Abstract
Purpose
Peritumoral edema may potentially harbor sarcoma cells. The extent of suspicious edema (SE) included in the treatment volume is subject to clinical judgment, balancing the risk of missing tumor cells with excess toxicity. Our goal was to determine variability in SE delineation by sarcoma radiation oncologists (RO).
Material and Method
Twelve expert ROs were provided with T1 Gadolinium and T2-weighted MR images of 10 patients with high-grade extremity soft tissue sarcoma. Gross tumor volume, CTV3cm (3cm longitudinal and 1.5cm radial margin) and CTV2cm (2cm longitudinal and 1cm radial margin) were contoured by a single observer. Suspicious peritumoral edema, defined as abnormal signal on T2 images, was independently delineated by all 12 ROs. Contouring agreement was analyzed using the Simultaneous Truth and Performance Level Estimation (STAPLE) algorithm and kappa statistics.
Results
The mean volumes of GTV, CTV2cm and CTV3cm were respectively 130 cm3 (7–413 cm3), 280cm3 and 360cm3. The mean consensus volume computed using the STAPLE algorithm at 95% confidence interval was 188cm3 (24–565cm3) with a substantial overall agreement corrected for chance (mean kappa =0.71; range: 0.32–0.87). The minimum, maximum and mean volume of suspicious edema (excluding the GTV) were 4cm3, 182cm3 and 58 cm3 (representing a median of 29% of the GTV volume). The median volume of suspicious edema not included in the CTV2cm and in the CTV3cm was 5 cm3 and 0.3cm3 respectively. There were 3 large tumors with >30cm3 of suspicious edema not included in the CTV3cm volume.
Conclusion
Despite the fact that SE would empirically seem to be a more subjective volume, a substantial or near-perfect inter-observer agreement was observed in SE delineation in most cases with high-grade STS of the extremity. A median of 97% of the consensus SE is within the CTV2cm (99.8% within the CTV3cm). In a minority of cases, however, significant expansion of the CTVs is required to cover SE.
Introduction
Preoperative radiotherapy followed by limb-preserving surgery is a standard treatment option now favored for most primary extremity soft tissue sarcomas (STS). Although it carries a higher risk of serious wound complications, pre-operative radiotherapy is preferred over post-operative radiotherapy as it is associated with lower rates of late fibrosis, joint stiffness and limb edema (1, 2). Larger radiation fields result in greater rates of late toxicity (2). These toxicities translate in the longer term into lower limb function scores and decreased quality of life.
The growing availability of newer radiotherapy techniques including intensity modulated radiotherapy (IMRT) and image-guided radiotherapy (IGRT) has lead to an increased interest in reducing radiation treatment volume to limit morbidity from treatment toxicities. Two multi-center studies are evaluating the effect of radiation volume reduction: the phase III two-armed VORTEX United Kingdom trial that compares conventional radiotherapy to reduced field radiotherapy in the postoperative setting and the recently completed RTOG 0630 phase II trial of IGRT for soft tissue sarcoma of the extremity. The primary objective of the RTOG trial is to determine the effect of a reduced radiation volume on ≥ grade 2 lymphedema, subcutaneous fibrosis, and joint stiffness at 2 years from the treatment. The COG ARST 0332 is another ongoing trial that uses limited CTV margins in young patients (30 years old and less) requiring radiotherapy.
Soft tissue sarcoma grows in a centrifugal manner with compression of peripheral cells in parallel layers (3). Secondary adjacent normal tissue atrophy often gives the gross appearance of a pseudocapsule surrounding the tumor (3, 4). Beyond the pseudocapsule there can be a “reactive zone” which is characterized by neovascularization, edema and possibly satellite tumor cells (4). On MR imaging, this will correspond to a variable degree of peritumoral T2 weighted signal changes (3, 5). Although not a statistically significant finding, White et al. described a strong correlation between peri-tumoral edema and microscopic tumor deposits. In their series of 15 patients (6), tumor cells were found beyond the tumor margin/capsule in 10 out of the 15 patients at a distance of up to 4 cm. In 9 of the 10 cases with tumor beyond the primary tumor mass, the tumor cells were identified histologically in areas with corresponding high T2-weighted signal changes on MRI. In the era of IGRT and IMRT, volume reduction to achieve healthy tissue sparing therefore needs to be weighed against the risk of missing tumor cells. Moreover, interpretation of MRI findings and contouring of SE for inclusion in the treatment volume is frequently rather subjective.
