolite of each of these neurotransmitters, DOPAC and 5HIAA from whole brain extracts. Using a novel video-tracking software application, we demonstrate significant strain dependent changes in the maturation of shoaling between day 7 and day 87 post-fertilization. Using high-precision liquid chromatography specifically adapted to zebrafish, we uncover a significant age x strain interaction in dopamine and DOPAC that apparently correlates well with the behavioral differences found between the strains. We also report on strain differences in serotonin and 5HIAA. We discuss possible mechanistic analyses that will address causality and conclude that zebrafish will be a useful tool with which the neurobiological and genetic bases of social behavior may be analyzed in vertebrates.
Keywords: development of social behavior, dopamine, serotonin, shoaling, zebrafish
INTRODUCTION
The zebrafish is becoming an important model organism in behavioral brain research [1]. One prominent feature of zebrafish is their propensity to form groups, a social behavior termed shoaling [2, 3]. Numerous human neuropsychiatric and neurodevelopmental diseases are associated with abnormal social behavior [4–6], and the zebrafish has been suggested for modeling and the analysis of the mechanisms of such diseases [7–9]. This suggestion is not far fetched given the well documented translational relevance of this species [10–14]. Particularly, the nucleotide sequence of zebrafish genes has been found homologous enough to that of human genes to aid identification of orthologs between these two species in hundreds of genetic studies, and conserved syntenic chromosomal regions between zebrafish and human have also been identified [15].
The embryonic development of the zebrafish brain has been well studied [16, 17], and attempts to understand neuronal mechanisms underlying simple behaviors have also been successfully made using this species [18]. Recently, researchers have also started to map changes that occur after the first 5 days of development of zebrafish (embryonic and “larval” stages), i.e. after the fish reach free swimming stage [19, 20]. For example, Buske and Gerlai [19] have demonstrated that shoaling (forming tight groups) is practically absent for the first week of the free swimming stage of zebrafish but subsequently gradually develops, matures with age. Also importantly, another study has found that correlating with this behavioral change the dopaminergic and serotoninergic systems also mature, i.e. the levels (weight) of corresponding neurotransmitters and their metabolites increase relative to total brain protein weight [21].
A potentially fruitful way with which one can start the analysis of mechanisms underlying brain function and behavior is to identify differences among inbred strains. This approach has been utilized numerous times, for example, in the quantitative and molecular behavior genetic analysis of rodents [22, 23], which includes studies that characterized strain differences in mesolimbic and nigrostriatal dopamine binding sites [24], in dopamine transporter sites [25], and, for example, in the distribution of dopamine receptors [26]. Although most genetically well defined zebrafish strains are not pure bred, in some strains the percentage of homozygous loci is as much as 80 [27] and thus these strains, including the two studied here (i.e., AB and TU) may be appropriate for comparative analyses. Strain comparison studies have already demonstrated significant genetic differences in zebrafish. For example, Carvan et al. [28] have shown differential strain dependent survival when zebrafish were exposed to teratogens. Barba-Escobedo & Gould [29] detected strain differences in visual social preference and anxiety-like behaviors. Pan et al. [30] revealed strain differences in gene expression levels in and in neurochemical properties of the zebrafish brain. Also noteworthy is a study that showed dopamine receptor antagonism to-induce changes in the level of neurotransmitters in the brain of AB strain zebrafish but not in a genetically heterogeneous population called SF [31]. Interestingly, AB and SF zebrafish were also found to show significant differences in alcohol induced behavioral responses [32]. While AB showed significant acute and chronic alcohol treatment induced changes, including adaptation to extended exposure to alcohol both at the level of behavior and neurochemistry, SF was found to be buffered against such effects and showed no, or only significantly blunted, alcohol induced changes. These strain differences strongly suggest the role of genes in a range of brain and behavior related functions in zebrafish.
In the current paper we decided to analyze potential differences between two of the most frequently researched zebrafish strains, AB and TU with regard to how shoaling matures in these strains. Because large number of genetic markers has been identified for these strains, finding differences between the AB and the TU strains in this behavioral trait would allow one to conduct quantitative trait locus (QTL) analysis, which may lead one to the identification of gene(s) underlying the observed differences. Finding no differences between these strains would also be an important result. In the latter case, one could suggest a follow up analysis of F2 hybrids between these strains. If one found no segregation in the target phenotype (shoaling in this case) in such an F2 generation, one could utilize the parental strains in a mutagenesis study, for example employing ethyl nitroso urea (ENU), as the host of mutation and mapping strains for subsequent linkage analysis-based positional cloning. Thus, irrespective of the outcome of the comparison, we argue that characterization of these and other zebrafish strains is important.
In addition to analyzing the maturation of shoaling, we also decided to quantify potential age-dependent changes in the levels of dopamine, serotonin and a metabolite of each of these neurotransmitters. The rationale behind this was that previously embryonic alcohol exposure was shown both to disrupt shoaling and to impair the dopaminergic and the serotoninergic systems [33]. Furthermore, maturation of shoaling has also been found to be accompanied with increases in the levels of these neurochemicals in zebrafish [21]. The dopaminergic system has been implicated in shoaling in zebrafish [31,34] and the serotoninergic system is involved in fear in mammals and zebrafish, among other functions, and fear is believed to influence shoaling in zebrafish [34,35]. However, whether strain differences in the age-dependent changes in levels of these neurochemicals exist has not been analyzed.
METHODS
Animals and Housing
Zebrafish of the AB and TU strains were used in this experiment. Both strains originated from the Zebrafish International Research Centre (ZIRC, Eugene, OR). All fish used in the experiments were bred in our vivarium (University of Toronto Mississauga) and housed in groups of ten under identical conditions in the same holding room. Behavioral analysis was conducted by measuring the fish in shoals. Each shoal had ten fish, the same ten individuals that were housed together. The unit of statistical analysis for the behavioral data was the shoal and the sample size represents the number of shoals tested. We tested a total of 56 shoals, 28 for AB and 27 for TU, in our behavioral study in a longitudinal manner. That is, each shoal was followed throughout development. Gender could not visually be determined when testing began (7 days post-fertilization). However, the gender composition of each shoal was confirmed to be approximately 50–50% male-female. Fish were bred, raised and maintained as described before [19]. Briefly, the fish were housed using a recirculating ltration aquaculture rack system with mechanical, biological, and activated carbon (chemical) filtration and a UV sterilizing unit (Aquaneering Inc. (San Diego, Ca, USA). Water was maintained at 27 °C. The system water used on the rack and in the test tanks was reverse osmosis purified and was supplemented with 60mg/l Instant Ocean Sea Salt to achieve optimal water chemistry. Zebra sh were kept on a 12h light - 12h dark cycle with lights on at 7:00 h. All fish were fed twice daily with Larval Arti cial Plankton 100 (particle size below 100 μm, ZeiglerBros, Inc., Gardners, PA, USA) until 2 weeks post fertilization, and subsequently with nauplii of brine shrimp (Artemia salina) until 4 weeks post fertilization. Older and adult fish were fed twice daily with a 1:1 mixture of ake food (Tetramin Tropical sh ake food, Tetra Co, Melle, Germany) and powdered spirulina algae (Jehmco Inc., Lambertville, NJ, USA).
