Abstract
Persistent patency of the ductus arteriosus (PDA) is a common problem in preterm infants. The antacid cimetidine is a potent antagonist of the H2 histamine receptor but also inhibits certain cytochrome P450 enzymes (CYPs), which may affect DA patency. We examined whether cimetidine contributes to PDA and is mediated by CYP inhibition rather than H2 blockade. Analysis of a clinical trial to prevent lung injury in premature infants revealed a significant association between cimetidine treatment and PDA. Cimetidine and ranitidine, both CYP inhibitors as well as H2 blockers, caused relaxation of the term and preterm mouse DA. CYP enzymes that are inhibited by cimetidine were expressed in DA subendothelial smooth muscle. The selective CYP3A inhibitor ketoconazole induced greater DA relaxation than cimetidine, whereas famotidine and other H2 antagonists with less CYP inhibitory effects caused less dilation. Histamine receptors were developmentally regulated and localized in DA smooth muscle. However, cimetidine caused DA relaxation in histamine-deficient mice, consistent with CYP inhibition, not H2 antagonism, as the mechanism for PDA. Oxygen-induced DA constriction was inhibited by both cimetidine and famotidine. These studies show that antacids and other compounds with CYP inhibitory properties pose a significant and previously unrecognized risk for PDA in critically ill newborn infants.
Keywords: ductus arteriosus, cytochrome P450, CYP, cimetidine, histamine, H2 antagonist
1. INTRODUCTION
The ductus arteriosus (DA) is a central vascular shunt in the fetus that allows oxygenated blood returning from the placenta to perfuse the systemic circulation, bypassing the unaerated lung. Rapid postnatal closure of the DA is required for successful circulatory transition and adaptation to newborn life. Failure of postnatal DA constriction may have life-threatening consequences [1, 2]. This condition, termed persistent patency of the ductus arteriosus (PDA), occurs in nearly 40% of infants <1000 g birth weight and is one of the most common cardiac disorders in infancy [3]. Despite decades of study, management of the PDA remains a clinical dilemma.
Maintenance of DA relaxation during fetal life and its constriction soon after birth are physiologic events that are mediated, in part, by changes in oxygen, nitric oxide, prostaglandins, and certain ion channels [2]. However, regulation of DA patency is complex and most likely involves other mechanisms. In this regard, Coceani and colleagues have proposed that postnatal DA constriction may involve the cytochrome P450 enzyme system (CYP) since a variety of CYP inhibitors were shown to relax the oxygen-constricted newborn lamb DA in vitro [4–6]. Although drugs that inhibit CYP enzymes are not usually prescribed for neonates, the widespread use of antacids in the neonatal intensive care unit [7–9] represents a special risk since their CYP inhibitory properties are not frequently considered. In this study, we hypothesized that cimetidine produces relaxation of the DA, acting via CYP inhibition rather than through its actions as an antagonist of the histamine H2 receptor.
CYP enzymes have been reported to mediate hyperoxic lung injury under various experimental conditions. For example, cimetidine, through its CYP inhibitory properties, prevents the severe failure of pulmonary gas exchange that occurs in newborn lambs after breathing 95% oxygen for 72 hours [10]. To examine whether these promising results would apply to human infants, we conducted a randomized clinical trial testing the efficacy of cimetidine to prevent CYP-mediated oxidant injury to the lung of premature infants at risk for chronic lung disease. There was no protective effect of cimetidine on lung injury in that trial [11]. By subgroup analysis, we now report that the incidence of symptomatic PDA was significantly greater in infants treated with cimetidine when compared to a placebo. An association between cimetidine exposure and PDA has not been previously described.
The mechanisms by which cimetidine might induce relaxation of the DA are unclear. We evaluated the expression of CYP isoforms and histamine receptors in mouse fetuses with advancing gestational age and in newborn mice. To avoid the confounding effects of systemic metabolism, cannulated vessel myography was used to study the isolated term and preterm DA under fetal and newborn oxygen conditions. Ductus response was analyzed after exposure to: 1) cimetidine, 2) histamine and specific histamine receptor agonists, 3) H2 antagonists that have CYP inhibitory effects (cimetidine, ranitidine), 4) H2 antagonists with minimal CYP inhibitory effects (famotidine, nizatidine, roxatidine), 5) a selective CYP3A4 inhibitor (ketoconazole), 6) cimetidine treatment in histamine-deficient mice, and 7) oxygen-induced constriction of the DA following pretreatment with clinically relevant CYP inhibitors (cimetidine, famotidine). Due to limitations in the availability and viability of human DA specimens, term and preterm mouse DAs were used for these experiments. Our results indicate that “cimetidine-associated PDA” is a concerning entity in vulnerable neonates and is mediated via inhibition of specific CYP enzymes, independent of H2 receptor effects. These findings demonstrate an important role for CYP enzymes in perinatal vascular regulation, and may help to prevent unintended drug effects in fragile newborn infants.
2. METHODS
2.1 Human studies
The clinical findings of this study are based on a retrospective subgroup analysis of data obtained during a randomized, double blind, placebo-controlled trial that was reviewed and approved by the Vanderbilt Institutional Review Board [11]. In that trial, a 10-day i.v. infusion of either cimetidine (0.5 mg/kg/h IV following a loading dose of 2.374 mg/kg) or saline placebo was given beginning 12–24 hours after birth. Inclusion criteria were a postnatal age between 12 and 24 hours, birth weight between 500 and 1250 g, gestational age ≤ 32 weeks and ventilator dependence at the time of randomization. Symptomatic PDA was diagnosed according to previously published criteria [12]. The primary outcome variable was severity of lung disease at 10 days of age assessed using a Respiratory Insufficiency Index. Detailed information about study design, patient care, clinical definitions, outcome variables, data management, randomization, and power analysis are contained in the earlier publication [11].
2.2 Animals and tissues
All animal experiments were conducted in accordance with National Institutes of Health animal care standards and were approved by the Institutional Animal Care and Use Committee at Vanderbilt University Medical Center. Adult female CD1 or C57BL6 mice were bred to produce timed pregnancies. Mice with targeted deletion of the histamine decarboxylase (Hdc) gene [13] were maintained on a C57BL6 background. Dams were euthanized by cervical dislocation at 0830–0900h on day 15, day 17, or day 19 (term gestation) of pregnancy. Tissues were also collected from anesthetized newborn animals after cervical transection on postpartum day 1 (P1). Isolation of the fetal DA for myography studies was performed as described [14].
2.3 Quantitative PCR
Analysis of DA-specific expression of histamine receptors and mouse homologues of representative CYP isoforms that are inhibited by cimetidine [15, 16] was performed by quantitative PCR (Roche Diagnostics, Indianapolis, IN) with rat or mouse isoform-specific primers for Cyp1a2, Cyp2c19, Cyp2d22, Cyp3a11, Cyp3a13, Cyp3a16, Cyp3a25, Cyp3a41, Cyp3a44, Cyp3a57, and Cyp3a59as well as the histamine receptors H1 (Hrh1), H2 (Hrh2), H3 (Hrh3), H4 (Hrh4), and ribosomal protein L7 (rpl7), a housekeeping gene with stable expression levels in the mouse DA [17] (Supplement, Table 1).
2.4 Immunohistochemistry
A goat anti-mouse polyclonal antibody (sc-33974; Santa Cruz Biotechnology) that detects the C-terminal domain of HRH2 was used to examine the cellular localization of the H2 receptor. A goat polyclonal antibody (sc-30621; Santa Cruz Biotechnology) that detects the mouse homologues of human CYP3A4 was used to examine CYP3A expression. Frozen cross-sections of the thorax (10µm) from d19 fetuses and from P1 mice were thaw-mounted onto poly-L-lysine coated slides and fixed in 4% paraformaldehyde for 10 minutes at 4° C, and immunostained per protocol (see Supplemental Methods).
2.5 Pressurized vessel myography
Freshly isolated outflow tract segments were placed in custom microvessel perfusion chambers (Instrumentation and Model Facility, University of Vermont) and positioned onto ~120–150 µm glass pipette tips, resulting in isolation of the DA between the proximal and distal cannulae, as previously described [14]. Intra-luminal diameter was continuously recorded (IonOptix, Milton, MA). Vessels were pressurized in a stepwise manner to 20 mmHg, allowed to equilibrate at 37°C for 60–90 minutes, and then exposed to 50 mM KCl for 10 minutes (x2) to determine the contractile response for each vessel [14]. After return to baseline, the bath was changed to a recirculating 20 ml circuit for treatment with various drugs or change in experimental conditions.