Whereas the wider margins used in conventional post-operative radiotherapy will generally include SE volume, the experimental use of reduced margins may require additional CTV expansions. In this context, interpretation of SE could become a significant source of contouring disagreement. The goal of this study was to compare the delineation of suspicious edema among sarcoma radiation oncologists from the protocol participant centers in order to quantify variations in the inclusion of peritumoral edema in the clinical target volume (CTV).
Method
Diagnostic gadolinium enhanced T1-weighted and T2-weighted MR images from 10 patients with primary STS of the extremity were provided to twelve experienced sarcoma radiation oncologists. The images were all obtained with the patients in supine position. All tumors were intermediate or high grade. The sarcoma subtypes are listed in Table 1. Mean tumor size was 6 cm (range: 3–9 cm). The GTV was pre-contoured by a single observer in each MR series using the gadolinium enhanced T1-weighted images. T1 and T2 images shared common DICOM coordinates and were thus pre-registered. The data sets were anonymized and made available for download from the Image-Guided Therapy QA Center (ITC) website. Participating physicians were provided with case histopathology, MR images with pre-contoured GTVs and instructions for suspicious edema delineation. They were asked to delineate on the T2 images the suspicious edema they would include in the CTV in order to comply with RTOG 0630 protocol guidelines. The contouring was performed using each participant’s institutional treatment planning system. The resulting DICOM-RT contours were then returned to the ITC for central analysis.
Table 1.
Study Cases
| Case | Histopathology | Limb | Max diameter (cm) |
GTV (cm3) |
|---|---|---|---|---|
| Case 1 | Leiomyosarcoma | Distal lower | 3.1 | 7 |
| Case 2 | Round cell liposarcoma | Distal lower | 7.5 | 169 |
| Case 3 | Malignant peripheral nerve sheath tumor | Proximal lower | 6.8 | 165 |
| Case 4 | Pleomorphic liposarcoma | Distal lower | 6.2 | 132 |
| Case 5 | Malignant peripheral nerve sheath tumor | Proximal lower | 5.1 | 51 |
| Case 6 | Malignant peripheral nerve sheath tumor | Proximal upper | 6.2 | 133 |
| Case 7 | Myxofibrosarcoma | Distal upper | 7.1 | 128 |
| Case 8 | Leiomyosarcoma | Proximal upper | 4.2 | 20 |
| Case 9 | Pleomorphic leiomyosarcoma | Proximal lower | 9.0 | 413 |
| Case 10 | Undifferentiated pleomorphic sarcoma | Proximal lower | 5.4 | 83 |
For the purpose of analysis, two CTV contours were delineated by a single observer per RTOG 0630 guidelines (7). A CTV3cm was created using a 3 cm proximal and distal expansion with a 1.5 cm radial margin, and a CTV2cm was created using a 2 cm proximal and distal margin and a 1 cm radial margin (Figures 1 and 2). The larger 3 cm was used for >8cm intermediate-to-high grade tumors in RTOG 0630 and the RTOG consensus CTV definition, and the smaller 2 cm margin was used for low grade or small tumors on RTOG 0630.