Behavioral testing
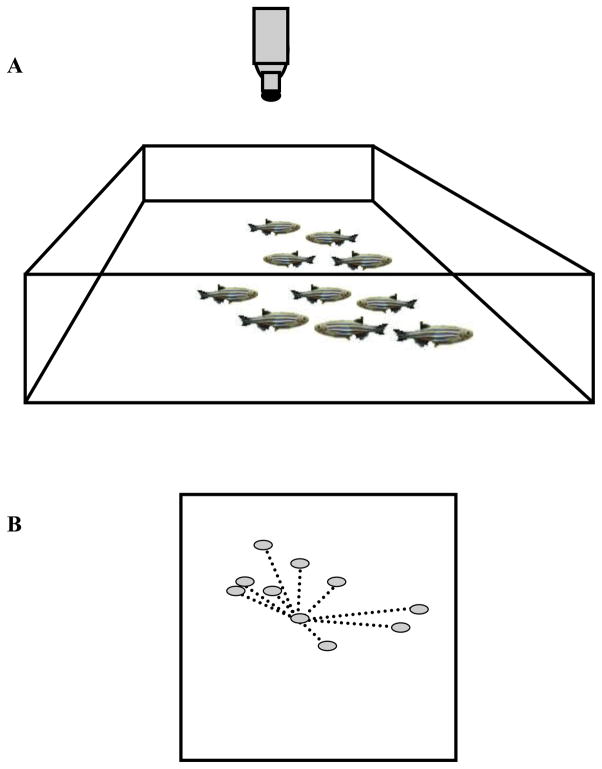
The linear dimensions of the test tank into which the group of ten experimental fish (the shoal) was placed were 28 times the body length of the zebrafish subject (figure 1). This tank/fish length ratio was found to be large enough to avoid forcing the fish artificially close to each other but small enough to allow proper detection of fish and thus proper quantification of their behavior [19, 36–38]. The order of testing the shoals was randomized.
Figure 1.
The experimental set up (panel A) and quantification of shoal cohesion (panel B). The experimental tank was a square bottom tank whose length and width were 28 times the body length of the experimental zebrafish. The ten zebrafish released into the tank for testing as well as the overhang camera are indicated. Panel B illustrates the method of quantification of inter-individual distance, a measure of shoal cohesion. The subjects are indicated by the grey ovals. A single focal fish is considered and its distances from its shoal members are indicated by the dotted lines. Note that in a ten member shoal nine distances are measured for the given focal fish and the average of these distances are calculated giving this focal fish a single inter-individual distance value. This procedure is conducted for all fish of the shoal. For further details of methods and data analysis see Methods.
First, fish were allowed to habituate to the experimental tank for 1 minute. Subsequently, an overhead video camera (JVC HD) was remotely turned on and the behavior of the shoal was recorded for 8 minutes. Testing took place between 10:00 and 17:00 h, i.e. during the middle of the light phase of the light cycle of the fish.
Each shoal was tested on post- fertilization day (dpf) 7, 23, 39, 55, 71 and 87, a longitudinal developmental analysis. A video-tracking software application, developed in-house, was employed to quantify numerous parameters of shoaling. This program is an automated video tracking system which is similar in principle and in its functionality to commercially available systems but it was specifically designed to be able to tracks multiple fish in the same arena (with a sampling rate of 29 hertz). The application outputted a file containing the xy coordinates of each fish. This raw data file was analyzed using the open source language “R”. For the current paper, we calculated the distance between each focal fish and all of its 9 shoal members in a given shoal (Inter-Individual Distance) as described before [2] (also see figure 1). We express inter-individual distance relative to body length. Similarly to keeping the linear dimensions of the test tank proportional to the length of the fish, shoal cohesion is also considered relative to body length because motor activity, speed of movement, i.e. the time to traverse a set distance is linearly proportional to the length of the animal ([2, 39] and references therein).
HPLC Analysis
For the high precision liquid chromatography (HPLC) analysis a separate set of fish were used. A longitudinal analysis could not be performed since sample taking required sacrificing the fish. HPLC was conducted at age 15, 40, 70 and 102 dpf. These age-points were chosen to approximate and encompass the developmental stages analyzed in our behavioral study. (Note that the 15 dpf stage is the earliest we can obtain sufficient amount and quality of brain tissue samples for HPLC analysis. Also note that after 87 dpf the fish are fully grown and mature.) Fish were decapitated rapidly at these time points and their brains were removed under a dissecting microscope and placed on ice as described before [40]. Brains were kept frozen in a microcentrifuge tube at −80°C) until further processing. To perform HPLC, the samples were thawed and suspended in artificial cerebral spinal fluid (ACSF, Harvard, 20μl/sample e.g. 20 μl per 5 brains of 15 dpf, 20 μl per 2 brains of 40 dpf, 20 μl per 1 brain of 70 dpf and 20 μl per 1 brain of 102 dpf). Brains were sonicated and 2μl of the solution was analyzed for protein content. 1μl of stabilizer was added to the sample and centrifuged. The supernatant was collected and stored at −80°C. HPLC analysis for dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), serotonin and 5- hydroxyindoleacetic acid (5-HIAA) of the supernatants was carried out using a BAS 460 MICROBORE HPLC system with electrochemical detection (Bio-analytical Systems Inc., West Lafayette, IN, USA) together with a Uniget C-18 reverse phase microbore column as the stationary phase (BASi, Cat no. 8949), a method specifically adapted to zebrafish and described before [41]. At 15 dpf 5 brains pooled per sample, at 40 dpf 2 brains pooled per sample, and at 70 and 102 dpf 1 brain per sample were sufficient to reach appropriate detection thresholds. The sample size (n= 8–11) indicated in the figure legends represents the number of samples (pooled for the younger fish) used per age group and not the number of individual brains used.