2.6 Drugs and compounds
Isolated DAs were exposed to increased oxygen tension (bath reservoir bubbled with 12% O2; designated “postnatal O2 conditions”, PaO2 90–120 Torr) or the synthetic thromboxane receptor agonist, U-46619 (10−8 M) (bath reservoir bubbled with 0% O2; designated “fetal O2 conditions”, PaO2 38–45 Torr) to submaximally preconstrict the DA prior to treatment with potential vasodilators [14]. Vessels were then exposed to cimetidine (10−9-10−2 M), ranitidine (10−9-10−2 M), famotidine (10−9-10−2 M), nizatidine (10−9-10−2 M) or roxatidine (10−6-10−2 M; Santa Cruz Biotechnology), or the proton pump inhibitors, omeprazole and lansoprazole (10−9-10−3 M). In other experiments, response to the selective CYP3A inhibitors ketoconazole (10−9-10−3 M), or fluconazole (10−9-10−4 M) was studied. The response to histamine (10−9-10−3 M) or an H1 agonist (2-(3-trifluromethyl)phenyl histamine), an H2 agonist (amthamine), or an H3 agonist (R(-)-alpha-methylhistamine) was also measured (10−9-10−3 M). Compounds were obtained from Sigma-Aldrich (St Louis, MO) unless otherwise specified. In some studies, drug-pretreated vessels were exposed to increasing oxygen tension (2, 5, 12, 21 or 95%O2) or to contractile stimuli (50mM KCl or 10−8 M U-46619). Vessels were observed at each step until a stable baseline diameter was reached (typically 20–30 minutes).
2.7 Statistical Methods
For analysis of clinical data, categorical variables were compared between groups using Pearson chi square analysis, or Fisher’s exact test when the value in a cell was less than 5. A 2- sample t test was used to test group differences between variables. Multivariate logistic regression was used to adjust outcome (PDA) for covariates. A p-value <0.05 was considered statistically significant. Data analysis was carried out using Systat Version 13 (Systat Software, Richmond, CA).
For myography studies, contractile changes were expressed as percent reduction in lumen diameter compared to baseline diameter at resting tone. Vasodilatory changes were expressed as percent reversal from a submaximally preconstricted caliber (mean ± SE). Dose-response relationships for each compound were illustrated using best-fit curves and sigmoidal approximation (Prism 4, GraphPad Software, La Jolla, CA). Repeated measurements of vessel diameter were analyzed using a linear mixed effects regression model controlling for baseline vessel diameter. A random intercept was included in each mixed effects model to account for the correlation arising from measuring the diameter of the same vessel at multiple concentrations. Regression models were fit using R (R Foundation, Vienna, Austria), Stata (StataCorp, College Station, TX) and Systat (Richmond, CA) statistics software. A p-value < 0.05 was considered significant.
3. RESULTS
3.1 Cimetidine treatment is associated with PDA in premature infants
Re-examination of the data that was generated during our earlier trial [11] revealed little or no significant associations between the cimetidine and placebo groups for any one of 12 adverse outcomes (Table 1). However, trends were noted toward an increased incidence of 3 adverse effects: any intraventricular hemorrhage, severe intraventricular hemorrhage, or symptomatic patent DA among patients treated with cimetidine.
Table 1.
Adverse Outcomes Following Cimetidine or Placebo Includes Survivors and Non-Survivors.
| Outcome | Cimetidine (n=41) |
Placebo (n=43) |
p-value |
|---|---|---|---|
| RDS Severity (d 10) | 248 ± 223 | 229 ± 231 | 0.713 |
| ↓ BP (req. pressors) | 26 (56) | 19 (44) | 0.275 |
| Methylxanthines | 29 (71) | 36 (84) | 0.155 |
| Sepsis | 21 (51) | 23 (53) | 0.835 |
| Antibx Rx (≥ d) | 35 (85) | 33 (77) | 0.315 |
| NEC | 2 (5) | 3 (7) | 1.000 |
| ROP w/ laser Rx | 1 (2) | 2 (5) | 1.000 |
| Days in hospital | 66 ± 33 | 74 ± 50 | 0.416 |
| Death | 6 (15) | 4 (9) | 0.515 |
| IVH (1–4) | 19 (46) | 12 (28) | 0.080 |
| IVH (3–4) | 9 (22) | 3 (7) | 0.050 |
| Symptomatic PDA | 13 (32) | 6 (14) | 0.052 |
Data expressed as n (%) or as mean ± standard deviation.
In that early death might have caused symptomatic PDA to be underestimated, analysis of the 12 adverse outcomes in Table 1 was repeated with the inclusion of only those infants who were alive at 28 days postnatal age (n = 74). As shown in Table 2, the association between cimetidine treatment and any IVH, severe IVH, and symptomatic PDA became statistically significant when the analysis was limited to surviving infants. The remaining 9 adverse outcomes were not statistically different between the cimetidine groups either before or after limiting the analysis to 28-day survivors (data not shown).
Table 2.
Adverse Outcomes Following Cimetidine or Placebo Infants Alive at 28 days Postnatal Age.
| Outcome | Cimetidine (n=35) |
Placebo (n=39) |
p-value |
|---|---|---|---|
| IVH (1–4) | 17 (49) | 9 (23) | 0.022 |
| IVH (3–4) | 8 (23) | 0 (0) | 0.002 |
| Symptomatic PDA | 12 (34) | 4 (10) | 0.012 |
Data expressed as n (%) or as mean ± standard deviation. Table includes only those adverse outcomes which were significantly associated with cimetidine treatment (p < 0.05).
Multivariable logistic regression was used to estimate the association of cimetidine with symptomatic PDA while adjusting for potential confounding factors. Tocolytic indomethacin and RDS Severity at day 10 were included in the multivariable model, as they were known to be associated with symptomatic PDA among our NICU patient population, along with a propensity score. We used the propensity score approach because it allows for simultaneous adjustment of multiple confounding variables even though the sample size was limited to 74 infants who were alive at 28 days of postnatal age. In this study, the propensity score was the probability of receiving cimetidine based on birth weight, gestational age, race, sex, presence of IVH, and 5-minute Apgar score. Variables included in this multivariable model were selected among 18 risk factors: exposure to antenatal indomethacin, maternal treatment with antenatal corticosteroids, rupture of membranes > 24 hours before birth, gestational age, birth weight, race, gender, inborn, Apgar scores at 1 and at 5 minutes, diagnosis of respiratory distress syndrome, severity of respiratory distress on days 1, 4, and 10, FiO2 on day 1, treatment with prophylactic indomethacin, intraventricular hemorrhage (grades 1 – 4), and severe intraventricular hemorrhage (grades 3 – 4).
When no adjustment was made for exposure to antepartum indomethacin, RDS severity on day 10, and the propensity score, the odds of symptomatic PDA in infants receiving cimetidine were 4.6 (95% CI: [1.3, 15.9]) fold higher than in infants not receiving cimetidine (Table 3). When adjusted for these risk factors, the odds ratio for cimetidine increased to 5.5 (95% CI: [1.1, 28.3]) and remained statistically significant. As expected, indomethacin use (OR = 7.2, 95% CI = [1.5, 34.4]) and a 1-point increase in RDS severity at day 10 (OR = 1.004, 95% CI = [1.001, 1.01]) were associated with increased odds of symptomatic PDA.
Table 3.
Odds Ratio, 95% Confidence Interval and p-value for Adjusted Risk Factors Predicting PDA. Cimetidine Remains a Significant Risk Factor after Adjustment for Other Significant Risk Factors.
| Risk Factor | OR | 95% CI | p-value |
|---|---|---|---|
| Cimetidine* | 4.6 | 1.3 – 15.9 | 0.017 |
| Cimetidine | 5.5 | 1.1 – 28.3 | 0.031 |
| Tocolytic Indocin | 7.2 | 1.5 – 34.4 | 0.010 |
| IVH (1–4) | 4.290 | 1.004 – 18.3 | 0.049 |
| RDS Severity (d 10) | 1.004 | 1.001 – 1.01 | 0.011 |
Not adjusted for other risk factors.