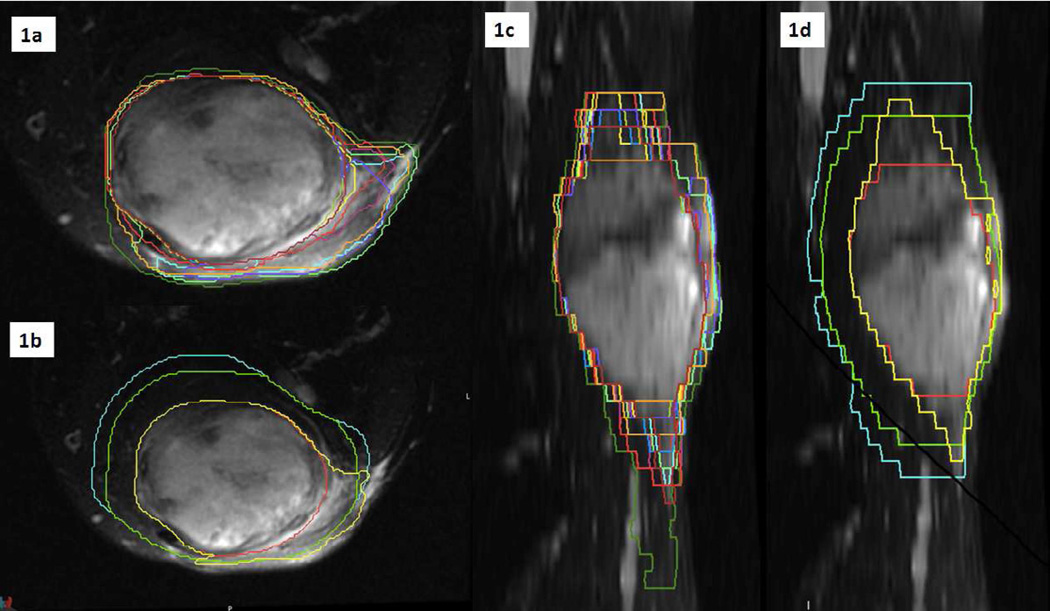
Figure 1. Case 3: Representation of volumes on T2-weighted MRI.
Fig 1a: Individual contours in coronal view. Fig 1b: GTV (red), CTV2cm (green), CTV3cm (cyan) and STAPLE95 (yellow) in coronal view. Fig 1c: Individual contours in sagittal view. Fig 1d: GTV (red), CTV2cm (green), CTV3cm (cyan) and STAPLE95 (yellow) in sagittal view. CTV2cm= GTV+ (2 cm proximal/distal margin and 1 cm radial margin). CTV3cm= GTV+ (3 cm proximal/distal margin and 1.5 cm radial margin).
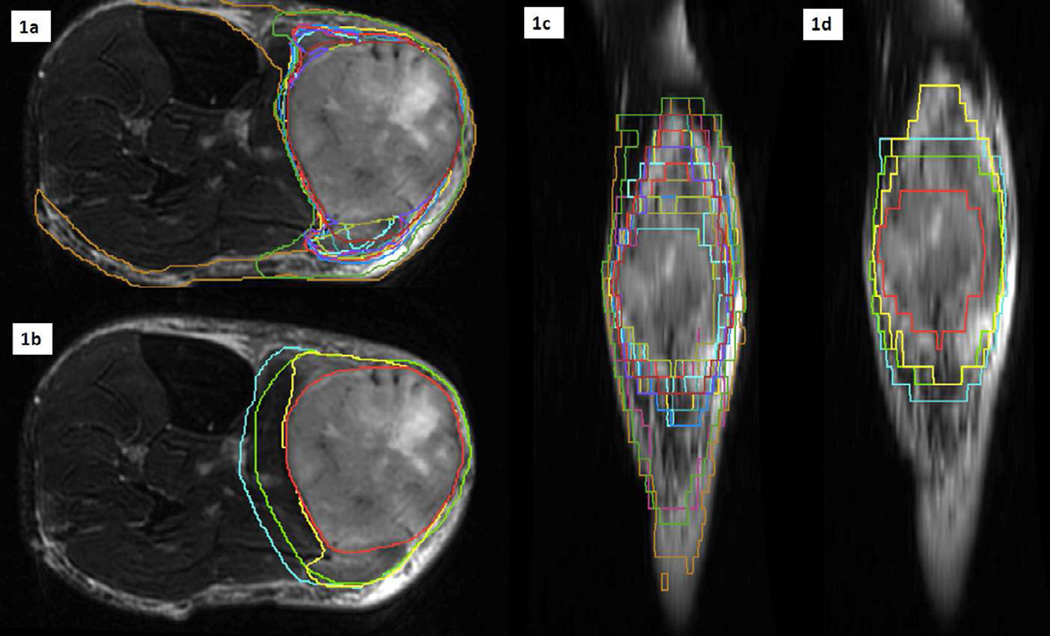
Figure 2. Case 4: Representation of volumes on T2-weighted MRI.