Statistical analysis
SPSS version 14 written for the PC was employed to conduct the analyses. The behavioral data were analyzed using repeated measure ANOVA with age as the repeated measure factor and strain as the between subject factor. The developmental analysis of neurochemicals was a cross sectional and not a longitudinal analysis (for this analysis the fish had to be sacrificed at the particular age) and accordingly a 2 factorial non-repeated ANOVA was used with Age and Strain as the between subject factors. In case of significant main effects or interactions, post-hoc Tukey Honestly Significant Difference (HSD) or t-tests with Bonferroni correction were conducted to minimize type-I error. Significance was accepted when the probability of null hypothesis was less than 5% (p < 0.05).
RESULTS
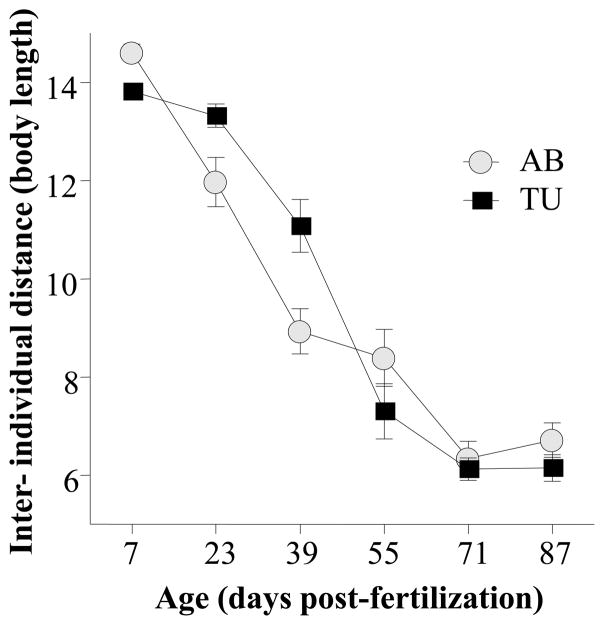
The current study confirmed previous findings and showed a robust age-dependent strengthening of shoaling in zebrafish. Figure 2 demonstrates that the inter-individual distance robustly decreased as zebrafish matured between 14 and 87 days post-fertilization. The figure also shows that the age-dependent increase of shoal cohesion was apparently different between the two zebrafish strains studied. These observations were confirmed by repeated measure ANOVA, which revealed a significant Age effect (F(5, 265) = 154.082, p < 0.001) and Age x Strain interaction (F(5, 265) = 5.935, p < 0.001) but found the Strain effect non-significant (F(1, 53) = 0.248, p > 0.60). Post hoc multiple range comparison tests including Tukey HSD analysis are inappropriate for repeated measures designs. To avoid committing type I error we compared the strains at every age using independent samples t-tests with Bonferroni correction. These analyses revealed that the inter-individual distance among shoal members was significantly higher in AB vs. TU at age 7 dpf (t = +3.485, df = 53, p < 0.01), was marginally lower in AB vs. TU at 23 dpf (t = −2.436, df = 53, p >= 0.05), and was significantly lower at 39 dpf (t = −3.031, df = 53, p < 0.05). The two strains were not significantly (p > 0.05) different at the older age points examined (55 to 87 dpf). Previously, using a Monte Carlo simulation run 10,000 times we estimated the average inter-individual distance for 10-member shoals in arenas whose linear dimensions were 28 times of the body length of the fish swimming in them [19] and found that in case of random distribution the average inter-individual distance (i.e. the mean of the distances between a focal fish and all the other shoal members) would be 14.6 body lengths. We compared each strain at each age point to this value using one sample t-tests with Bonferroni correction and found that fish of all ages except AB at 7 dpf (t = 0.033, df = 27, p > 0.95) were significantly (|t| > 5.408, df > 26, p < 0.05) below the value representing random chance.
Figure 2.
Strain dependent reduction of inter-individual distance among shoal members as zebrafish mature. Mean ± S.E.M. are shown. Black squares represent strain TU and grey circles strain AB. Note the quasi-linear decrease of inter-individual distance (strengthening of shoal cohesion) in AB zebrafish from 7 to 71 days post-fertilization, and also note the inverted S-shaped trajectory with the most rapid change between ages 39 and 55 days post-fertilization in TU zebrafish. Last, note that inter-individual distance is expressed relative to body length of the experimental subjects.
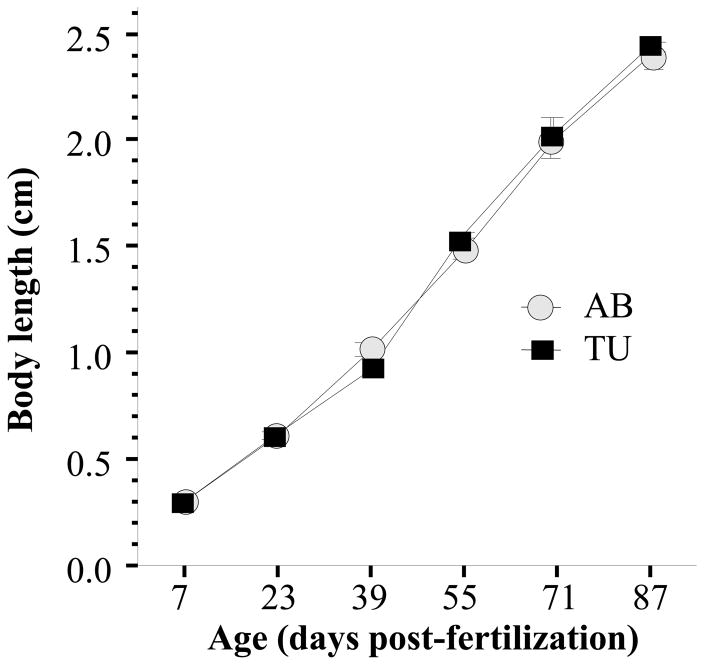
The strain differences in shoaling performance might be due to differential development or growth rates. If, for example, TU fish grew faster for some reason, their larger size would allow them to move faster, which itself could influence the distance they may keep from each other in the shoal. It is also possible that differential growth rate may directly influence the maturation of shoaling. To investigate if growth differences exist between the strains we measured the standard body length (from the tip of the nose to the base of the caudal fin) of all fish tested in our behavioral paradigm. The results are shown on figure 3 and they demonstrate apparently identical growth in the two strains. ANOVA confirmed this observation and found Age to have a highly significant effect Age F(5, 265) = 1135.6, p < 0.001). But no significant Age x Strain interaction (F(5, 265) = 1.465, p > 0.20) or Strain effects (F(1, 53) = 0.117, p > 0.70) were detected.