3.2 Histamine has mixed vasoactive effects on the isolated ductus arteriosus
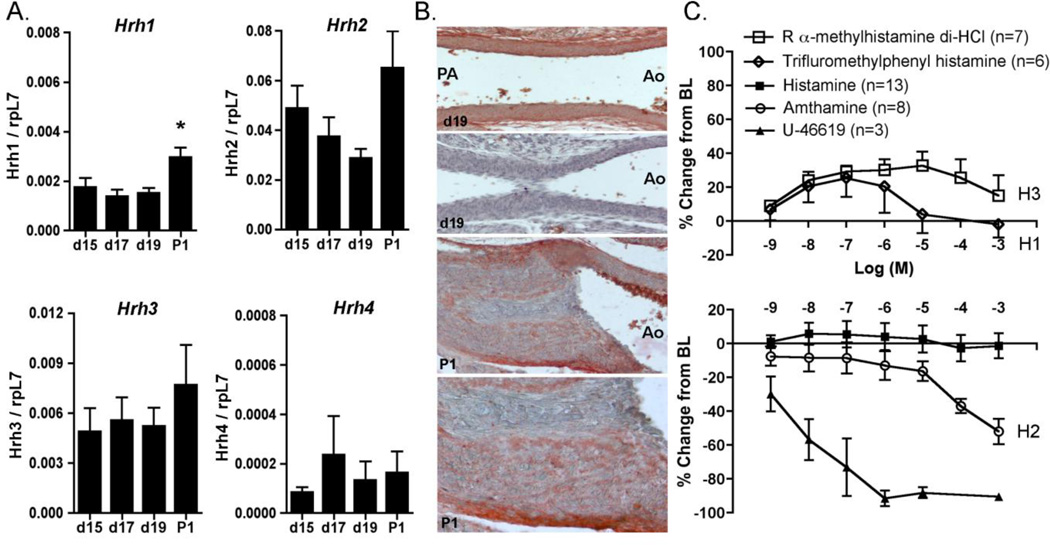
Because cimetidine is a known histamine H2 receptor antagonist, we examined whether cimetidine-associated PDA was mediated by perturbations of this pathway. The expression of histamine receptors was examined by quantitative PCR. Results show that H1, H2, H3, and H4 receptors were present in the fetal and newborn mouse DA, with predominance of the H2 receptor and limited expression of H4 (Fig. 1A). Cell-specific expression of the H2 receptor protein was noted in DA smooth muscle cells, with increased concentration of immunoreactive HRH2 in the outer layers of the muscular media and the tunica adventitia (Fig. 1B). No immunostaining was detected in the absence of primary antibody (Fig. 1B). Despite the presence of histamine receptors in the DA wall, exposure of the isolated DA to increasing concentrations of histamine did not induce a change in vessel tone (Fig. 1C). The use of different histamine formulations made no difference in these results, nor did inhibition of NO and prostaglandin synthesis prior to histamine treatment (Supplement Fig. 1). In contrast, the H2-selective agonist amthamine caused significant constriction of the DA lumen (Fig. 1C), suggesting that H2 receptors are active in the DA and that H2 blockers like cimetidine have the potential to relax DA tone via this mechanism. Exposure to selective H1 or H3 receptor agonists produced a vasodilatory response at lower concentration and a return to baseline lumen diameter at higher concentrations (Fig. 1C). These studies indicate that the absence of an overall effect of histamine on the isolated DA may represent competing effects of histamine acting via both contractile and vasodilatory receptors.
Figure 1. Histamine receptors in regulation of DA vasomotor tone.
A. Histamine receptor expression as determined by qPCR in the developing DA; values are expressed in relationship to the housekeeping gene, rpL7. P1, postnatal day 1. * p<0.01. B. Localization of immunoreactive H2 receptor protein in the unconstricted fetal DA (d19, top panel) and in the closed neonatal DA (P1; 200×, 400×). Negative control study on a parallel section of d19 tissues is shown in the second panel (200×). C. Three specific histamine receptor agonists caused significant changes in DA diameter compared to baseline (H1: trifluromethylphenyl histamine, H3: R alpha-methylhistamine di-HCl, and H2: amthamine, all p<0.01). DA diameter was unchanged in response to increasing concentrations of histamine (p=0.203). Increasing concentrations of U-46619, a thromboxane receptor agonist serving as a vasoconstrictive control, caused marked reduction in DA diameter (p<0.05). These results indicate that specific histamine receptor agonists, but not histamine itself, stimulate concentration-dependent changes in tone of the term fetal mouse DA. BL, baseline diameter.
3.3 CYP enzymes that are inhibited by cimetidine are expressed in the developing ductus arteriosus
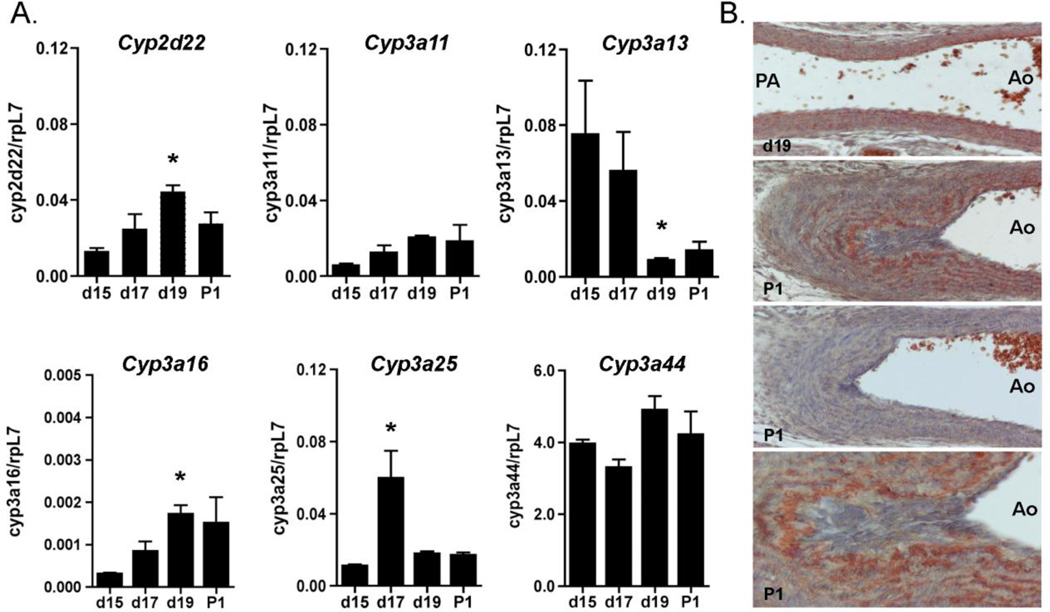
Cimetidine has recognized inhibitory effects on human CYP3A4, as well as CYP1A2, CYP2C19, and CYP2D6, among others. However, only limited information is available on the expression of CYP enzymes in the DA [18, 19]. Conventional PCR demonstrated the presence of Cyp2d22 (similar to human CYP2D6), and the CYP3A mouse homologues Cyp3a11, Cyp3a13, Cyp3a16, Cyp3a25and Cyp3a44 in the fetal and newborn DA. Cyp1a2, Cyp2c19, Cyp3a41, Cyp3a57and Cyp3a59 were present in liver but not expressed in the DA (data not shown). Quantitative PCR revealed maturation-associated increases in the expression of mRNAs for Cyp2d22 and Cyp3a16whereas Cyp3a13 was significantly downregulated with advancing gestation (Fig. 2A). Cyp3a25 gene expression was only elevated on day 17 of gestation. Cyp3a44 was the most highly expressed CYP in the DA, but was not developmentally regulated. An antibody that detects mouse CYP3A4 isoforms showed localization of immunoreactive CYP3A in concentric layers of DA smooth muscle cells. In contrast to the pattern of H2 receptor localization, CYP3A proteins were consistently expressed at higher levels in the inner layers of the muscular media (Fig. 2B), and were concentrated in the subendothelial smooth muscle cells of the widely patent fetal DA on day 19 as well as the closed postnatal DA on P1. No immunostaining was detected in the absence of primary antibody (Fig. 2B). In contrast to prior reports on limited CYP expression in the DA [18, 19], these results demonstrate the presence of multiple CYP enzymes that may be inhibited by cimetidine.
Figure 2. Expression of CYP isoforms inhibited by cimetidine in the DA.
A. Stage-specific expression of the CYP enzymes that are inhibited by cimetidine was analyzed by qPCR. Expression levels are displayed in relationship to the housekeeping gene rpL7. * p<0.01. B. Localization of the mouse homologues of CYP3A4 protein in the unconstricted fetal DA (d19) and in the closed neonatal DA (P1; 200×, 400×). Negative control study on a parallel section of P1 tissues is shown in the third panel (200×).
3.4 Cimetidine and ranitidine induce concentration-dependent dilation of the isolated ductus arteriosus
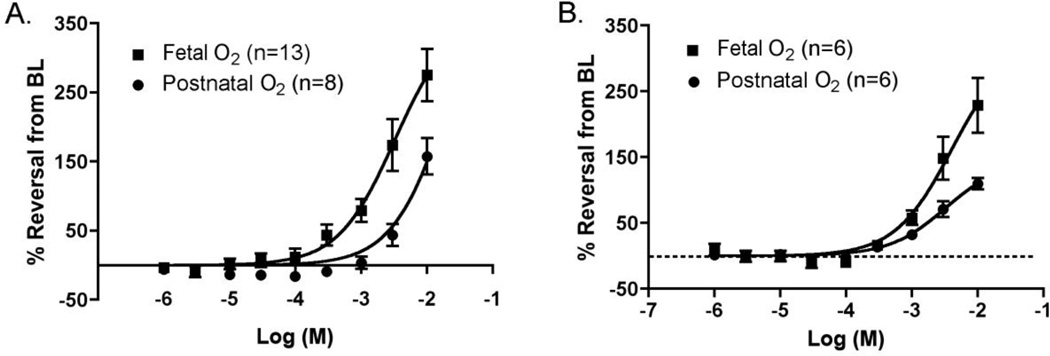
The functional consequences of CYP enzyme expression were examined in the ex vivo DA by pressurized vessel myography. Exposure of the term gestation (day 19) mouse DA to increasing concentrations of cimetidine under fetal oxygen conditions produced a significant concentration-dependent dilation (Fig. 3A). Complete (100%) reversal of preconstriction occurred at 10−3 M. At the highest concentration studied, the DA lumen was nearly four-fold dilated compared to preconstricted baseline diameter (446±27µm vs. 116±13µm). In comparison, cimetidine induced significantly less dilation under conditions that simulate postnatal oxygen tension (p<0.01, fetal vs. postnatal O2 conditions), where maximal dilation was approximately 2.8 fold greater than preconstricted baseline diameter (329±29µm vs. 117±19 µm). In the preterm (day 15) DA (Fig. 3B), cimetidine also induced significant concentration-dependent relaxation compared to baseline (240±30µm vs. 149±18µm; p<0.01), however, the difference between vessels under fetal versus postnatal O2 conditions was not significant (p=0.567). Cimetidine-induced vasorelaxation was less potent in the preterm than the term DA under either fetal (p<0.01) or postnatal (p<0.01) O2 conditions.