Fig 2a: Individual contours in coronal view. Fig 2b: GTV (red), CTV2cm (green) and CTV3cm (cyan) and STAPLE95 (yellow) in coronal view. Fig 2c: Individual contours in sagittal view. Fig 2d: GTV (red), CTV2cm (green), CTV3cm (cyan) and STAPLE95 (yellow) in sagittal view. CTV2cm= GTV+ (2 cm proximal/distal margin and 1 cm radial margin). CTV3cm= GTV+ (3 cm proximal/distal margin and 1.5 cm radial margin).
Suspicious edema contours and CTV contours were analyzed using the Computerized Environment for Radiation Research environment (8). Three statistical tools were used to measure agreements between physicians: the apparent agreement, the kappa algorithm and the expectation-maximization algorithm for simultaneous truth and performance level estimation (STAPLE). The apparent agreement represents the overlap contour obtained by average agreement probability of a voxel selection by the radiation oncologists (9). The kappa algorithm is an inter-rater metric that corrects for agreement that could be obtained by chance and is calculated as:
Kappa= (Apparent agreement−Chance Agreement)/(1−Chance agreement)
Kappa metrics values are between −1 and 1 with a value of −1 accounting for a complete disagreement, 0 for no agreement above chance and 1 for perfect agreement. Table 2 presents Kappa metric interpretation for strength of agreement as per the Landis and Koch’s criteria (10). The STAPLE algorithm is used to generate a consensus contour from a voxel by voxel maximal likelihood estimate based on the contours that the 12 observers produced for each case (11). The STAPLE95 volume, which represents a probabilistic estimate of the “true” suspicious edema contour at a 95% confidence level is thus obtained (Figures 1 and 2).
Table 2.
Landis & Koch’s criteria for kappa strength of agreement interpretation
| Kappa | Agreement interpretation |
|---|---|
| <0 | Poor |
| 0–0.20 | Slight |
| 0.21–0.40 | Fair |
| 0.41–0.60 | Moderate |
| 0.61–0.80 | Substantial |
| 0.81–1.00 | Almost perfect |
Comparison of CTV2cm and CTV3cm to the Staple95 volume
The dice similarity coefficient (DSC) index is considered a special case of Kappa coefficient and was used for comparison of CTV2cm and CTV3cm overlap with the Staple95 volume. A voxel by voxel comparison of the 2 volumes was achieved by calculating the ratio of the intersection volume of the 2 contours (CTV ∩ Staple 95) divided by the average size of the 2 volumes. A DSC of 1 represents perfect overlap and thus perfect agreement whereas DSC is 0 if there is no overlap (9, 12).
Results
Twelve radiation oncologists involved in the phase II RTOG 0630 trial participated in this study. Ten participants (83%) completed >80% of the required contours and 8 (67%) completed all contours. Two participants returned only 2 out of the 10 MR datasets. The mean volumes of GTV, CTV2cm and CTV3cm were respectively 130 cm3 (range: 7–413 cm3), 306 cm3 (range: 53–860 cm3) and 360 cm3 (range: 91–1055 cm3). The overall mean consensus volume computed using the STAPLE algorithm at 95% confidence interval was 188 cm3 (range 24.4–564.8 cm3) with a net consensus suspicious edema volume of 58 cm3 (range 4–182 cm3) when the GTV was subtracted. The mean kappa was 0.73 (range 0.36–0.89) representing a substantial overall agreement corrected for chance. A summary of suspicious edema contour statistics is reported in Table 3.
