Abstract
INTRODUCTION
We report the long-term results of RTOG 9801, a randomized trial investigating the ability of amifostine, a radioprotector, to reduce chemoradiation-induced esophagitis.
METHODS
Patients with stage II and IIIA/B non-small-cell lung cancer received induction paclitaxel 225 mg/m2 intravenously (IV) and carboplatin area under the curve (AUC) 6 both days 1 and 22, followed by concurrent weekly paclitaxel (50 mg/m2) and carboplatin (AUC 2), with hyperfractionated radiation therapy (69.6 Gy at 1.2 Gy BID). Patients were randomly assigned to amifostine (AM) 500 mg IV four times per week or no-AM during chemoradiotherapy. Stratification factors included age (< 70 v ≥ 70 years), stage and performance status.
RESULTS
243 patients (pts) were enrolled; 120 received AM, 123 received no-AM. Two pts on each arm were found ineligible. Overall, 85% of patients were ≤70 years; 75% had a KPS ≥90. 34% had squamous histology. With median follow-up of 96.3 months (for patients still alive), overall survival was identical (Hazard ratio 1.03 (0.79–1.34), NS): five-year survival 17% in both arms. The incidence of late grade 3–5 toxicities was 16% in the AM arm and 19% in the control arm (Hazard ratio 1.24 (0.66–2.32), NS). There was no significant difference between the arms regarding overall survival, disease-free survival or long-term toxicity.
CONCLUSION
The chemoradiation regimen of carboplatin and paclitaxel produced long-term results in the multi-institutional setting comparable to other regimens. Amifostine did not appear to compromise survival. Better data is required regarding the comparative long-term toxicity of different chemoradiation regimens. NCT00003313.
Keywords: NSCLC, non-small cell lung cancer, chemoradiation, carboplatin, paclitaxel, amifostine
Introduction
The treatment of locally advanced, unresectable non-small cell lung cancer (NSCLC) is challenging. Radiation therapy (RT) combined with chemotherapy is more efficacious than RT alone[1–3]; furthermore, concomitant chemoradiation produces longer overall survival than sequential chemotherapy and RT[4, 5]. The majority of chemoradiation randomized clinical trials have featured cisplatin based combination chemotherapy concurrent with RT with median survival times of 16–18 months, and 5 year survival rates of 15 to 20%[5, 6]. Debate continues about the relative merits of cisplatin versus carboplatin in NSCLC. Carboplatin-paclitaxel was given concurrently with radiation therapy in CALGB 39801, however the relatively low median overall survival (12–14 months) observed in that trial raised the concern that this regimen might be suboptimal in the combined modality setting[7].
Radiation Therapy Oncology Group (RTOG) 9801 was a randomized trial designed to assess the role of the cytoprotectant free-radical scavenger amifostine (AM) in combination with definitive chemoradiation for NSCLC. This trial employed carboplatin and paclitaxel both in the induction setting and concomitantly with radiation therapy. We previously reported that the rate of esophagitis as assessed by standard NCI-CTC toxicity criteria (the primary endpoint) was not significantly different between the two arms[8], although amifostine appeared to improve some patient reported outcomes[9]. In this manuscript, we update the long-term survival results, patterns of failure and late toxicities as of June 30, 2010, when data collection for this study was finalized.
Methods
Patient Selection and Eligibility
Eligibility requirements have already been described[8], briefly, subjects were required to have unresectable and/or loco-regionally advanced NSCLC (stages II, IIIA, or IIIB), age ≥ 18 years, Karnofsky performance status ≥ 70, and weight loss ≤ 5% in the prior 3 months. Laboratory criteria included a serum creatinine ≤ 1.5 mg/dL, hemoglobin ≥ 8 g/dL, absolute granulocyte count ≥ 2,000/uL. Exclusion criteria included prior chemotherapy or thoracic or neck radiation therapy, or a prior invasive malignancy within 3 years. Pretreatment evaluations included a computed tomography of chest, liver, and adrenals within 4 weeks; brain computed tomography (or magnetic resonance imaging) and bone scan within 6 weeks. Baseline pulmonary function tests were required. Toxicities were reported using the National Cancer Institute Common Toxicity Criteria version 2.0 and adverse event reporting. Late toxicities were scored using the RTOG / EORTC late radiation morbidity scoring.
Treatment Regimen
The treatment regimen has been described previously[8]. Briefly, treatment began with two cycles of induction chemotherapy (CT). Paclitaxel 225 mg/m2 was administered intravenously (IV) followed directly by carboplatin (AUC 6) IV on days 1 and 22. This was followed by concurrent weekly paclitaxel (50 mg/m2 IV) and carboplatin (AUC 2) with hyperfractionated RT starting day 43. RT was administered at 1.2 Gy twice daily at least 5 hours apart (5 days a week) to a total dose of 69.6 Gy. A total of 42 fractions (50.4 Gy) were delivered to the primary tumor and mediastinum plus a boost to the primary tumor and involved nodes of 19.2 Gy in 16 fractions. The boost volume was treated every Friday (on days without AM in both arms), as well as the last three treatment days. Computer tomography–based treatment planning was recommended, but not required.
At registration, patients were randomly assigned to receive or not to receive AM, which was administered at a flat dose of 500 mg IV primarily before the afternoon (PM) treatment 4 days per week (Monday to Thursday). AM, when given, was always administered before the chemotherapy infusion. The protocol was approved by each institutions review board, and all patients provided informed consent.
