Resveratrol has been proclaimed as an anti-aging compound to prevent and treat chronic conditions, including cardiovascular disease, diabetes mellitus and neurodegenerative disorders[1]. Though resveratrol’s potential utility in preventive medicine has been demonstrated using animal models [2], few clinical trials have evaluated the effects of resveratrol on gene expression or clinically relevant biomarkers in healthy individuals. Trials evaluating cardiovascular risk generally measure plasma inflammatory biomarkers, including interleukins (IL) 1β, IL-6, Interferon Gamma (IFN-γ), Tumor Necrosis Factor alpha (TNF-α), but do not consider how components in plasma may interactively drive convergent endothelial cellular responses that contribute to the pathogenesis of atherosclerosis, including release of chemokines, such as IL-8 and activation of Vascular Cell Adhesion Molecule (VCAM) and Intercellular Adhesion Molecule (ICAM) [3–5]. This clinical trial evaluated the effects of one month resveratrol treatment on endothelial response and plasma biomarkers in healthy individuals using a a novel unbiased assay to assess the overall inflammatory capacity of plasma on expression of genes associated with inflammation and atherosclerosis.
This double-blind, randomized, placebo-controlled clinical trial comprised of 44 healthy subjects (ClinicalTrials.gov: NCT01244360). Informed consent was obtained from each subject and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee. Exclusion criteria were: age <18 years; history of significant general medical conditions; history of drug or supplement use which could alter metabolic or cardiovascular physiology; and use of grape-related supplements within one year. Subjects took either RESV (400mg trans-resveratrol, 400mg grapeskin extract, and 100mg quercetin) or a cellulose placebo (PLA) for 30 days and were instructed to report side effects. Fasting blood was collected from each subject at baseline and the morning following the last day of treatment for biomarker assays.
Plasma concentrations of IL-1β, IL-6, IFN-γ, TNF-α, insulin, and leptin were analyzed using an electrochemiluminescence multiplex immunoassay (MesoScale, MD). To gain further insight into how RESV might affect atherosclerosis, we employed a novel assay whereby cultured human coronary artery endothelial cells (hCAECs; Lonza, NJ) were incubated with diluted plasma from participants. Gene expression of VCAM, ICAM, and IL-8 were quantified using our previously published methods[6]. Baseline versus post-treatment changes within each group were evaluated using paired t-tests when data met the assumptions of parametric statistics or the Wilcoxon signed-rank test when these assumptions were not met
One subject did not complete baseline testing, another withdrew due to scheduling conflicts, and a third receiving PLA withdrew due to gastrointestinal side effects, resulting in 41 completers (RESV: n= 7 male, 13 female; PLA: n= 6 male, 15 female). Two RESV subjects and one PLA subject reported mild gastrointestinal side effects. Side effects were consistent with previously reported data[1].