Table 3.
Summary of suspicious edema statistics
| Maximum volume (cm3) | 684.1 | ||
| Minimum volume (cm3) | 8.3 | ||
| Average volume (cm3) | 197.8 | ||
| Standard volume (cm3) | 66.1 | ||
| CTV2cm & S95 | Mean | Min | Max |
| Union volume (cm3) | 327.1 | 60.7 | 931.7 |
| Intersection volume (cm3) | 167.2 | 16.6 | 493.3 |
| DSC (cm3) | 0.63 | 0.43 | 0.76 |
| CTV3cm & S95 | |||
| Union volume (cm3) | 397.9 | 96.2 | 1115.1 |
| Intersection volume (cm3) | 172.8 | 18.7 | 504.7 |
| DSC (cm3) | 0.56 | 0.32 | 0.74 |
| Overall kappa | 0.71 | 0.32 | 0.88 |
S95 = Staple 95, DSC= Dice Similarity Coefficient
Table 4 presents a summary of the comparison of STAPLE95 volumes with CTV2cm and CTV3cm. Only one of the patients had a tumor size ≥8 cm (9.0 cm) and therefore, according to the RTOG 0630 protocol instructions, would require a 3 cm proximal and distal expansion to the CTV (CTV3cm). The median volume of suspicious edema not included in the CTV2cm was 5 cm3 (range: 0–77 cm3) and not included in the CTV3cm was 0.3 cm3 (range: 0–60 cm3). For three tumors measuring 6.2, 6.2 and 9 cm, large suspicious edema volumes of 40, 48 and 60 cm3 respectively were not included the CTV3cm volume. The mean volume included in the CTV3cm and that was not involved with suspicious edema (CTV3cm-S95) was 210 cm3 (Table 4).
Table 4.
Comparison of STAPLE95 volumes with CTV2cm and CTV3cm
| Case | Tumor Size (cm) |
Volume of S95 not included in CTV2cm (cm3) |
Volume of S95 not included in CTV3cm (cm3) |
Volume of CTV3cm not included in S95 (cm3) |
|---|---|---|---|---|
| 1 | 3.1 | 7.8 | 5.7 | 72 |
| 2 | 7.5 | 0.0 | 0.0 | 294 |
| 3 | 6.8 | 1.4 | 0.3 | 330 |
| 4 | 6.2 | 76.6 | 47.8 | 163 |
| 5 | 5.0 | 0.0 | 0.0 | 96 |
| 6 | 6.2 | 42.7 | 39.7 | 183 |
| 7 | 6.7 | 2.4 | 0.2 | 106 |
| 8 | 4.2 | 0.0 | 0.0 | 98 |
| 9 | 9 | 71.6 | 60.1 | 550 |
| 10 | 5.4 | 6.8 | 0.0 | 205 |
| Mean | 6.1 | 4.6 | 0.3 | 210 |
Discussion
The peritumoral zone found in STS is often associated with vascular congestion, hyperperfusion and increased extracellular fluid (4) as manifested by edema visualized on T2 weighted MRI series. Small studies evaluating the histology of peritumoral edema have shown satellite tumor cells within this zone (6, 13). Rates of reported malignant cells within the edematous peri-lesional area are variable based on the limited reports (6, 13, 14) and true rates remain unknown. Beltran (14) et al. reported biopsy proven malignant cells in the edematous area surrounding musculoskeletal malignant tumors in 3 out of 11 patients. In another series of 41 tumors with peritumoral edema, that included osteosarcomas, Ewing’s sarcomas and STS, malignant cells were found in only one patient with massive edema surrounding an osteosarcoma (13).
