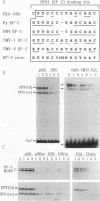
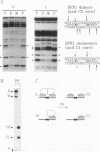
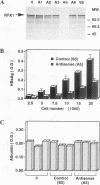
Abstract
Hepatitis B virus gene expression is to a large extent under the control of enhancer I (EnhI). The activity of EnhI is strictly dependent on the enhancer factor C (EF-C) site, an inverted repeat that is bound by a ubiquitous nuclear protein known as EF-C. Here we report the unexpected finding that EF-C is in fact identical to RFX1, a novel transcription factor previously cloned by virtue of its affinity for the HLA class II X-box promoter element. This finding has allowed us to provide direct evidence that RFX1 (EF-C) is crucial for EnhI function in HepG2 hepatoma cells; RFX1-specific antisense oligonucleotides appear to inhibit EnhI-driven expression of the hepatitis B virus major surface antigen gene, and in transfection assays, RFX1 behaves as a potent transactivator of EnhI. Interestingly, transactivation of EnhI by RFX1 (EF-C) is not observed in cell lines that are not of liver origin, suggesting that the ubiquitous RFX1 protein cooperates with liver-specific factors.
Full text
PDF
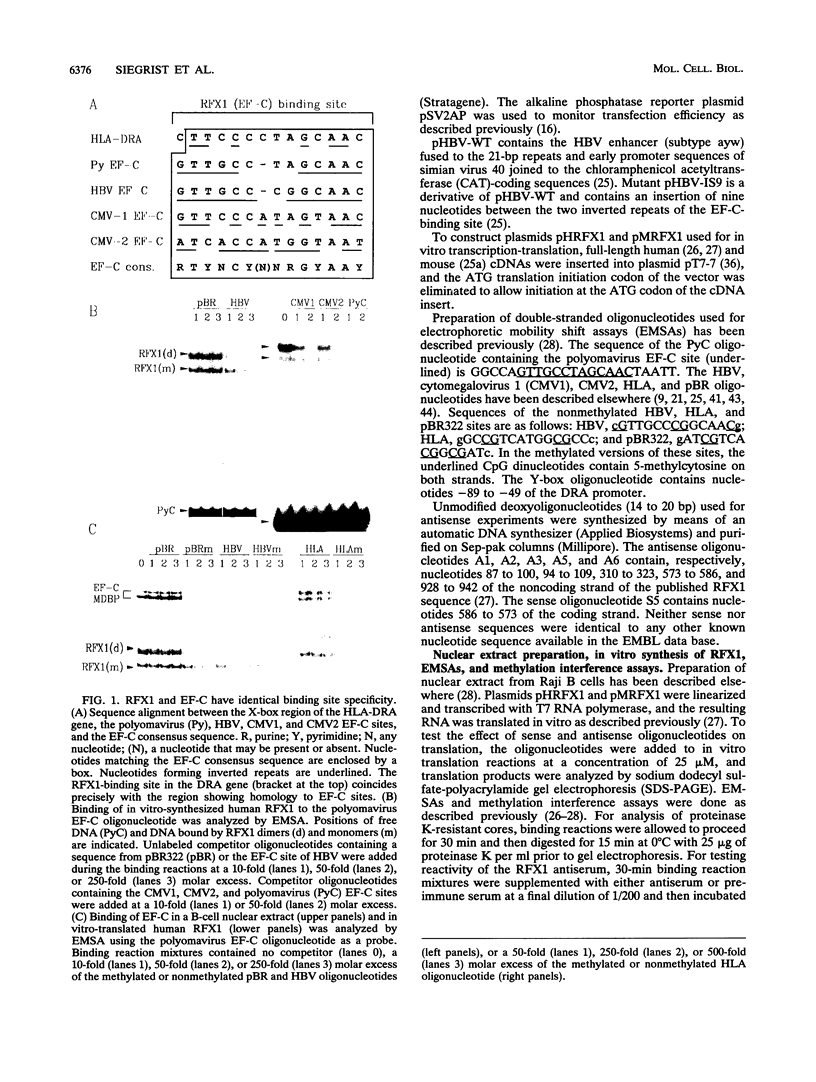






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Levy R., Faktor O., Berger I., Shaul Y. Cellular factors that interact with the hepatitis B virus enhancer. Mol Cell Biol. 1989 Apr;9(4):1804–1809. doi: 10.1128/mcb.9.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgrove R., Simon G., Ganem D. Transcriptional activation of homologous and heterologous genes by the hepatitis B virus X gene product in cells permissive for viral replication. J Virol. 1989 Sep;63(9):4019–4026. doi: 10.1128/jvi.63.9.4019-4026.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikstein R., Faktor O., Ben-Levy R., Shaul Y. Functional organization of the hepatitis B virus enhancer. Mol Cell Biol. 1990 Jul;10(7):3683–3689. doi: 10.1128/mcb.10.7.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikstein R., Heffetz D., Ben-Neriah Y., Shaul Y. c-abl has a sequence-specific enhancer binding activity. Cell. 1992 May 29;69(5):751–757. doi: 10.1016/0092-8674(92)90287-m. [DOI] [PubMed] [Google Scholar]
- Ehrlich K. C., Ehrlich M. Highly repeated sites in the apolipoprotein(a) gene recognized by methylated DNA-binding protein, a sequence-specific DNA-binding protein. Mol Cell Biol. 1990 Sep;10(9):4957–4960. doi: 10.1128/mcb.10.9.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfassi E. Broad specificity of the hepatitis B enhancer function. Virology. 1987 Sep;160(1):259–262. doi: 10.1016/0042-6822(87)90069-9. [DOI] [PubMed] [Google Scholar]
- Faktor O., Budlovsky S., Ben-Levy R., Shaul Y. A single element within the hepatitis B virus enhancer binds multiple proteins and responds to multiple stimuli. J Virol. 1990 Apr;64(4):1861–1863. doi: 10.1128/jvi.64.4.1861-1863.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. D., Ostapchuk P., Hearing P. Functional interaction of nuclear factors EF-C, HNF-4, and RXR alpha with hepatitis B virus enhancer I. J Virol. 1993 Jul;67(7):3940–3950. doi: 10.1128/jvi.67.7.3940-3950.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. D., Ostapchuk P., Hearing P. Methylation-dependent and -independent DNA binding of nuclear factor EF-C. Virology. 1991 Jun;182(2):857–860. doi: 10.1016/0042-6822(91)90629-p. [DOI] [PubMed] [Google Scholar]