Figure 3.
Growth rate does not significantly differ between the AB and TU strains. Mean ± S.E.M. are of the standard length of the fish shown. Black squares represent strain TU and grey circles strain AB. Note the highly similar age dependent increase of body length of zebrafish in both strains.
As the development of shoaling was previously found to be accompanied by changes in levels of dopamine, serotonin and the metabolites of these neurotransmitters [21], and because alcohol has been shown to affect both shoaling and these neurochemicals in zebrafish [33], we compared the zebrafish strains AB and TU as to potential strain x age dependent changes in dopamine, DOPAC (dopamine’s metabolite), serotonin and 5HIAA (serotonin’s metabolite), respectively. Figure 4 shows the results we obtained for dopamine. The results suggest a robust and liner age-dependent increase in zebrafish of the AB strain and a different trajectory (step wise change between age 40 and 70 dpf) in zebrafish of the TU strain. These observations were confirmed by ANOVA, which revealed a significant Age (F(3, 60) = 97.708, p < 0.001) and Strain effect (F(1, 60) = 21.759, p < 0.001) as well as a significant Age x Strain interaction (F(3, 60) = 2.595, p < 0.001). Subsequent post hoc ANOVA with Tukey HSD test showed that in case of AB the age effect was indeed significant (F(3, 30) = 103.073, p < 0.001) and that every age group significantly differed from the other (p < 0.001). Although ANOVA also found a significant age-dependent increase for TU (F(3, 30) = 24.857, p < 0.001), Tukey HSD demonstrated what is apparent on figure 3: 15 vs. 40 dpf and 70 vs. 102 dpf fish of the TU strain do not significantly (p > 0.05) differ but 15 and 40 are significantly (p < 0.001) different from 70 and 102 dpf fish.
Figure 4.
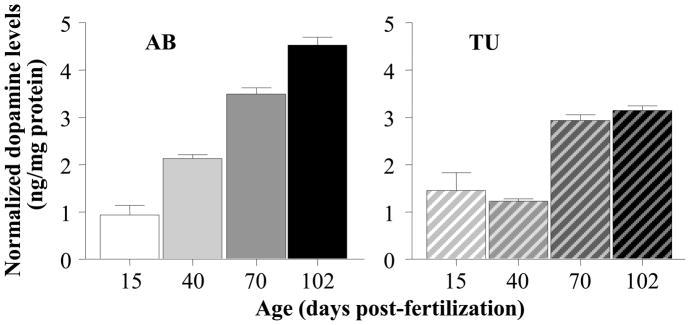
Strain dependent increase of normalized dopamine levels in the brain as zebrafish mature. Mean ± S.E.M. are shown. The solid colored bars on the left represent the results obtained for strain AB and the striped bars on the right the results obtained for strain TU. Note the linear increase of relative dopamine levels in AB zebrafish and the step wise increase between ages 40 and 70 days-post-fertilization in TU zebrafish. Last, note that dopamine levels are expressed as relative to total brain protein weight.
A similar pattern of age x strain dependent changes was observed in the level of DOPAC (figure 5). ANOVA found a significant Age effect (F(3, 60) = 24.166, p < 0.001) a significant Strain effect (F(1, 60) = 9.469, p < 0.01) and a significant interaction between these factors (F(3, 60) = 4.283, p < 0.01). Post hoc ANOVA conducted separately for the AB strain also found Age to have a significant effect (F(3, 30) = 20.820, p < 0.001) and Tukey HSD showed that all age group differences, except age groups 15 vs. 40 dpf and 70 vs. 102 dpf, were significant (p < 0.01). For TU, ANOVA also confirmed a significant Age effect (F(3, 30) = 8.472, p < 0.001). Tukey HSD showed that 15 dpf TU fish significantly (p < 0.05) differed from 70 dpf old fish, and 40 dpf fish significantly (p < 0.05) differed from 70 as well as from 102 dpf old, while other differences were found non-significant (p > 0.05).
Figure 5.
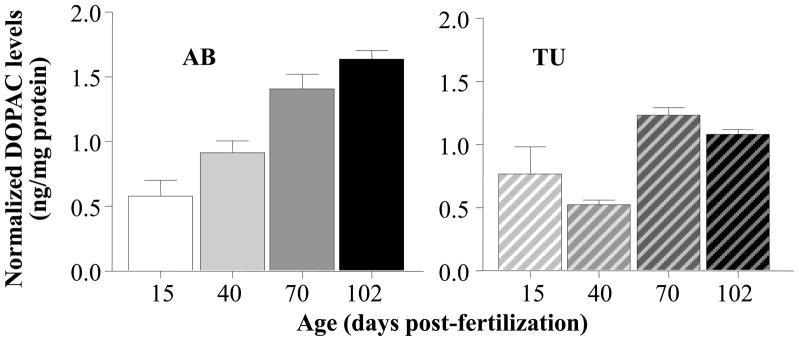
Strain dependent increase of normalized DOPAC levels in the brain as zebrafish mature. Mean ± S.E.M. are shown. The solid colored bars on the left represent the results obtained for strain AB and the striped bars on the right the results obtained for strain TU. Note the quasi-linear increase of relative DOPAC levels in AB zebrafish and the step wise increase between ages 40 and 70 days-post-fertilization in TU zebrafish. Last, note that DOPAC levels are expressed as relative to total brain protein weight.
HPLC analysis revealed a pattern of age-dependent change in serotonin different from what we found for dopamine and DOPAC (figure 6). Serotonin showed an apparent inverted U-shaped developmental trajectory with 70 dpf old fish having the highest level of this neurotransmitter. ANOVA confirmed a significant Age effect (F(3, 60) = 69.631, p < 0.001) and a significant Strain effect (F(1, 60) = 15.843, p < 0.01), but, in accordance with the similar developmental trajectory seen for both strains (figure 6), found the Age x Strain interaction non-significant (F(3, 60) = 1.670, p > 0.15). Subsequent Tukey HSD test showed that 15 dpf fish were significantly (p < 0.05) different from all other age groups in both strains, and that 70 dpf old fish were also significantly (p < 0.05) different from all other age groups in both strains, while other differences were not significant (p > 0.05).