Figure 3. Response of the isolated mouse DA to cimetidine.
A. Relaxation of the term gestation (d19) DA under fetal or postnatal oxygen (O2) conditions in the perfusion bath. B. Relaxation of the preterm gestation (d15) DA under fetal or postnatal oxygen conditions. Each dose-response curve shows significant concentration-dependent DA relaxation (p<0.01) compared to the preconstricted baseline (BL) diameter. In d19 mice, relaxation was significantly greater in fetal oxygen conditions than in postnatal oxygen conditions (p<0.01). This difference was not significant in d15 mice (p=0.567). DA relaxation was significantly greater with d19 mice than d15 mice for either fetal oxygen conditions (p<0.01) or for postnatal oxygen conditions (p=0.01). Thus, cimetidine-induced DA relaxation is dependent on cimetidine concentration, gestational maturation, and oxygen level.
To determine whether the vasodilatory effects of cimetidine are unique to this compound, the isolated term DA was also exposed to ranitidine, which shares H2 blocker and CYP-inhibitory properties with cimetidine. Ranitidine induced concentration-dependent dilation of the DA and complete reversal of preconstriction at approximately 10−3 M, similar to cimetidine (Supplement, Fig. 2). These findings indicate that various antacids with CYP inhibitory properties have the potential to induce DA relaxation.
3.5 CYP inhibition, but not H2 antagonism, dilates the isolated ductus arteriosus in a concentration-dependent manner
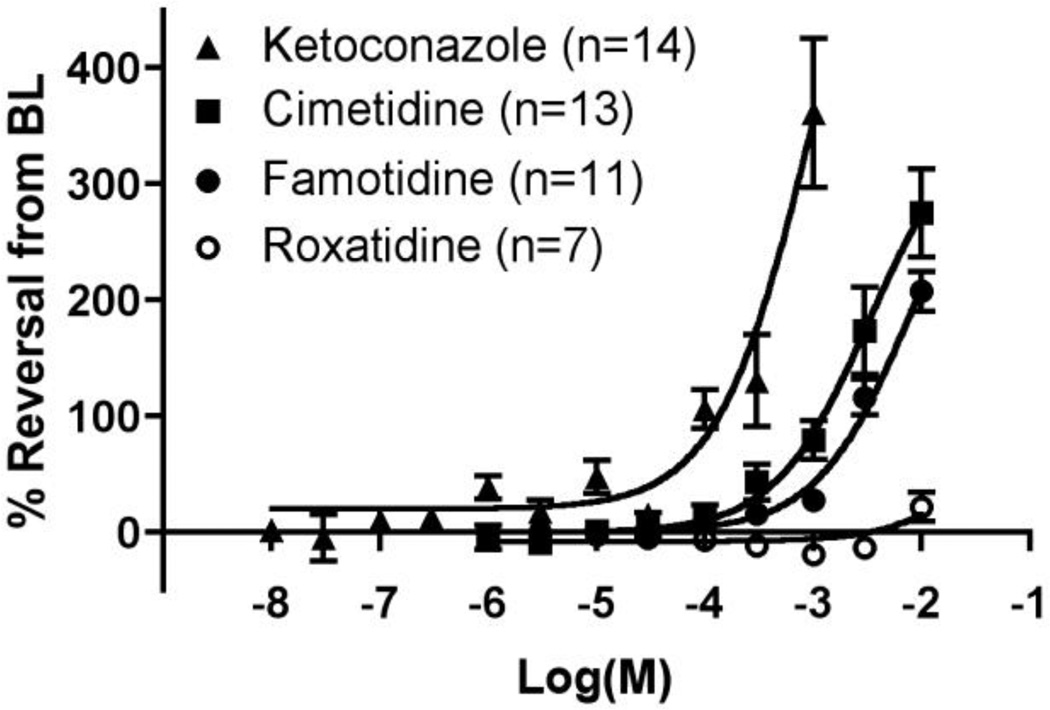
To examine whether the vasodilatory effects of cimetidine and ranitidine are due to their effects on CYP enzyme inhibition rather than H2 antagonism, inhibitors with more selective effects were studied. Treatment of the isolated DA with ketoconazole, which selectively and potently inhibits CYP3A enzymes, produced a significant increase in DA diameter (393±21µm at 10−3 M) compared to preconstricted baseline (115±18µm) (p<0.01) (Fig. 4). Ketoconazole exposure induced significantly greater relaxation than cimetidine-treated vessels over increasing concentrations (p<0.05; Fig. 4). Complete (100%) reversal of preconstriction required only 10−4 M ketoconazole, whereas approximately 10−3 M cimetidine (or ranitidine) was required for this effect. Fluconazole, an antifungal agent with similar CYP3A inhibitory properties, had less significant effects on DA tone than ketoconazole. Fluconazole produced comparable DA relaxation to cimetidine although the dose range that could be studied excluded the concentrations where the peak vasodilatory effects of cimetidine were observed (Supplement, Fig. 3).
Figure 4. Relationship of cimetidine-induced DA relaxation to CYP3A- and H2-selective inhibitors.
Cimetidine stimulated less concentration-dependent DA dilation than the CYP3A inhibitor ketoconazole (p<0.05), and a greater concentration-dependent relaxation than roxatidine, a highly selective H2 antagonist (p<0.01). Famotidine, an H2 blocker with modest CYP inhibitory properties, induced greater DA dilation than roxatidine (p<0.01) and significantly less than ketoconazole (p<0.01). These results suggest that DA relaxation associated with cimetidine is more likely mediated by CYP inhibition than by H2 blockade. BL, preconstricted baseline diameter.
To further investigate the contribution of H2 antagonism to cimetidine-induced effects, isolated DA preparations were treated with famotidine, nizatidine, or roxatidine, H2 blockers that have limited inhibition of CYP enzymes (Fig. 4). Famotidine treatment produced a modest degree of vasorelaxation at higher doses, although less than that of cimetidine (p<0.01). Exposure to nizatidine produced similar results (data not shown). Roxatidine, a highly selective H2 antagonist with different biochemical structure and properties than other H2 blockers and little or no effects on the CYP enzyme system [20–22], caused a small dilation of the isolated DA (p<0.05), but was significantly less vasodilatory than cimetidine or famotidine (p<0.01). Taken together, these experiments suggest that cimetidine-induced dilation of the isolated DA is most likely related to CYP inhibition rather than H2 antagonism.
Proton-pump inhibitors (PPIs) are antacids that avoid H2 receptor antagonism and have limited CYP inhibitory properties. In contrast to the vasodilatory effects of cimetidine, ranitidine, and famotidine, exposure to the PPIs omeprazole or lansoprazole (10−6–10−3 M) had a constrictive effect on the isolated DA, although minor DA dilation was noted at the highest doses (Supplement Fig. 4).
3.6 Cimetidine-mediated dilation of the ductus arteriosus occurs in the absence of histamine
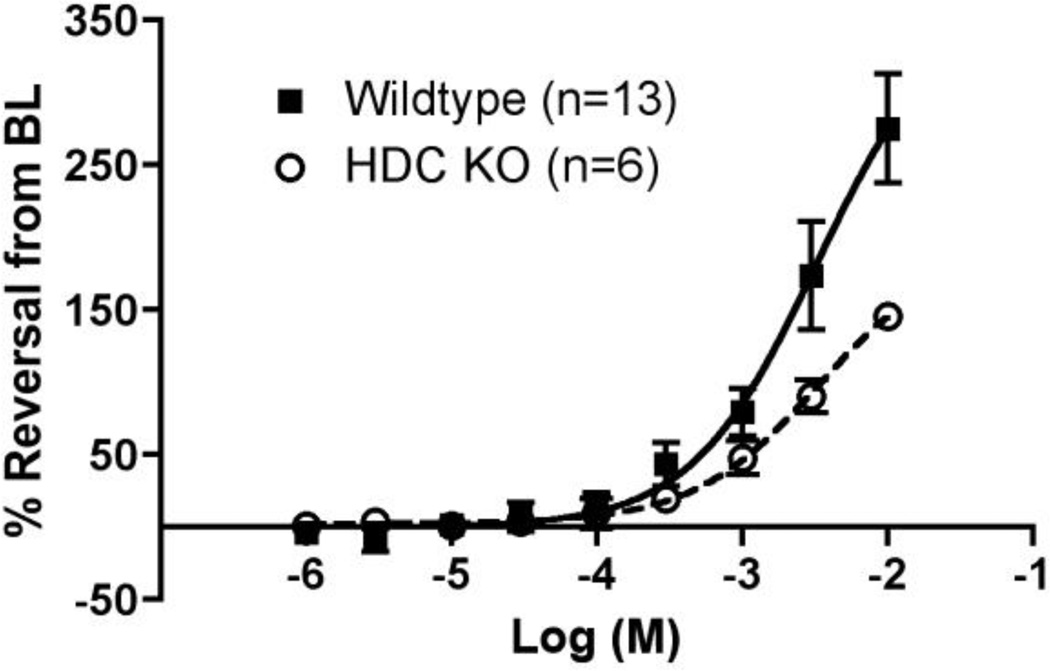
To better distinguish the roles of CYP enzymes and histamine receptor blockade in DA function, the response to cimetidine was examined in mice lacking histidine decarboxylase (HDC, EC 4.1.1.22). HDC null mice were chosen for study rather than H2 receptor knockout mice, since the latter may have compensatory signaling via other histamine receptor family members [23]. HDC is the sole enzyme responsible for biosynthesis of histamine; tissue histamine levels depend on pyridoxal phosphate-dependent decarboxylation of L-histidine to histamine by HDC. Mice with deletion of the HDC enzyme have undetectable histamine levels, as well as impairments of gastric acid secretion, cutaneous anaphylaxis, mast cell degranulation and altered bone formation [24], but an abnormal DA phenotype (fetal constriction or postnatal PDA) has not been reported. Exposure to cimetidine induced significant concentration-dependent relaxation of the HDC null DA (379±25µm at 10−2 M) compared to its preconstricted baseline (98±13µm; p<0.01) (Fig. 5). There was no significant difference in overall relaxation of DA tone between wild type and HDC null animals (p=0.468). These results indicate that cimetidine-induced DA relaxation is unlikely to involve the actions of histamine.