Moreover, even if these rates become better defined, the significance of isolated tumor cells is unclear since they may not all have the potential to form recurrent tumor. In a study by White, looking specifically at soft tissue sarcoma of the extremity (6), satellite tumor cells were seen in 10 of 15 patients. In 9 of these, tumor cells were found within the edema zone as seen on MRI. On pathological examination, the furthest that satellite tumor cells were found from gross tumor was 4 cm (6). Although this was a small study and the potential of these satellite cells to grow and form an actual tumor was not studied and remains controversial, the presence of satellite tumor cells in 9 out of 10 cases warranted the inclusion of edema in RTOG 0630 volumes.
Radiation volume reduction aims at sparing healthy surrounding tissues to potentially decrease treatment toxicities. In this context, accurate definition of radiation target volumes (GTV, CTV) and an understanding of the extent of normal tissue that can be safely spared without compromising local control and survival become critical. Three prospective trials, the RTOG 0630, the United Kingdom Vortex trial and the COG ARST 0332 trial are currently investigating the impact of limited radiation volumes on toxicity rates and local control. The results of these studies have the potential to modify the current standard radiotherapy volumes for STS of the extremity. In the meantime, the extent of edema inclusion within the radiation treatment volume should be carefully evaluated, weighing the risk of missing tumor cells against risk of causing important toxicities translating into decreased limb function and quality of life. RTOG sarcoma panel currently agrees that the CTV should be manually contoured to encompass any suspicious peritumoral edema that is not already included in this margin (7) but the extent of inclusion remains subject to clinical judgment. Edema will typically already be included when 3 cm longitudinal margins are used and the benefit of including edema more than 4 cm from the GTV is unclear.
A study by Roberge (15) showed significant inter-observer variation in GTV definition with an average of 50–90% of the GTV being common to all observers and a standard deviation across observers of 6.1% of the average volume. However, remarkable consistency was found between centers of mass of the target contoured by the observers. In a recent study from the RTOG group evaluating the variability in definition of GTV and CTV as delineated by sarcoma radiation oncologists, substantial to almost perfect agreement was found between physicians when specific instructions were provided (16). An RTOG atlas for GTV and CTV definition aiming at improving target volume consistency was subsequently developed and the current consensus is to combine the edema volume (in this case, the STAPLE95) and the anatomical expansion (in this case the CTV2cm or CTV3cm) to form the clinical target volume (to which a PTV margin would be added) (7). Clinical judgment is required to decide how much of the T2 edema is suspicious. We compared uniform CTV expansions to STAPLE95 volumes in order to isolate the additional SE volume not included in the uniform reduced CTV volume.
Conventional post-operative radiotherapy margins (5 to 7 cm expansion in the cranio-caudal direction and 2 cm expansion axially) will usually include SE volume. With the use of smaller margins, inconsistent volume definition due to disagreements in SE delineation was perceived as a potential issue. However, in our study, we found substantial agreement in contouring suspicious peritumoral edema among radiation oncologists from selected RTOG 0630 participant institutions. We therefore believe that consistency of radiotherapy volume definition can be preserved despite margin reduction. This being said, we found a lower level of agreement than that found for GTV and CTV definition in previous studies. This is most likely attributed to the infiltrative appearance of peritumoral edema on MR images. In individual cases, variations can be large. Reduced variation likely would be achieved through routine peer-review of treatment volumes as is performed centrally on clinical trials such as RTOG 0630.
Conclusion
We found substantial agreement in contouring of peritumoral edema among RTOG sarcoma radiation oncologists. Clinical target volumes formed from anatomic expansion of 3 cm longitudinally and 1.5 cm radially are likely to include peritumoral edema in the majority of cases. In some cases, however, clinical judgment, weighing the risks for geographic miss versus radiation morbidity, may be required to determine if CTV volumes should be expanded to include all peritumoral edema observed on the MRI T2 images.
Summary.
With the introduction of intensity modulated radiotherapy and image-guided radiotherapy, there is growing interest in reducing radiation treatment volume to decrease treatment toxicities. In soft tissue sarcoma of the extremity, the extent of suspicious peritumoral edema included in the treatment volume is subject to clinical judgment, balancing the risk of missing tumor cells with excess toxicity. The aim of this study was to determine variability in suspicious edema delineation by sarcoma radiation oncologists.