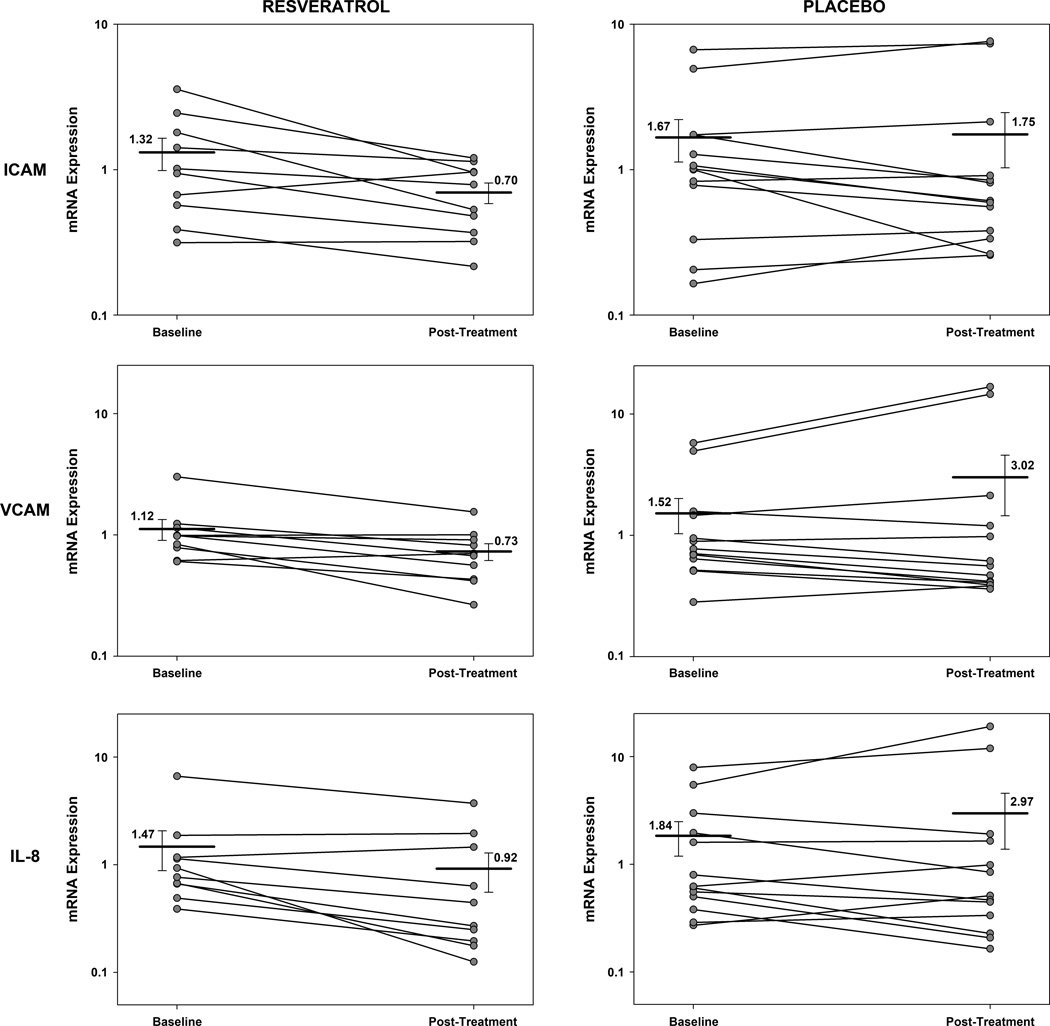
As demonstrated in Figure 1, exposing hCAECs to plasma drawn post-RESV resulted in significantly lower mRNA expression of VCAM (p=0.017), ICAM (p=0.037), and IL-8 (p=0.022) than plasma drawn from the same subjects at baseline, whereas PLA had no significant effect (p=0.65, 0.65, 0.753, respectively). There was a significant reduction in plasma IFN-γ in RESV (p=0.033), but not in PLA (p=0.96), and a significant reduction in fasting insulin concentration in RESV (p=0.045 vs. p=0.16 for PLA, one-tailed). These findings are in agreement with recent reports of resveratrol reducing inflammation and improving glucose tolerance in overweight subjects [7, 8]. There was a trend toward increased IL-1β in PLA (p=0.062), but not in RESV (p=0.62). Fasting glucose, leptin, IL-1β, IL-6, and TNFα were within normal limits, and not significantly affected by either treatment. Lack of significant changes in plasma IL-1β, IL-6, TNFα may be attributable to differences in subject population, dosage, and duration compared to other protocols [7, 8].
Figure 1. Comparison of ICAM, VCAM, and IL-8 mRNA expression in human coronary endothelial artery cells incubated using plasma from subjects in this trial.
Each pair of data points represents data from one subject at baseline and 30-days post treatment. The solid horizontal line and corresponding number is mean value for baseline and post-treatment data. Please note, the y-axis uses a logarithmic scale and represents mRNA expression in relative units (described previously[6]). There were significant (p<0.05) decreases in mRNA expression for ICAM, VCAM, and IL-8 for RESV, but not PLA. (ICAM = intracellular adhesion molecule; IL-8 = interleukin eight; mRNA = messenger ribonucleic acid; PLA = placebo; RESV = resveratrol; VCAM = vascular cell adhesion molecule)
Conventional plasma biomarkers of inflammation exhibited few changes, but endothelial cells were less activated by the complete plasma profile following RESV treatment. Various cytokines, proteins, and lipid intermediates converge mechanistically to influence the expression of ICAM, VCAM, and IL-8 [9], molecules that are directly related to atherosclerosis initiation[3, 5]. RESV may have increased plasma concentration of resveratrol and its metabolites[1] within an individual, leading to a systemic milieu within plasma that significantly suppressed expression of these biomarkers. Endothelial cell responses may reflect changes in individual plasma components and / or complex interactions due to changes in multiple components which offer cardioprotective effects that may be otherwise undetectable using conventional biomarker assays. While ICAM, VCAM and IL-8 are involved in the promotion of atherosclerosis, the novel assay paradigm requires further characterization in terms of cardiovascular disease risk.