Figure 5. Comparison of cimetidine-induced relaxation in the wild type and HDC null DA.
A significant concentration-dependent increase in DA relaxation by cimetidine was noted for either the HDC knockout mice (p<0.01) or the wild type mice (p<0.01) compared to their preconstricted baseline (BL) diameter (wild type data is from Fig. 3). The difference in cimetidine-induced DA relaxation between wild type and HDC null mice was not significant (p=0.468), suggesting that DA relaxation in d19 mice does not depend on H2 signaling.
3.7 Prevention of oxygen-induced ductus arteriosus constriction by cimetidine and famotidine
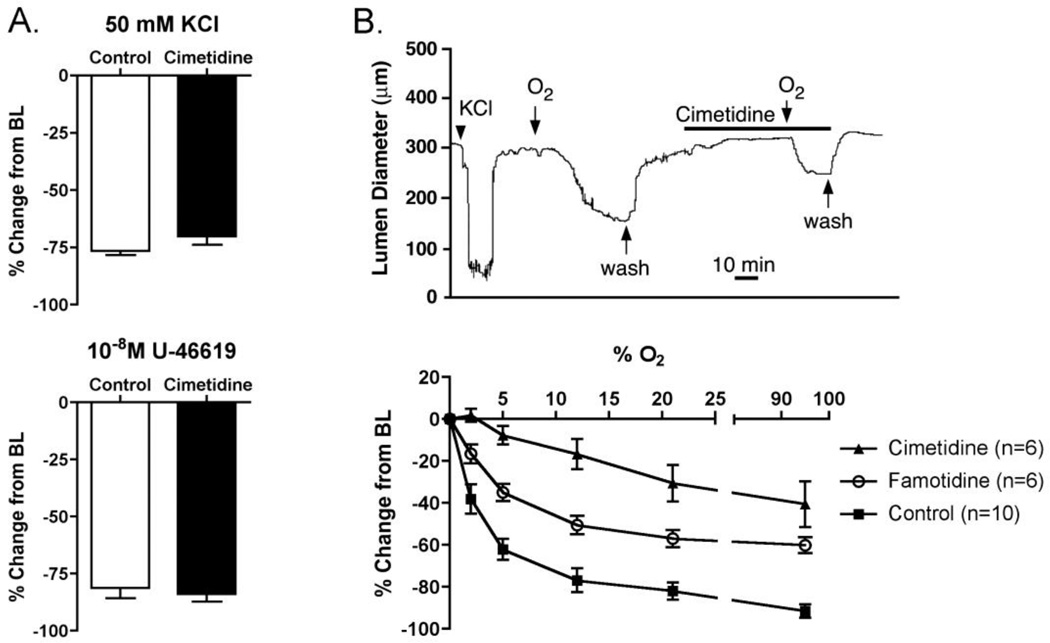
The DA of most species is exquisitely sensitive to oxygen tension both in vivo and in vitroincluding pressurized DA preparations from fetal mice [14]. Exposure of the pressurized fetal mouse DA to either to 50mM KCl or U-46619 induced strong constriction of the DA, which was not inhibited by pre-treatment with 10−3 M cimetidine (Fig. 6A). In contrast, oxygen-induced constriction was blunted by a 50-minute exposure to cimetidine (Fig. 6B, top panel), suggesting that cimetidine has a selective inhibitory effect on oxygen sensing or oxygen-specific contractile pathways in the DA.
Figure 6. Inhibition of oxygen-induced DA constriction by cimetidine and famotidine.
Incubation of the isolated DA with cimetidine (10−3 M; 50 min.) did not inhibit DA constriction induced by 50mM KCl or 10−8 M U-46619 (A), but attenuated the DA constriction induced by exposure to 12% oxygen (B, top panel). Exposure to increasing oxygen (O2) concentration caused progressive DA constriction (B, bottom panel). DA constriction was dependent on oxygen concentration for cimetidine (p<0.01), famotidine (p<0.01) and control DAs (p<0.05), compared to baseline (BL) diameter. Pretreatment with either famotidine or cimetidine reduced the constrictive effect of oxygen when compared to untreated controls (p<0.01).
Isolated DAs exposed to incremental increases in oxygen showed progressive constriction (Fig. 6B, bottom panel). An increase in oxygen mixture from 0 to 2.5% caused approximately 40% constriction from resting baseline diameter (Fig. 6B). Complete closure of the DA lumen was frequently observed in 21% oxygen conditions, although additional constriction of the vessel wall was noted under 95% oxygen. Pre-incubation of the isolated DA with 10−3 M famotidine diminished the contractile response to increasing oxygen tension (p<0.01). Famotidine-treated vessels, however, were able to develop luminal constriction to approximately three-fourths of the oxygen-constricted control vessel dimensions. Vessels pretreated with 10−3 M cimetidine had marked impairment of oxygen-induced DA constriction compared to untreated DAs (p<0.01) and were significantly less constricted than famotidine-treated vessels (p<0.01) (Fig. 6B). Although increasing oxygen exposure eventually led to partial constriction, lumen closure was never observed in cimetidine-treated vessels, which were still approximately 50% patent compared to control vessels after exposure to 95% oxygen (Fig. 6B).
4. DISCUSSION
Despite recent advances in our understanding of DA development and function, PDA remains a difficult problem for premature infants. The present study implicates cimetidine as a previously unrecognized cause of symptomatic PDA in infants <1250g. Using a mouse model, we found that cimetidine induces concentration-dependent relaxation of the isolated DA. The term gestation DA was more susceptible than preterm, and the vasodilatory response was more pronounced under fetal than newborn oxygen conditions. Cimetidine effects were similar to ranitidine, another antacid that is frequently used in preterm infants. Although H2 receptors were expressed and functional in the DA, histamine had limited effects on DA tone and does not appear to be the key mechanism for cimetidine-associated PDA. Studies in HDC null mice that are histamine-deficient further demonstrated that cimetidine-induced DA relaxation occurs without antagonism of histamine actions on H2 receptors. Selective CYP3A inhibitors that lack H2 antagonism had more potent effects on DA tone than cimetidine. In contrast, famotidine and roxatidine (H2 antagonists with limited CYP inhibitory effects) produced significantly less DA dilation than cimetidine. Together, these findings indicate that cimetidine–associated PDA is an important clinical entity that is primarily mediated via inhibition of CYP enzymes rather than through H2 receptor antagonism.
PDA is one of the most common cardiac disorders in infancy. Efforts to better define the risk factors that predispose to PDA have not identified cimetidine or other antacids as significant risk factors [25, 26]. The current study examined preterm infants <1250g, who have the greatest predilection to symptomatic PDA and are most likely to require pharmacological or surgical treatment [27]. Even after controlling for common risk factors, we found a 5.5-fold increased risk for symptomatic PDA in infants treated with a 10-day course of cimetidine.