Acknowledgments
This trial was conducted by the Radiation Therapy Oncology Group (RTOG), and was supported by RTOG grant U10 CA21661 and CCOP grant U10 CA37422 from the National Cancer Institute (NCI). This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None.
References
- 1.Davis AM, O'Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Davis AM, O'Sullivan B, Bell RS, et al. Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma. J Clin Oncol. 2002;20:4472–4477. doi: 10.1200/JCO.2002.03.084. [DOI] [PubMed] [Google Scholar]
- 3.Ilaslan H, Schils J, Nageotte W, et al. Clinical presentation and imaging of bone and soft-tissue sarcomas. Cleve Clin J Med. 2010;77(Suppl 1):S2–S7. doi: 10.3949/ccjm.77.s1.01. [DOI] [PubMed] [Google Scholar]
- 4.Enneking WF, Spanier SS, Malawer MM. The effect of the Anatomic setting on the results of surgical procedures for soft parts sarcoma of the thigh. Cancer. 1981;47:1005–1022. doi: 10.1002/1097-0142(19810301)47:5<1005::aid-cncr2820470532>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Kroon HM, Bloem JL, Holscher HC, et al. MR imaging of edema accompanying benign and malignant bone tumors. Skeletal Radiol. 1994;23:261–269. doi: 10.1007/BF02412359. [DOI] [PubMed] [Google Scholar]
- 6.White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2005;61:1439–1445. doi: 10.1016/j.ijrobp.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Bosch W, Roberge D, et al. RTOG sarcoma radiation oncologists reach consensus on gross tumor volume and clinical target volume on computed tomographic images for preoperative radiotherapy of primary soft tissue sarcoma of extremity in Radiation Therapy Oncology Group studies. Int J Radiat Oncol Biol Phys. 2011;81:e525–e528. doi: 10.1016/j.ijrobp.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deasy JO, Blanco AI, Clark VH. CERR: a computational environment for radiotherapy research. Med Phys. 2003;30:979–985. doi: 10.1118/1.1568978. [DOI] [PubMed] [Google Scholar]
- 9.Allozi R, Li XA, White J, et al. Tools for consensus analysis of experts' contours for radiotherapy structure definitions. Radiother Oncol. 2010;97:572–578. doi: 10.1016/j.radonc.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 11.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): an algorithm for the validation of image segmentation. IEEE Trans Med Imaging. 2004;23:903–921. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang T, Chi Y, Meldolesi E, et al. Automatic delineation of on-line head-and-neck computed tomography images: toward on-line adaptive radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:522–530. doi: 10.1016/j.ijrobp.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 13.Hanna SL, Fletcher BD, Parham DM, et al. Muscle edema in musculoskeletal tumors: MR imaging characteristics and clinical significance. J Magn Reson Imaging. 1991;1:441–449. doi: 10.1002/jmri.1880010408. [DOI] [PubMed] [Google Scholar]
- 14.Beltran J, Simon DC, Katz W, et al. Increased MR signal intensity in skeletal muscle adjacent to malignant tumors: pathologic correlation and clinical relevance. Radiology. 1987;162:251–255. doi: 10.1148/radiology.162.1.3786772. [DOI] [PubMed] [Google Scholar]
- 15.Roberge D, Skamene T, Turcotte RE, et al. Inter- and intra-observer variation in soft-tissue sarcoma target definition. Cancer Radiother. 2011;15:421–425. doi: 10.1016/j.canrad.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Bosch W, Kirsch DG, et al. Variation in the gross tumor volume and clinical target volume for preoperative radiotherapy of primary large high-grade soft tissue sarcoma of the extremity among RTOG sarcoma radiation oncologists. Int J Radiat Oncol Biol Phys. 2011;81:e775–e780. doi: 10.1016/j.ijrobp.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]