Our findings suggest that altered plasma composition leading to decreased expression of endothelial cell ICAM, VCAM and IL-8 may be an important mechanism contributing to resveratrol’s beneficial effects on cardiovascular function. Further, RESV may reduce inflammatory biomarkers in individuals whose inflammatory biomarkers are already within normal limits, as demonstrated by decreases in plasma IFN-γ and insulin. Lastly, our novel assay demonstrates the potential value of evaluating the entire plasma milieu to quantify how various treatments alter endothelial response, and ultimately, atherosclerotic risk.
These data are the first to indicate that resveratrol may have protective effects against atherosclerosis in individuals who would not be considered high risk with the current screening criteria, suggesting resveratrol could receive consideration as a primary preventive agent. We are in the process of designing and conducting a large-scale trial examining the effects of resveratrol supplementation to identify mechanisms of clinical benefits and how these effects can be maximized.
Supplementary Material
ACKNOWLEDGEMENTS
Acknowledgement of Grant Support:
Financial support for this study was provided by National Institutes of Health (Bethesda, MD: ES014639 to MJC and AG031182 to JAB), Vinomis Laboratories (Sewickley, PA), and Marywood University (Scranton, PA). ES014639, National Institute of Environmental Health (Grant) was used to develop, validate, and conduct the endothelial cell assays used in this study. Beamon Agarwal is supported, in part, by AG031182. Vinomis Laboratories supplied RESV and PLA, provided funding for subject honoraria and data collection personnel, and along with Marywood University, provided funding to perform plasma biomarker assays.
The authors would like thank Gerald S. Zavorsky, PhD, Kathy Uhranowsky, RN, CCRP, and Charles G. Fisher, MS at Marywood University for their contributions to this study. The authors would also like to thank technical director Heather Collins, PhD of the Radioimmunoassay and Biomarkers Core of the University of Pennsylvania Diabetes Research Center (P30-DK19525).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Each of the eight authors takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Conflicts of Interests: The authors of this study have no conflicts of interest
The author(s) of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology: reference: Coats AJS and Shewan LG. Statement on Authorship and Publishing Ethics in the International Journal of Cardiology. Int J Cardiol 2011; 153: 239-40.
REFERENCES
- 1.Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health--a comprehensive review of human clinical trials. Mol Nutr Food Res. 2011;55:1129–1141. doi: 10.1002/mnfr.201100143. [DOI] [PubMed] [Google Scholar]
- 2.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt C, Hulthe J, Fagerberg B. Baseline ICAM-1 and VCAM-1 are increased in initially healthy middle-aged men who develop cardiovascular disease during 6.6 years of follow-up. Angiology. 2009;60:108–114. doi: 10.1177/0003319708316899. [DOI] [PubMed] [Google Scholar]
- 4.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–e636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 5.Luc G, Arveiler D, Evans A, Amouyel P, Ferrieres J, Bard JM, et al. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and incident coronary heart disease: the PRIME Study. Atherosclerosis. 2003;170:169–176. doi: 10.1016/s0021-9150(03)00280-6. [DOI] [PubMed] [Google Scholar]
- 6.Channell MM, Paffett ML, Devlin RB, Madden MC, Campen MJ. Circulating factors induce coronary endothelial cell activation following exposure to inhaled diesel exhaust and nitrogen dioxide in humans: Evidence from a novel translational in vitro model. Toxicol Sci. 2012;127(1):179–186. doi: 10.1093/toxsci/kfs084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, et al. One-Year Consumption of a Grape Nutraceutical Containing Resveratrol Improves the Inflammatory and Fibrinolytic Status of Patients in Primary Prevention of Cardiovascular Disease. Am J Cardiol. 2012;110(3):356–363. doi: 10.1016/j.amjcard.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.