We considered whether cimetidine acting as an H2 blocker may have mediated the relaxant effect of this agent on the DA. Only limited studies exist on the direct contribution of histamine or its receptors to DA tone. Experiments on the isolated DA of preterm fetal lambs showed that histamine had little or no effect on DA tone, although very high doses caused a small contraction [28]. Indirect evidence from pulmonary blood flow studies in fetal sheep supported a possible role for histamine in DA constriction, most likely through the actions of the H1 receptor [29]. Our data demonstrate the predominance of H2 receptor expression in the fetal and newborn mouse DA, although H1 and H3 expression was also appreciable. Surprisingly, histamine itself did not cause a significant change in DA tone. This was most likely due to the opposing constrictive and vasodilatory effects of the H2 versus H1 and H3 receptors, respectively, but suggests the possibility that a selective H2 antagonist could stimulate relaxation of DA tone. These are potentially important findings since H2 receptor antagonists are among the most frequently prescribed drugs in the NICU [7–9]. One survey found that ranitidine use in the NICU was surpassed only by metoclopromide, ampicillin and caffeine [8]. In spite of this, HDC null mice that are incapable of histamine synthesis do not have an abnormal DA phenotype at birth and had cimetidine-induced relaxation that was not different than the wild type DA. Histamine blockade could also have an adverse effect on postnatal DA closure via impairment of platelet function and inhibition of platelet-mediated sealing of the ductus lumen [30, 31] although intact DA closure in HDC null mice argues against this possibility. Together, our findings support the concept that cimetidine-associated PDA is related to the CYP inhibitory properties of this compound rather than its effects as an H2 blocker.
Coceani and colleagues were the first investigators to establish an important regulatory role for the cytochrome P450 system in the DA [4–6, 32]. Their early studies showed that carbon monoxide (CO) acts as a potent vasodilatory signaling molecule in the DA at both low and high oxygen tensions. CO-induced relaxation was completely reversed by monochromatic light at 450nm wavelength, implicating the actions of a hemoprotein in the CYP family [4, 5]. Additional evidence suggested that the CYP moiety was localized in the DA smooth muscle since endothelium-stripped vessels were equally relaxed by CO and required an intact plasma membrane [4, 5, 33]. Subsequent electron microscopy studies showed immunoreactive localization of CYP3A enzymes in the plasma membrane and sarcoplasmic reticulum of cultured DA smooth muscle cells [32]. Our data show that CYP3A proteins are localized in the concentric smooth muscle layers of the DA wall. Smooth muscle cells in the innermost layers of the DA muscular media had the greatest CYP3A expression, with only low-level expression in the endothelium. This pattern was unchanged on postpartum day one, after DA closure and the onset of fibromuscular remodeling. Baragatti et al., also found more intense staining of the cytochrome P450 enzyme, CYP2J6, in the intimal layers of the mouse DA, whereas the expression of another family member, CYP2J9, was more evenly distributed across the DA wall [18]. More recent studies show that CYP3A13 is concentrated in the inner layers of the DA at term gestation [19]. We also found increasing expression of most CYP3A homologues with advancing gestation. The purpose for CYP3A expression in this spatiotemporal distribution is unclear. The data of Coceani and colleagues provide strong evidence that a hemoprotein in the CYP3A family of enzymes endows the ductus with a fundamental mechanism for oxygen-sensing [4–6, 32, 33]. In this regard, we observed that the term gestation DA was more susceptible than the preterm DA to cimetidine-induced relaxation, corresponding to maturation-related changes in DA sensitivity to oxygen. Moreover, cimetidine selectively blocked constriction of the DA by increased oxygen tension but not by potassium-induced depolarization or thromboxane-stimulated DA constriction. These results coincide with the enhanced susceptibility of the DA to oxygen with advancing gestation, and support the concept that maturation of DA oxygen-sensing is related to CYP function.
The purpose for ongoing CYP expression in the innermost muscular layers of the closed postnatal DA is less clear. Oxygen and diffusible nutrients are more concentrated in the inner layers of the DA in small or preterm animals, in which vasa vasorum are not required for perfusion of the muscular media [17]. However, the closed postnatal DA at term gestation undergoes nutrient and energy depletion along with tissue hypoxia and cellular apoptosis to maintain permanent closure [34, 35]. CYP3A is known to interact with the hypoxia-regulated molecules HIF1α [36, 37] and erythropoietin [38]. Hypoxia-specific CYP3A isoforms are linked with HIF1α, HIF2α, VEGF and other hypoxia signaling mechanisms that are active in the DA wall [34, 39]. Hypoxia is also known to upregulate the expression of CYP3A1, CYP3A6, and certain CYP2C enzymes [36, 40–45] that contribute to vasoconstriction in the lung, but not other vascular beds [45, 46], suggesting that hypoxia signaling via CYP enzymes may contribute to permanent DA constriction in the postnatal period. Thus, drugs with CYP inhibitory properties may prevent both the initial phase of oxygen-induced DA constriction and a later phase of oxygen sensing that contributes to final DA closure.
CYP enzymes metabolize a wide variety of compounds, whose derivatives have vasoactive properties and potential implications for DA patency and closure. Although CYPs generally act as hepatic microsomal detoxification enzymes, certain CYP enzymes serve as monooygenases in the vascular system. These CYPs catalyze the formation of epoxyeicosatrienoic acids (EETs) and hydroxyeicosatetraenoic acids (HETEs or their metabolites) from arachidonic acid. The synthesis of EETs by epoxygenases of the CYP2C and CYP2J family, and HETEs by ω-hydroxylases of the CYP4 family, contribute to vascular homeostasis or pathologic vascular conditions [47, 48]. Baragatti et al., demonstrated the presence of Cyp2j6, Cyp2j9, Cyp4a31and Cyp4b1 in the mouse DA. However, the EET and HETE species that were suspected to act as hyperpolarizing factors to induce DA relaxation could not be confirmed [18]. It is likely that the DA contains additional CYP genes for local metabolism of endogenous substrates. For example, we identified heightened expression of Cyp1b1, Cyp26b1, Cyp51, Cyp20a1, Cyp2b10, Cyp2s1, Cyp4v3and Cyp39a1 in the DA by microarray analysis (unpublished data). Coceani et al., also found DA-specific expression of Cyp51the critical enzyme for sterol biosynthesis, and the CYP1A1-associated factor Bteb1 in the neonatal mouse DA using a microarray approach [49]. Similar to our findings for significant Cyp2d22 expression, Dagle et al. identified a potentially informative polymorphism in CYP2D6 (the human corollary to mouse CYP2D22) in a screen for risk factors that predispose to PDA in preterm infants [50].
The current studies focused on CYP3A family members, since these isoforms are strongly inhibited by cimetidine [15, 16]. Cimetidine also has inhibitory effects on other CYPs, including CYP1A2, CYP2B1, CYP2C6, CYP2C9, CYP2C11, CYP2C19, and CYP2D6. Here, we found expression of five of the eight known mouse homologues for human CYP3A enzymes [51, 52]. In agreement with Baragatti et al., we observed declining expression of Cyp3a13 with advancing gestation [19]. In contrast to their results, we also found developmental stage-specific patterns for increases in the expression of Cyp3a16and Cyp3a25. The expression of Cyp3a44 was predominant, but did not change with advancing gestation, suggesting that this isoform may be involved in other processes in addition to oxygen-sensing. Although CYP3A4 usually functions in drug and steroid metabolism, it can also act as a vasoactive mediator and is capable of 5,6-, 8,9-, 11,12- and 14,15-EET synthesis from arachidonic acid [53, 54]. CYP enzymes are monooxygenases that are often considered a third pathway for arachidonic acid metabolism, similar to the formation of prostaglandins and leukotrienes by the cyclooxygenase and lipoxygenase pathways, respectively. Thus, the presence of multiple CYP3A family members in the DA and the varying expression pattern of each CYP enzyme implies that numerous drugs with CYP inhibitory properties could alter DA tone and contribute to PDA [26], not only by impairment of oxygen-sensing, but also though inhibition of homeostatic vasoregulatory processes.
5. LIMITATIONS
While this study found that cimetidine treatment was associated with PDA in preterm infants and DA relaxation in mice, it does have limitations. One shortcoming is the difficulty in obtaining human fetal DA samples that are appropriate for functional studies. Another caveat was the insolubility of some CYP inhibitory compounds and our inability to establish complete dose-response curves to determine EC50 values or other pharmacologic parameters. Whenever possible, alternative solvents were used to optimize solubility. Multiple agents in each drug category were also examined, to verify observations within a drug class. Despite solubility limitations, the overall effect of these drugs on vasomotor tone of the DA was clear. An additional concern was the high concentration of CYP inhibitors required to elicit an effect on the isolated DA. In our study of preterm infants <1250g, plasma cimetidine levels 24h after beginning a continuous infusion were 3.84 ± 1.59 µg/mL [11], corresponding to approximately 15 µM cimetidine concentrations, which did not stimulate DA relaxation in our myography experiments. Other investigators have reported that the concentration of cimetidine required for in vitro inhibition of CYP activity is typically 100–1000 times greater than serum levels that are associated with inhibition of drug metabolism in vivo [55, 56]. This finding has been confirmed in both human and rodent experiments. Consistent with these observations, our studies required cimetidine concentrations that were 10–100 times higher than expected to induce DA relaxation and prevent oxygen-induced DA constriction in vitro. We also anticipated that higher doses might be required, since short-term in vitro drug exposure may not fully reflect the conditions experienced by preterm infants that are exposed to long-term infusions of H2 blockers in vivo. Although the results of animal studies should be interpreted with caution, the findings in our clinical trial agree with the data obtained from our in vitro experiments.