- Ghazal P., Lubon H., Hennighausen L. Multiple sequence-specific transcription factors modulate cytomegalovirus enhancer activity in vitro. Mol Cell Biol. 1988 Apr;8(4):1809–1811. doi: 10.1128/mcb.8.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi G., Ohno H., Adams R., Darabi A., Tewari A., Watabe M., Watabe K. Mutational analysis of enhancer domains responsive to trans-activation by the X gene of human hepatitis B virus. Arch Virol. 1990;114(3-4):237–242. doi: 10.1007/BF01310752. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W. T., Bell K. D., Ou J. H. Characterization of the hepatitis B virus EnhI enhancer and X promoter complex. J Virol. 1991 Dec;65(12):6686–6692. doi: 10.1128/jvi.65.12.6686-6692.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa S. L., Sloan J. H., Reith W., Mach B., Boss J. M. Regulatory factor-X binding to mutant HLA-DRA promoter sequences. Nucleic Acids Res. 1991 Mar 25;19(6):1243–1249. doi: 10.1093/nar/19.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn P., Zervos P., Raducha M., Harris H., Kadesch T. Expression of a human placental alkaline phosphatase gene in transfected cells: use as a reporter for studies of gene expression. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6342–6346. doi: 10.1073/pnas.85.17.6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero Sanchez C., Reith W., Silacci P., Mach B. The DNA-binding defect observed in major histocompatibility complex class II regulatory mutants concerns only one member of a family of complexes binding to the X boxes of class II promoters. Mol Cell Biol. 1992 Sep;12(9):4076–4083. doi: 10.1128/mcb.12.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K. Q., Siddiqui A. Regulation of the hepatitis B virus gene expression by the enhancer element I. Virology. 1991 Apr;181(2):721–726. doi: 10.1016/0042-6822(91)90906-r. [DOI] [PubMed] [Google Scholar]
- Huang L. H., Wang R., Gama-Sosa M. A., Shenoy S., Ehrlich M. A protein from human placental nuclei binds preferentially to 5-methylcytosine-rich DNA. Nature. 1984 Mar 15;308(5956):293–295. doi: 10.1038/308293a0. [DOI] [PubMed] [Google Scholar]
- Kekulé A. S., Lauer U., Weiss L., Luber B., Hofschneider P. H. Hepatitis B virus transactivator HBx uses a tumour promoter signalling pathway. Nature. 1993 Feb 25;361(6414):742–745. doi: 10.1038/361742a0. [DOI] [PubMed] [Google Scholar]
- Khan R., Zhang X. Y., Supakar P. C., Ehrlich K. C., Ehrlich M. Human methylated DNA-binding protein. Determinants of a pBR322 recognition site. J Biol Chem. 1988 Oct 5;263(28):14374–14383. [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Maguire H. F., Hoeffler J. P., Siddiqui A. HBV X protein alters the DNA binding specificity of CREB and ATF-2 by protein-protein interactions. Science. 1991 May 10;252(5007):842–844. doi: 10.1126/science.1827531. [DOI] [PubMed] [Google Scholar]
- Ostapchuk P., Diffley J. F., Bruder J. T., Stillman B., Levine A. J., Hearing P. Interaction of a nuclear factor with the polyomavirus enhancer region. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8550–8554. doi: 10.1073/pnas.83.22.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapchuk P., Scheirle G., Hearing P. Binding of nuclear factor EF-C to a functional domain of the hepatitis B virus enhancer region. Mol Cell Biol. 1989 Jul;9(7):2787–2797. doi: 10.1128/mcb.9.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith W., Barras E., Satola S., Kobr M., Reinhart D., Sanchez C. H., Mach B. Cloning of the major histocompatibility complex class II promoter binding protein affected in a hereditary defect in class II gene regulation. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4200–4204. doi: 10.1073/pnas.86.11.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith W., Herrero-Sanchez C., Kobr M., Silacci P., Berte C., Barras E., Fey S., Mach B. MHC class II regulatory factor RFX has a novel DNA-binding domain and a functionally independent dimerization domain. Genes Dev. 1990 Sep;4(9):1528–1540. doi: 10.1101/gad.4.9.1528. [DOI] [PubMed] [Google Scholar]