Figure 6.
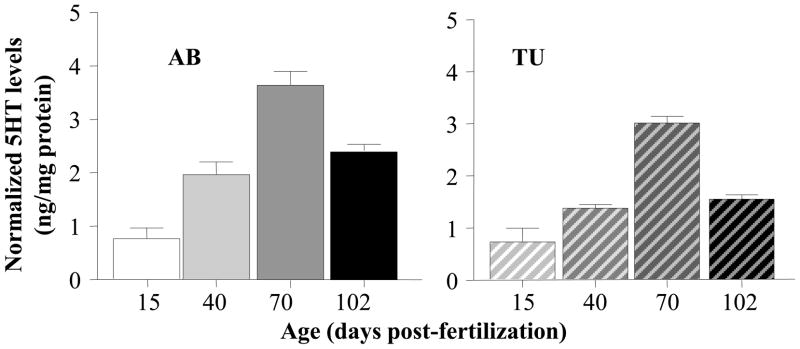
Age dependent changes of normalized serotonin levels in the zebrafish brain. Mean ± S.E.M. are shown. The solid colored bars on the left represent the results obtained for strain AB and the striped bars on the right the results obtained for strain TU. Note the-inverted U-shaped developmental trajectory in both strains. Also note that although less apparent, AB fish were found to exhibit significantly higher values compared to TU. Last, note that serotonin levels are expressed as relative to total brain protein weight.
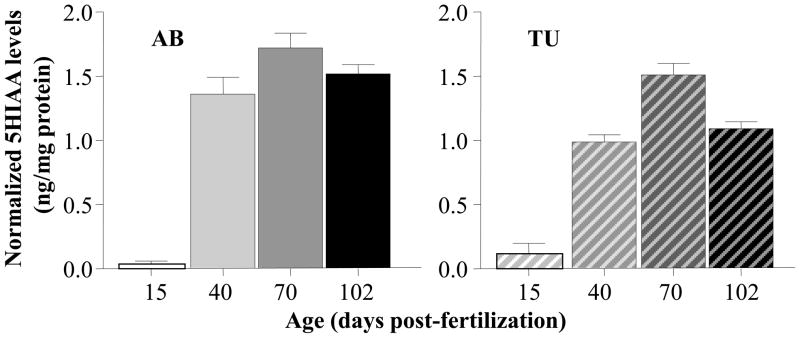
The metabolite of serotonin, 5HIAA showed a pattern of age dependent change similar to that of serotonin’s, with the 70 dpf old fish exhibiting the highest level in both strains (figure 7). ANOVA confirmed a significant Age effect (F(3, 60) = 116.325, p < 0.001), Strain effect (F(1, 60) = 13.942, p < 0.001) and unlike for serotonin, also found the Age x Strain interaction significant (F(3, 60) = 3.206, p < 0.05). Subsequent, post hoc ANOVA conducted separately for AB fish confirmed a significant Age effect for this strain (F(3, 30) = 59.160, p < 0.001) and the Tukey HSD test found age group 15 dpf to differ significantly (p < 0.001) from all other age groups, while these older age groups were found not to differ (p > 0.05) from each other. For TU the results were only slightly different. ANOVA found the Age effect significant (F(3, 30) = 60.798, p < 0.001) and Tukey HSD showed the 15 dpf fish to significantly (p < 0.05) differ from all other age groups but also detected a significant (p < 0.05) difference between the 70 dpf old fish as compared to all other age groups, while other age group differences were non-significant (p > 0.05).
Figure 7.
Age dependent changes of normalized 5HIAA levels in the zebrafish brain. Mean ± S.E.M. are shown. The solid colored bars on the left represent the results obtained for strain AB and the striped bars on the right the results obtained for strain TU. Note the-inverted U-shaped developmental trajectory in both strains. Also note that although less apparent, AB fish were found to exhibit significantly higher values compared to TU. Last, note that 5HIAA levels are expressed as relative to total brain protein weight.
DISCUSSION
The first unequivocal demonstration of maturation of shoaling in zebrafish was published only recently [19]. Our current paper reaffirms this previous finding and now also adds the new finding of significant strain dependent differences in the developmental trajectory of shoaling. Similarly to what was described before, we found a robust reduction of inter-individual distance among shoal members, i.e. age-dependent increase of shoal cohesion in zebrafish. From 7 days post-fertilization (2 days after reaching the free swimming stage) to 87 days post-fertilization (sexually mature young adult) zebrafish reduced their inter-individual distance within the studied ten-member shoals from about 14 body lengths to about 6–7 body lengths. It is notable that the experimental procedures used in the previous study [19] were identical to what we employed here (for example, there were 10 fish in each shoal, the linear dimensions of the test tank were 28x the body length of the subject, water chemistry was the same) and the strain this prior study used (AB) was also the same as one of the strains we measured here. Also notably, the similar findings were obtained despite the different methods of quantification of shoaling behavior employed by the previous and the current study [19]. The latter employed a manual tracking method and here we used a novel automated tracking software application.
Strain differences in the development of shoaling have not been demonstrated before. Here we provide evidence for such a strain difference. Although fish of both the AB and the TU strains showed a robust reduction of inter-individual distance with age, significant strain differences were detected at certain time points throughout the maturation of these fish. For example, as found before [19], AB fish exhibited inter-individual distances statistically indistinguishable from random chance at 7 dpf, whereas TU showed significantly shorter distances even at this young age. Thus, it appears that TU strain zebrafish shoal as early as 7 dpf, but AB fish do not. It is also notable that the developmental trajectory of shoaling was found steeper in AB as compared to TU. By the age of 23 dpf AB zebrafish started to form tighter shoals compared to TU, a trend that became significant by the age of 39 dpf. Last, after this age, i.e. by the age of 55 dpf, the two strains became indistinguishable and shoaled similarly. Given that the experimental subjects from both strains were bred, raised and maintained in an identical manner in the same vivarium room at the same time, and given that all fish were measured in a randomized, blind and identical manner and at the same time, we propose that the above differences observed in shoaling between these two distinct strains are due to genetic factors.