6. CONCLUSIONS
In summary, we detected a significant association between PDA and cimetidine treatment in a prospective, randomized clinical trial that was performed to examine the effects of CYP inhibition in preterm infants. Mechanistic studies in mice revealed an important role for specific CYP enzymes in DA function and determined that cimetidine and similar H2 antagonists inhibit DA constriction by actions that are largely independent of their antihistamine effects. Our findings suggest that the use of H2 antagonists or PPIs that have minimal effects on the CYP enzyme system will provide a greater margin of safety for infants that require antacid therapy. These results also reveal new information on the contribution of CYP enzymes to vascular regulation during circulatory adaptation at birth and may benefit the premature neonates that are most susceptible to PDA.
Supplementary Material
Highlights.
-
-
Preterm infants exposed to cimetidine have increased patent ductus arteriosus (PDA)
-
-
Antacids that inhibit CYP enzymes relax the term and preterm mouse DA
-
-
CYP inhibition, but not H2 antagonism, alters DA tone in histamine deficient mice
-
-
Oxygen-induced DA constriction is selectively inhibited by cimetidine and famotidine
ACKNOWLEDGMENTS
FINANACIAL SUPPORT
Supported by NIH grants HD44741 (Paria) and HL77395, HL96967, HL109199 (Reese).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES AND CONFLICT OF INTEREST
None.
REFERENCES
- 1.Cotton RB, Stahlman MT, Kovar I, Catterton WZ. Medical management of small preterm infants with symptomatic patent ductus arteriosus. J Pediatr. 1978 Mar;92(3):467–473. doi: 10.1016/s0022-3476(78)80451-x. [DOI] [PubMed] [Google Scholar]
- 2.Smith GC. The pharmacology of the ductus arteriosus. Pharmacol Rev. 1998 Mar;50(1):35–58. [PubMed] [Google Scholar]
- 3.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002 Jun 19;39(12):1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 4.Coceani F, Hamilton NC, Labuc J, Olley PM. Cytochrome P 450-linked monooxygenase: involvement in the lamb ductus arteriosus. Am J Physiol. 1984 Apr;246(4 Pt 2):H640–H643. doi: 10.1152/ajpheart.1984.246.4.H640. [DOI] [PubMed] [Google Scholar]
- 5.Coceani F, Breen CA, Lees JG, Falck JR, Olley PM. Further evidence implicating a cytochrome P-450-mediated reaction in the contractile tension of the lamb ductus arteriosus. Circ Res. 1988 Mar;62(3):471–477. doi: 10.1161/01.res.62.3.471. [DOI] [PubMed] [Google Scholar]
- 6.Coceani F, Kelsey L, Seidlitz E, Korzekwa K. Inhibition of the contraction of the ductus arteriosus to oxygen by 1-aminobenzotriazole, a mechanism-based inactivator of cytochrome P450. Br J Pharmacol. 1996 Apr;117(7):1586–1592. doi: 10.1111/j.1476-5381.1996.tb15325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006 Jun;117(6):1979–1987. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- 8.Kumar P, Walker JK, Hurt KM, Bennett KM, Grosshans N, Fotis MA. Medication use in the neonatal intensive care unit: current patterns and off-label use of parenteral medications. J Pediatr. 2008 Mar;152(3):412–415. doi: 10.1016/j.jpeds.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 9.Malcolm WF, Gantz M, Martin RJ, Goldstein RF, Goldberg RN, Cotten CM. Use of medications for gastroesophageal reflux at discharge among extremely low birth weight infants. Pediatrics. 2008 Jan;121(1):22–27. doi: 10.1542/peds.2007-0381. [DOI] [PubMed] [Google Scholar]
- 10.Hazinski TA, France M, Kennedy KA, Hansen TN. Cimetidine reduces hyperoxic lung injury in lambs. J Appl Physiol. 1989 Dec;67(6):2586–2592. doi: 10.1152/jappl.1989.67.6.2586. [DOI] [PubMed] [Google Scholar]
- 11.Cotton RB, Hazinski TA, Morrow JD, Roberts LJ, Zeldin DC, Lindstrom DP, et al. Cimetidine does not prevent lung injury in newborn premature infants. Pediatr Res. 2006 Jun;59(6):795–800. doi: 10.1203/01.pdr.0000219397.35473.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotton RB, Haywood JL, FitzGerald GA. Symptomatic patent ductus arteriosus following prophylactic indomethacin. A clinical and biochemical appraisal. Biol Neonate. 1991;60(5):273–282. doi: 10.1159/000243418. [DOI] [PubMed] [Google Scholar]
- 13.Tan X, Essengue S, Talreja J, Reese J, Stechschulte DJ, Dileepan KN. Histamine directly and synergistically with lipopolysaccharide stimulates cyclooxygenase-2 expression and prostaglandin I(2) and E(2) production in human coronary artery endothelial cells. J Immunol. 2007 Dec 1;179(11):7899–7906. doi: 10.4049/jimmunol.179.11.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reese J, O'Mara PW, Poole SD, Brown N, Tolentino C, Eckman DM, et al. Regulation of the fetal mouse ductus arteriosus is dependent on interaction of nitric oxide and COX enzymes in the ductal wall. Prostaglandins Other Lipid Mediat. 2009 Apr;88(3–4):89–96. doi: 10.1016/j.prostaglandins.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flockhart DA. Drug interactions: Cytochrome P450 drug interaction table. Indiana University School of Medicine; 2009. [Accessed June, 2011]. http://medicineiupuiedu/clinpharm/ddis/tableasp. [Google Scholar]
- 16.Rendic S. Summary of information on human CYP enzymes: human P450 metabolism data. Drug metabolism reviews. 2002 Feb-May;34(1–2):83–448. doi: 10.1081/dmr-120001392. [DOI] [PubMed] [Google Scholar]
- 17.Richard C, Gao J, LaFleur B, Christman BW, Anderson J, Brown N, et al. Patency of the preterm fetal ductus arteriosus is regulated by endothelial nitric oxide synthase and is independent of vasa vasorum in the mouse. Am J Physiol Regul Integr Comp Physiol. 2004 Sep;287(3):R652–R660. doi: 10.1152/ajpregu.00049.2004. [DOI] [PubMed] [Google Scholar]
- 18.Baragatti B, Schwartzman ML, Angeloni D, Scebba F, Ciofini E, Sodini D, et al. EDHF function in the ductus arteriosus: evidence against involvement of epoxyeicosatrienoic acids and 12S-hydroxyeicosatetraenoic acid. Am J Physiol Heart Circ Physiol. 2009 Dec;297(6):H2161–H2168. doi: 10.1152/ajpheart.00576.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baragatti B, Ciofini E, Scebba F, Angeloni D, Sodini D, Luin S, et al. Cytochrome P-450 3A13 and endothelin jointly mediate ductus arteriosus constriction to oxygen in mice. Am J Physiol Heart Circ Physiol. 2011 Mar;300(3):H892–H901. doi: 10.1152/ajpheart.00907.2010. [DOI] [PubMed] [Google Scholar]
- 20.Morita K, Konishi H, Ono T, Shimakawa H. A comparison of the inhibitory effects of roxatidine acetate hydrochloride and cimetidine on cytochrome P-450-mediated drug-metabolism in mouse hepatic microsomes and in man in vivo. Journal of pharmacobio-dynamics. 1987 Jul;10(7):287–295. doi: 10.1248/bpb1978.10.287. [DOI] [PubMed] [Google Scholar]
- 21.Scholtholt J, Bickel M, Herling AW. A review of the animal pharmacology of roxatidine acetate. Drugs. 1988;35(Suppl 3):30–40. doi: 10.2165/00003495-198800353-00008. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka E, Nakamura K. The effect of roxatidine acetate and cimetidine on hepatic drug clearance assessed by simultaneous administration of three model substrates. British journal of clinical pharmacology. 1989 Aug;28(2):171–174. doi: 10.1111/j.1365-2125.1989.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakurai E, Kuramasu A, Watanabe T, Yanai K. Multiple histamine receptor gene knockout mice and their phenotypes. Inflamm Res. 2009 Apr;58(Suppl 1):41–42. doi: 10.1007/s00011-009-0659-5. [DOI] [PubMed] [Google Scholar]
- 24.Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS letters. 2001 Jul 27;502(1–2):53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- 25.Chorne N, Jegatheesan P, Lin E, Shi R, Clyman RI. Risk factors for persistent ductus arteriosus patency during indomethacin treatment. J Pediatr. 2007 Dec;151(6):629–634. doi: 10.1016/j.jpeds.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Reese J, Veldman A, Shah L, Vucovich M, Cotton RB. Inadvertent relaxation of the ductus arteriosus by pharmacologic agents that are commonly used in the neonatal period. Semin Perinatol. 2010 Jun;34(3):222–230. doi: 10.1053/j.semperi.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemerofsky SL, Parravicini E, Bateman D, Kleinman C, Polin RA, Lorenz JM. The ductus arteriosus rarely requires treatment in infants >1000 grams. American journal of perinatology. 2008 Nov;25(10):661–666. doi: 10.1055/s-0028-1090594. [DOI] [PubMed] [Google Scholar]