- Reith W., Satola S., Sanchez C. H., Amaldi I., Lisowska-Grospierre B., Griscelli C., Hadam M. R., Mach B. Congenital immunodeficiency with a regulatory defect in MHC class II gene expression lacks a specific HLA-DR promoter binding protein, RF-X. Cell. 1988 Jun 17;53(6):897–906. doi: 10.1016/s0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Rossner M. T., Jackson R. J., Murray K. Modulation of expression of the hepatitis B virus surface antigen gene by the viral X-gene product. Proc Biol Sci. 1990 Jul 23;241(1300):51–58. doi: 10.1098/rspb.1990.0065. [DOI] [PubMed] [Google Scholar]
- Seto E., Mitchell P. J., Yen T. S. Transactivation by the hepatitis B virus X protein depends on AP-2 and other transcription factors. Nature. 1990 Mar 1;344(6261):72–74. doi: 10.1038/344072a0. [DOI] [PubMed] [Google Scholar]
- Shaul Y., Rutter W. J., Laub O. A human hepatitis B viral enhancer element. EMBO J. 1985 Feb;4(2):427–430. doi: 10.1002/j.1460-2075.1985.tb03646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandau D. F., Lee C. H. trans-activation of viral enhancers by the hepatitis B virus X protein. J Virol. 1988 Feb;62(2):427–434. doi: 10.1128/jvi.62.2.427-434.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Yee J. K. Regulation of hepatitis B virus gene expression by its two enhancers. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2708–2712. doi: 10.1073/pnas.89.7.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supakar P. C., Zhang X. Y., Githens S., Khan R., Ehrlich K. C., Ehrlich M. How different DNA sequences are recognized by a DNA-binding protein: effects of partial proteolysis. Nucleic Acids Res. 1989 Nov 11;17(21):8611–8629. doi: 10.1093/nar/17.21.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognoni A., Cattaneo R., Serfling E., Schaffner W. A novel expression selection approach allows precise mapping of the hepatitis B virus enhancer. Nucleic Acids Res. 1985 Oct 25;13(20):7457–7472. doi: 10.1093/nar/13.20.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo M. A., Letovsky J., Maguire H. F., Lopez-Cabrera M., Siddiqui A. Functional analysis of a liver-specific enhancer of the hepatitis B virus. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3797–3801. doi: 10.1073/pnas.88.9.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Schloemer R. H. Transcriptional trans-activating function of hepatitis B virus. J Virol. 1987 Nov;61(11):3448–3453. doi: 10.1128/jvi.61.11.3448-3453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger T., Shaul Y. The X protein of the hepatitis B virus acts as a transcription factor when targeted to its responsive element. EMBO J. 1990 Jun;9(6):1889–1895. doi: 10.1002/j.1460-2075.1990.tb08315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. Y., Zhang X. Y., Khan R., Zhou Y. W., Huang L. H., Ehrlich M. Methylated DNA-binding protein from human placenta recognizes specific methylated sites on several prokaryotic DNAs. Nucleic Acids Res. 1986 Dec 22;14(24):9843–9860. doi: 10.1093/nar/14.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee J. K. A liver-specific enhancer in the core promoter region of human hepatitis B virus. Science. 1989 Nov 3;246(4930):658–661. doi: 10.1126/science.2554495. [DOI] [PubMed] [Google Scholar]
- Zhang X. Y., Asiedu C. K., Supakar P. C., Khan R., Ehrlich K. C., Ehrlich M. Binding sites in mammalian genes and viral gene regulatory regions recognized by methylated DNA-binding protein. Nucleic Acids Res. 1990 Nov 11;18(21):6253–6260. doi: 10.1093/nar/18.21.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Y., Inamdar N. M., Supakar P. C., Wu K., Ehrlich K. C., Ehrlich M. Three MDBP sites in the immediate-early enhancer-promoter region of human cytomegalovirus. Virology. 1991 Jun;182(2):865–869. doi: 10.1016/0042-6822(91)90631-k. [DOI] [PubMed] [Google Scholar]