What may be these genetic factors and what biochemical and developmental mechanisms may underlie the observed behavioral differences are not known at this point. To start the investigation of such potential mechanisms we quantified levels of neurohemicals dopamine, DOPAC, serotonin and 5HIAA. One working hypothesis could be that as the brain matures the levels of all, including the above, neurochemicals should rise. However, this is not what we found. While the levels of dopamine and its metabolite, DOPAC, indeed increased with age, serotonin and its metabolite, 5HIAA, showed an inverted U-shaped age-trajectory with fish of 70 dpf exhibiting the highest value. Even more importantly, we detected significant strain differences in the neurochemical responses. Although fish of both the AB and TU strains showed the characteristic inverted U-shaped age trajectory in the measured serotoninergic neurochemicals, TU in general exhibited slightly (but significantly) smaller values as compared to AB. The strain differences in the dopaminergic responses were more robust and, most importantly, age-dependent. While AB showed a linear age-dependent increase in both dopamine and DOPAC levels, TU had a step-wise trajectory, a robust increase between 40 and 70 dpf but no significant change before or after this high-transition period.
The behavioral and neurochemical results of this study are only correlative. Nevertheless, one may hypothesize about potential causal relationships. For example, figure 2 shows that the developmental trajectory of shoaling in TU is S-shaped, i.e. it appears that these fish rapidly increased their shoaling tendency in between their ages 39 and 55 dpf, but the change was slower before and after this period. Unlike TU, AB fish appears to exhibit a more gradual and steady increase of shoaling tendency up to age 71 dpf. The rapid increase of shoaling seen in TU coincides well with the step wise increase of dopamine and DOPAC levels seen after 40 dpf. Also, the steady increase of shoaling seen in AB coincides well with the linear age-dependent increase of dopamine and DOPAC obtained for this strain. Granted, the demonstrated strain differences in the serotoninergic system may also influence shoaling behavior. Furthermore, there may be other neurotransmitter systems and several biochemical processes and neurobiological mechanisms underlying shoaling that may drive or influence the observed behavioral differences between AB and TU. Nevertheless, our data are in line with recent results suggesting that the dopaminergic system plays important roles in shoaling in zebrafish.
For example, a D1-R antagonist (SCH23390) has been shown to disrupt shoaling when administered acutely to adult zebrafish [31], and images of moving zebrafish were found to elicit significant increases in dopamine and DOPAC levels but not in serotonin or 5HIAA [34]. Also notably, the sight of conspecifics was shown to be rewarding in learning tasks developed for zebrafish [41,42]. The dopaminergic system has long been known to be involved in reward and, for example, increased dopaminergic neurotransmission is believed to underlie the rewarding properties of drugs of abuse [43]. Alcohol is a drug of abuse known to interact with a number of neurotransmitter systems and a large number of biochemical processes in multiple vertebrate species. But it has also been shown to disrupt both shoaling and influence the dopaminergic system in zebrafish [32]. When administered acutely, this substance was found to increase dopamine and DOPAC levels in a linearly dose dependent manner in AB zebrafish, and withdrawal from this substance after chronic exposure was also found to elevate dopamine and DOPAC levels [32]. Perhaps even more relevant to the current results are the studies that characterized developmental changes in shoaling as well as neurochemical responses in zebrafish. For example, linear age-dependent increase of shoaling in AB zebrafish was shown to be accompanied by a similarly linear increase of dopamine and DOPAC levels [21]. But these same authors found a rapid increase of the levels of serotonin and 5HIAA between 45 to 50 dpf peaking around 70 dpf, an age-dependent trajectory that correlated less well with the behavioral changes but was very similar to what we are reporting in the current study for the same (AB) strain of zebrafish. Also notably, another study [33] found that the effect of embryonic alcohol treatment correlated well between the ensuing impairment in shoaling and reduced dopamine and DOPAC levels but less so with the detected changes in the serotoninergic system. Although the above findings do implicate the dopaminergic system in the development of shoaling in zebrafish, we emphasize that the results do not prove a causal link between these phenomena.
Our current findings may be utilized in a number of ways to investigate potential causal relationships between the observed developmental changes in behavior and neurochemistry of the zebrafish brain. The strain differences demonstrated here now make it possible for one to conduct quantitative trait locus mapping. For example, one could generate an F2 segregating generation between the AB and TU strains and study linkage and segregation in this population. This analysis could accomplish two goals. One, the investigator could address whether the behavioral and neurochemical characteristics co-segregate with each other. Two, he/she could identify genetic markers that co-segregate with the trait(s) in question, which will facilitate the identification of genes that underlie the differences between the strains in the studied behavioral and neurochemical characteristics. One can also foresee a selection experiment that could be started from the F2 segregating generation. An artificial selection study could develop highly divergent lines that exhibit robust differences in the selected trait, for example, in the speed of maturation of shoaling, and such lines would allow one to study correlated changes in neurochemical responses. More direct manipulations targeting specific genes associated with particular neurotransmitter systems during early development using morpholinos would also be feasible [44]. Mapping of the neuroanatomical locale of gross or microstructural or gene expression level differences between the strains throughout the maturation of shoaling will also be informative. Clearly, a lot will have to be done before one will understand the mechanisms of shoaling in general and the mechanisms underlying the differences between these two strains in shoaling in particular. Nevertheless, our results now demonstrate that such studies are feasible: we argue that the now discovered genetic differences in the development of shoaling in zebrafish open new avenues for the analysis of these mechanisms. This brings us to the last point we wish to discuss: the potential translational relevance of these analyses.
Teleost fish and mammals are separated by at least 300 million years of biological evolution [45] and thus one may argue that analysis of zebrafish cannot yield any insights into the biology of our own species. However, the zebrafish has been proposed and proven to be an excellent laboratory organism for the modeling and the analysis of the potential mechanisms of a broad spectrum of human disorders from autism and schizophrenia through addiction to eye and cardiovascular diseases, to mention but a few examples [7, 8, 11–13]. Why is this so? It is because especially at the nucleotide sequence level of DNA one finds significant homologies between zebrafish and humans, and hundreds of zebrafish studies utilized this for the identification of human and zebrafish orthologs. Furthermore, it is often found that the homology extends beyond nucleotide sequences of the sister genes and reaches higher levels of the biological organization biologists would consider “function”. Given that the speed of discovery may be higher with the small and prolific zebrafish kept under the controlled laboratory environment and studied with the help of sophisticated recombinant DNA technologies [16, 46], we argue that this reductionist approach does have a place in medically oriented research.