- 28.Kovalcik V. The response of the isolated ductus arteriosus to oxygen and anoxia. J Physiol. 1963;169:185–197. doi: 10.1113/jphysiol.1963.sp007249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods JR, Jr, Brinkman CR, 3rd, Assali NS. Fetal and neonatal cardiopulmonary response to histamine. Obstet Gynecol. 1976 Aug;48(2):195–202. [PubMed] [Google Scholar]
- 30.Echtler K, Stark K, Lorenz M, Kerstan S, Walch A, Jennen L, et al. Platelets contribute to postnatal occlusion of the ductus arteriosus. Nat Med. 2010 Jan;16(1):75–82. doi: 10.1038/nm.2060. [DOI] [PubMed] [Google Scholar]
- 31.Sallmon H, Weber SC, Huning B, Stein A, Horn PA, Metze BC, et al. Thrombocytopenia in the first 24 hours after birth and incidence of patent ductus arteriosus. Pediatrics. 2012 Sep;130(3):e623–e630. doi: 10.1542/peds.2012-0499. [DOI] [PubMed] [Google Scholar]
- 32.Coceani F, Kelsey L, Ackerley C, Rabinovitch M, Gelboin H. Cytochrome P450 during ontogenic development: occurrence in the ductus arteriosus and other tissues. Can J Physiol Pharmacol. 1994 Mar;72(3):217–226. doi: 10.1139/y94-034. [DOI] [PubMed] [Google Scholar]
- 33.Coceani F, Wright J, Breen C. Ductus arteriosus: involvement of a sarcolemmal cytochrome P-450 in O2 constriction? Can J Physiol Pharmacol. 1989 Nov;67(11):1448–1450. doi: 10.1139/y89-232. [DOI] [PubMed] [Google Scholar]
- 34.Levin M, McCurnin D, Seidner SR, Yoder B, Waleh N, Goldbarg S, et al. Postnatal Constriction, ATP Depletion, and Cell Death in the Mature and Immature Ductus Arteriosus. Am J Physiol Regul Integr Comp Physiol. 2006 Oct 13; doi: 10.1152/ajpregu.00629.2005. [DOI] [PubMed] [Google Scholar]
- 35.Reese J. Death, dying, and exhaustion in the ductus arteriosus: prerequisites for permanent closure. Am J Physiol Regul Integr Comp Physiol. 2006 Feb;290(2):R357–R358. doi: 10.1152/ajpregu.00749.2005. [DOI] [PubMed] [Google Scholar]
- 36.Fradette C, du Souich P. Hypoxia-inducible factor-1 and activator protein-1 modulate the upregulation of CYP3A6 induced by hypoxia. Br J Pharmacol. 2003 Nov;140(6):1146–1154. doi: 10.1038/sj.bjp.0705543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Legendre C, Hori T, Loyer P, Aninat C, Ishida S, Glaise D, et al. Drug-metabolising enzymes are down-regulated by hypoxia in differentiated human hepatoma HepaRG cells: HIF-1alpha involvement in CYP3A4 repression. Eur J Cancer. 2009 Nov;45(16):2882–2892. doi: 10.1016/j.ejca.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Fradette C, Bleau AM, Pichette V, Chauret N, Du Souich P. Hypoxia-induced down-regulation of CYP1A1/1A2 and up-regulation of CYP3A6 involves serum mediators. Br J Pharmacol. 2002 Nov;137(6):881–891. doi: 10.1038/sj.bjp.0704933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivey KN, Sutcliffe D, Richardson J, Clyman RI, Garcia JA, Srivastava D. Transcriptional regulation during development of the ductus arteriosus. Circ Res. 2008 Aug 15;103(4):388–395. doi: 10.1161/CIRCRESAHA.108.180661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alkayed NJ, Goyagi T, Joh HD, Klaus J, Harder DR, Traystman RJ, et al. Neuroprotection and P450 2C11 upregulation after experimental transient ischemic attack. Stroke; a journal of cerebral circulation. 2002 Jun;33(6):1677–1684. doi: 10.1161/01.str.0000016332.37292.59. [DOI] [PubMed] [Google Scholar]
- 41.Earley S, Pastuszyn A, Walker BR. Cytochrome p-450 epoxygenase products contribute to attenuated vasoconstriction after chronic hypoxia. Am J Physiol Heart Circ Physiol. 2003 Jul;285(1):H127–H136. doi: 10.1152/ajpheart.01052.2002. [DOI] [PubMed] [Google Scholar]
- 42.Fradette C, Batonga J, Teng S, Piquette-Miller M, du Souich P. Animal models of acute moderate hypoxia are associated with a down-regulation of CYP1A1, 1A2, 2B4, 2C5, and 2C16 and up-regulation of CYP3A6 and P-glycoprotein in liver. Drug metabolism and disposition: the biological fate of chemicals. 2007 May;35(5):765–771. doi: 10.1124/dmd.106.013508. [DOI] [PubMed] [Google Scholar]
- 43.Michaelis UR, Fisslthaler B, Barbosa-Sicard E, Falck JR, Fleming I, Busse R. Cytochrome P450 epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced endothelial cell migration and angiogenesis. J Cell Sci. 2005 Dec 1;118(Pt 23):5489–5498. doi: 10.1242/jcs.02674. [DOI] [PubMed] [Google Scholar]
- 44.Michaelis UR, Xia N, Barbosa-Sicard E, Falck JR, Fleming I. Role of cytochrome P450 2C epoxygenases in hypoxia-induced cell migration and angiogenesis in retinal endothelial cells. Invest Ophthalmol Vis Sci. 2008 Mar;49(3):1242–1247. doi: 10.1167/iovs.07-1087. [DOI] [PubMed] [Google Scholar]
- 45.Pokreisz P, Fleming I, Kiss L, Barbosa-Sicard E, Fisslthaler B, Falck JR, et al. Cytochrome P450 epoxygenase gene function in hypoxic pulmonary vasoconstriction and pulmonary vascular remodeling. Hypertension. 2006 Apr;47(4):762–770. doi: 10.1161/01.HYP.0000208299.62535.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keseru B, Barbosa-Sicard E, Popp R, Fisslthaler B, Dietrich A, Gudermann T, et al. Epoxyeicosatrienoic acids and the soluble epoxide hydrolase are determinants of pulmonary artery pressure and the acute hypoxic pulmonary vasoconstrictor response. Faseb J. 2008 Dec;22(12):4306–4315. doi: 10.1096/fj.08-112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capdevila JH. Regulation of ion transport and blood pressure by cytochrome p450 monooxygenases. Current opinion in nephrology and hypertension. 2007 Sep;16(5):465–470. doi: 10.1097/MNH.0b013e32827ab48c. [DOI] [PubMed] [Google Scholar]
- 48.Fleming I. Vascular cytochrome p450 enzymes: physiology and pathophysiology. Trends in cardiovascular medicine. 2008 Jan;18(1):20–25. doi: 10.1016/j.tcm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Costa M, Barogi S, Socci ND, Angeloni D, Maffei M, Baragatti B, et al. Gene expression in ductus arteriosus and aorta: comparison of birth and oxygen effects. Physiol Genomics. 2006 Apr 13;25(2):250–262. doi: 10.1152/physiolgenomics.00231.2005. [DOI] [PubMed] [Google Scholar]
- 50.Dagle JM, Lepp NT, Cooper ME, Schaa KL, Kelsey KJ, Orr KL, et al. Determination of genetic predisposition to patent ductus arteriosus in preterm infants. Pediatrics. 2009 Apr;123(4):1116–1123. doi: 10.1542/peds.2008-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004 Jan;14(1):1–18. doi: 10.1097/00008571-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 52.van Herwaarden AE, Wagenaar E, van der Kruijssen CM, van Waterschoot RA, Smit JW, Song JY, et al. Knockout of cytochrome P450 3A yields new mouse models for understanding xenobiotic metabolism. J Clin Invest. 2007 Nov;117(11):3583–3592. doi: 10.1172/JCI33435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ayajiki K, Fujioka H, Toda N, Okada S, Minamiyama Y, Imaoka S, et al. Mediation of arachidonic acid metabolite(s) produced by endothelial cytochrome P-450 3A4 in monkey arterial relaxation. Hypertens Res. 2003 Mar;26(3):237–243. doi: 10.1291/hypres.26.237. [DOI] [PubMed] [Google Scholar]
- 54.Mitra R, Guo Z, Milani M, Mesaros C, Rodriguez M, Nguyen J, et al. CYP3A4 Mediates Growth of Estrogen Receptor-positive Breast Cancer Cells in Part by Inducing Nuclear Translocation of Phospho-Stat3 through Biosynthesis of ({+/−})-14,15-Epoxyeicosatrienoic Acid (EET) J Biol Chem. 2011 May 20;286(20):17543–17559. doi: 10.1074/jbc.M110.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang T, Levine M, Bellward GD. Selective inhibition of rat hepatic microsomal cytochrome P-450. II. Effect of the in vitro administration of cimetidine. J Pharmacol Exp Ther. 1992 Mar;260(3):1450–1455. [PubMed] [Google Scholar]
- 56.Levine M, Bellward GD. Effect of cimetidine on hepatic cytochrome P450: evidence for formation of a metabolite-intermediate complex. Drug metabolism and disposition: the biological fate of chemicals. 1995 Dec;23(12):1407–1411. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.