The biological mechanisms of social behavior in humans are far from understood and there is a plethora of neuropsychiatric and neurodevelopmental CNS disorders that are associated with, or whose core symptoms include, abnormal social behavior [47]. Autism spectrum disorders and schizophrenia are but only two examples of such diseases [5, 6, 48]. Despite their high prevalence, these latter diseases have been particularly difficult to treat because of the lack of understanding of their mechanisms. Even diagnosis or identification of individuals suffering from these CNS disorders has been difficult because at the clinical, i.e. behavioral phenotypical, level these diseases manifest as a range with varying severity [49, 50]. It is too early to tell whether analysis of zebrafish social behavior will yield biomarkers, alleles of genes and their protein products, which may be utilized as diagnostic markers leading to earlier intervention for patients suffering from autism or schizophrenia, but we hope that in addition to mechanistic insights, zebrafish research will increase our knowledge in this direction too.
Highlights.
Two zebrafish strains (AB & TU) differ in the maturation of shoaling
Dopamine levels in the brain increase differently with age in the two strains
DOPAC levels in the brain increase differently with age in the two strains
Serotonin & 5HIAA levels show no Age x Strain interaction
But the strains differ in overall serotonin and 5HIAA levels
Acknowledgments
We would like to thank Dr. James McCrea for developing our video-tracking software. This study was supported by an NIH/NIAAA grant awarded to RG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gerlai R. Using Zebrafish to unravel the genetics of complex brain disorders. Current Topics in Behavioral Neurosciences. 2012;12:3–24. doi: 10.1007/7854_2011_180. [DOI] [PubMed] [Google Scholar]
- 2.Miller N, Gerlai R. Quantification of shoaling behaviour in zebrafish (Danio rerio) Behavioural Brain Research. 2007;184:157–166. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Peichel CL. Social behavior: how do fish find their shoal mate? Current Biology. 2004;14:R503–4. doi: 10.1016/j.cub.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 4.Brady LS, Mansbach RS, Skurdal DN, Muldoon SM, Barrett JE. Reversal of the antinociceptive effects of centrally-administered morphine by the benzodiazepine receptor antagonist Ro 15-1788. Life Sciences. 1984;35:2593–2600. doi: 10.1016/0024-3205(84)90026-2. [DOI] [PubMed] [Google Scholar]
- 5.Grossman JB, Carter A. Social behavior in autism. Annals of the New York Academy of Science. 2006;807:440–454. doi: 10.1111/j.1749-6632.1997.tb51938.x. [DOI] [PubMed] [Google Scholar]
- 6.Morrison RL, Bellack AS. Social functioning of schizophrenic patients: clinical and research issues. Schizophrenia Bulletin. 1987;13:715–725. doi: 10.1093/schbul/13.4.715. [DOI] [PubMed] [Google Scholar]
- 7.Tropepe V, Sive HL. Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes, Brain and Behavior. 2003;2:268–281. doi: 10.1034/j.1601-183x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 8.Morris JA. Zebrafish: a model system to examine the neurodevelopmental basis of schizophrenia. Progress in Brain Research. 2009;179:97–106. doi: 10.1016/S0079-6123(09)17911-6. [DOI] [PubMed] [Google Scholar]
- 9.Norton W, Bally-Cuif L. Adult zebrafish as a model organism for behavioural genetics. BioMed Central Neuroscience. 2010;11:90. doi: 10.1186/1471-2202-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan CH. Zebrafish behavioural assays of translational relevance for the study of psychiatric disease. Reviews in the Neurosciences. 2011;22:37–48. doi: 10.1515/RNS.2011.006. [DOI] [PubMed] [Google Scholar]
- 11.Klee EW, Schneider H, Clark KJ, Cousin MA, Ebbert JO, Hooten WM, et al. Zebrafish: a model for the study of addiction genetics. Human Genetics. 2012;131:977–1008. doi: 10.1007/s00439-011-1128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurer CM, Huang Y-Y, Neuhauss SCF. Application of zebrafish oculomotor behavior to model human disorders. Reviews in the Neurosciences. 2011;22:5–16. doi: 10.1515/RNS.2011.003. [DOI] [PubMed] [Google Scholar]
- 13.Bakkers J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovascular Research. 2011;91:279–288. doi: 10.1093/cvr/cvr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodd A, Curtis PM, Williams LC, Love DR. Zebrafish: bridging the gap between development and disease. Human Molecular Genetics. 2000;9:2443–2449. doi: 10.1093/hmg/9.16.2443. [DOI] [PubMed] [Google Scholar]
- 15.Woods IG, Kelly PD, Chu F, Ngo-Hazelett P. A comparative map of the zebrafish genome. Genome Research. 2000;10:1903–1914. doi: 10.1101/gr.10.12.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driever W, Stemple D, Schier A, Solnica-Krezel L. Zebrafish: genetic tools for studying vertebrate development. Trends in Genetics. 1994;10:152–159. doi: 10.1016/0168-9525(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 17.Fulwiler C, Gilbert W. Zebrafish embryology and neural development. Current Opinion in Cell Biology. 1991;3:988–991. doi: 10.1016/0955-0674(91)90118-i. [DOI] [PubMed] [Google Scholar]
- 18.Ahrens MBM, Li JMJ, Orger MBM, Robson DN, Schier AF, Engert F, et al. Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature. 2012;485:471–477. doi: 10.1038/nature11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buske C, Gerlai R. Shoaling develops with age in Zebrafish (Danio rerio) Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:1409–1415. doi: 10.1016/j.pnpbp.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engeszer RE, Da Barbiano LA, Ryan MJ, Parichy DM. Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio. Animal Behaviour. 2007;74:1269–1275. doi: 10.1016/j.anbehav.2007.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buske C, Gerlai R. Maturation of shoaling behavior is accompanied by changes in the dopaminergic and serotoninergic systems in zebrafish. Developmental Psychobiology. 2012;54:28–35. doi: 10.1002/dev.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen PV, Gerlai R. Behavioural and physiological characterization of inbred mouse strains: prospects for elucidating the molecular mechanisms of mammalian learning and memory. Genes, Brain and Behavior. 2002;1:72–81. doi: 10.1034/j.1601-183x.2002.10202.x. [DOI] [PubMed] [Google Scholar]
- 23.Wehner JM, Radcliffe RA, Bowers BJ. Quantitative genetics and mouse behavior. Annual Reviews Neuroscience. 2001;24:845–867. doi: 10.1146/annurev.neuro.24.1.845. [DOI] [PubMed] [Google Scholar]
- 24.Boehme RE, Ciaranello RD. Dopamine receptor binding in inbred mice: strain differences in mesolimbic and nigrostriatal dopamine binding sites. Proceedings of the National Academy of Science of the United States of America. 1981;78:3255–3259. doi: 10.1073/pnas.78.5.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiao X, Paré WP, Tejani-Butt S. Strain differences in the distribution of dopamine transporter sites in rat brain. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27:913–919. doi: 10.1016/S0278-5846(03)00150-7. [DOI] [PubMed] [Google Scholar]
- 26.Yaroslavsky I, Colletti M, Jiao X, Tejani-Butt S. Strain differences in the distribution of dopamine (DA-2 and DA-3) receptor sites in rat brain. Life Sciences. 2006;79:772–776. doi: 10.1016/j.lfs.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 27.Guryev V. Genetic variation in the zebrafish. Genome Research. 2006;16:491–497. doi: 10.1101/gr.4791006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carvan MJ, III, Loucks E, Weber DN, Williams FE. Ethanol effects on the developing zebrafish: neurobehavior and skeletal morphogenesis. Neurotoxicology and Teratology. 2004;26:757–768. doi: 10.1016/j.ntt.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Barba-Escobedo PA, Gould GG. Visual social preferences of lone zebrafish in a novel environment: strain and anxiolytic effects. Genes, Brain and Behavior. 2012;11:366–373. doi: 10.1111/j.1601-183X.2012.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan Y, Chatterjee D, Gerlai R. Strain dependent gene expression and neurochemical levels in the brain of zebrafish: Focus on a few alcohol related targets. Physiology & Behavior. 2012;107:773–780. doi: 10.1016/j.physbeh.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scerbina T, Chatterjee D, Gerlai R. Dopamine receptor antagonism disrupts social preference in zebrafish: a strain comparison study. Amino Acids. 2012;43:2059–2072. doi: 10.1007/s00726-012-1284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerlai R, Chatterjee D, Pereira T, Sawashima T, Krishnannair R. Acute and chronic alcohol dose: population differences in behavior and neurochemistry of zebrafish. Genes, Brain and Behavior. 2009;8:586–599. doi: 10.1111/j.1601-183X.2009.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buske C, Gerlai R. Early embryonic ethanol exposure impairs shoaling and the dopaminergic and serotoninergic systems in adult zebrafish. Neurotoxicology and Teratology. 2011;33:698–707. doi: 10.1016/j.ntt.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saif M, Chatterjee D, Buske C, Gerlai R. Sight of conspecific images induces changes in neurochemistry in zebrafish. Behavioural Brain Research. 2013 doi: 10.1016/j.bbr.2013.01.020. (in press) doi.org/10.1016/j.bbr.2013.01.020. [DOI] [PMC free article] [PubMed]
- 35.Popova NK. From gene to aggressive behavior: the role of brain serotonin. Neuroscience and Behavioral Physiology. 2008;38:471–475. doi: 10.1007/s11055-008-9004-7. [DOI] [PubMed] [Google Scholar]
- 36.Gallego A, Heath MR. The development of schooling behaviour in Atlantic herring Clupea harengus. Journal of Fish Biology. 1994;45:569–588. [Google Scholar]
- 37.Hale ME. Locomotor mechanics during early life history: effects of size and ontogeny on fast-start performance of salmonid fishes. Journal of Experimental Biology. 1999 doi: 10.1242/jeb.202.11.1465. [DOI] [PubMed] [Google Scholar]
- 38.Kimmel CBC, Patterson JJ, Kimmel ROR. The development and behavioral characteristics of the startle response in the zebra fish. Developmental Psychobiology. 1974;7:47–60. doi: 10.1002/dev.420070109. [DOI] [PubMed] [Google Scholar]
- 39.Miller N, Gerlai R. Oscillations in shoal cohesion in Zebrafish (Danio rerio) Behavioural Brain Research. 2008;193:148–151. doi: 10.1016/j.bbr.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chatterjee D, Gerlai R. High precision liquid chromatography analysis of dopaminergic and serotoninergic responses to acute alcohol exposure in zebrafish. Behavioural Brain Research. 2009;200:208–213. doi: 10.1016/j.bbr.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Imari L, Gerlai R. Sight of conspecifics as reward in associative learning in zebrafish (Danio rerio) Behavioural Brain Research. 2008;189:216–219. doi: 10.1016/j.bbr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Pather S, Gerlai R. Shuttle box learning in zebrafish (Danio rerio) Behavioural Brain Research. 2009;196:323–327. doi: 10.1016/j.bbr.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fibiger HCH, Phillips AGA. Mesocorticolimbic dopamine systems and reward. Annals of the New York Academy of Science. 1988;537:206–215. doi: 10.1111/j.1749-6632.1988.tb42107.x. [DOI] [PubMed] [Google Scholar]
- 44.Bill BR, Petzold AM, Clark KJ, Schimmenti LA, Ekker SC. A primer for morpholino use in zebrafish. Zebrafish. 2009;6:69–77. doi: 10.1089/zeb.2008.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohno S, Wolf U, Atkin NB. Evolution from fish to mammals by gene duplication. Hereditas. 1968;59:169–187. doi: 10.1111/j.1601-5223.1968.tb02169.x. [DOI] [PubMed] [Google Scholar]
- 46.Gerlai R. High-throughput behavioral screens: the first step towards finding genes involved in vertebrate brain function using zebrafish. Molecules. 2010;15:2609–2622. doi: 10.3390/molecules15042609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anckarsäter H. Central nervous changes in social dysfunction: autism, aggression, and psychopathy. Brain Research Bulletin. 2006;69:259–265. doi: 10.1016/j.brainresbull.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Tantam D. Characterizing the fundamental social handicap in autism. Acta Paedopsychiatry. 1992;55:83–91. [PubMed] [Google Scholar]
- 49.Kendell RE. Schizophrenia: the remedy for diagnostic confusion. Br Journal of Psychiatry. 1975:11–17. [PubMed] [Google Scholar]
- 50.Volkmar FR, State M, Klin A. Autism and autism spectrum disorders: diagnostic issues for the coming decade. Journal of Child Psychological and Psychiatry. 2009;50:108–115. doi: 10.1111/j.1469-7610.2008.02010.x. [DOI] [PubMed] [Google Scholar]