Statistical Analysis
This non-blinded randomized study was powered to assess a change in the primary endpoint of NCI-CTC esophagitis rates as previously published[8]. The treatment allocation used a randomized permuted block within strata to balance for patient factors. The stratified variables included age (< 70 v ≥ 70 years), stage (II v IIIA or IIIB), and Karnofsky performance status score (70 – 80 v 90 – 100). Survival was measured from the date of randomization to the date of last follow-up or death. Point estimates were computed using the Kaplan-Meier method[10] with groups compared using the log-rank test[11]. Time to late toxicity was estimated using the competing risk method and compared using the Gray s test[12] an event was considered experiencing a given toxicity, and death without toxicity was considered as a competing risk. In tables of proportions, the two treatment groups were compared using the chi-square test. No adjustments were made for multiple comparisons. All analyses were performed using SAS 9.1 (Cary, NC). The trial was registered on ClinicalTrials.gov, NCT00003313.
Disease-free survival time was measured from the date of randomization to the date of first failure (local progression, regional progression, distant progression, death due to any cause); patients who did not experience a failure were censored at the date of last follow-up. Overall survival time was measured from the date of randomization to the date of death from any cause; patients who were not reported as deceased were censored at the date of last follow-up. Median follow-up was simply the median value of all follow-up times and is provided to give a general sense of the duration of follow-up.
Results
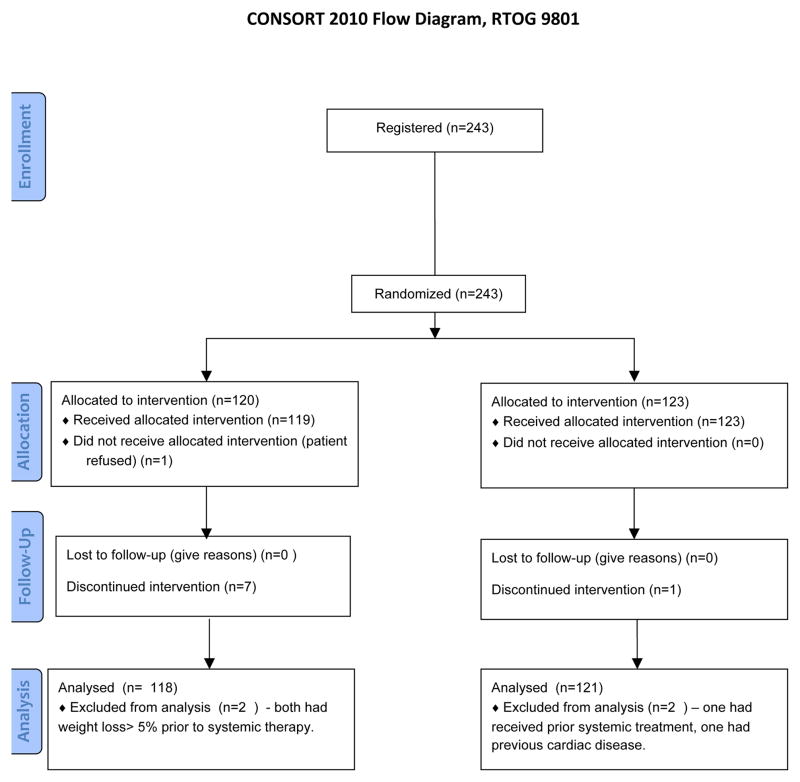
Between September 1998 and March 2002, 243 patients were accrued, 120 to the AM arm and 123 to the No-AM arm. Two patients in each arm were found to be ineligible: two patients had weight loss > 5% prior to study entry, one had received prior systemic chemotherapy, and one had pre-existing cardiac disease, (Fig. 1). A total of 239 patients were analyzable. The baseline demographics were well balanced; no significant differences existed between treatment arms with regard to patient age, sex, performance status, histology, stage, or mean esophageal length in the radiation field (Table I). Median follow-up for the 22 patients still alive is 96.3 months (range 4.0 – 132.2 months). Acute toxicities have been previously reported[8]; briefly, there was more nausea, vomiting, hypotension, and acute infections in the AM arm, but less weight-loss compared to the no-AM arm. There was a lower incidence of swallowing symptoms in the AM arm as recorded in patient diaries, but no reduction in rate of esophagitis as assessed by health care personnel.
Figure 1.
CONSORT flow diagram
Table I.
Pretreatment Characteristics
| Amifostine (n=118) | No Amifostine (n=121) | |||
|---|---|---|---|---|
| Age | ||||
| Median | 62 | 61 | ||
| Range | 34 – 81 | 38 – 79 | ||
| n | % | n | % | |
|
|
||||
| Age | ||||
| ≤ 70 | 99 | 84 | 103 | 85 |
| > 70 | 19 | 16 | 18 | 15 |
| Gender | ||||
| Male | 70 | 59 | 79 | 65 |
| Female | 48 | 41 | 42 | 35 |
| KPS | ||||
| 70–80 | 33 | 28 | 26 | 21 |
| 90–100 | 85 | 72 | 95 | 79 |
| Histology | ||||
| Squamous | 42 | 36 | 39 | 32 |
| Adenocarcinoma | 31 | 26 | 44 | 36 |
| Large cell | 9 | 8 | 12 | 10 |
| Mixed squamous/non-squamous | 2 | 2 | 1 | 1 |
| NSCLC NOS | 32 | 27 | 25 | 21 |
| Other | 2 | 2 | 0 | 0 |
| AJC Stage | ||||
| IIA | 1 | 1 | 1 | 1 |
| IIB | 7 | 6 | 6 | 5 |
| IIIA | 54 | 46 | 54 | 45 |
| IIIB | 56 | 47 | 60 | 50 |
A summary of late adverse events (AE) possibly related to radiation is presented in Table II; these were not significantly different between the two arms. There were 21, 3 and 2 late grade 3, 4 and 5 AEs in the AM arm, and 18, 4 and 2 late grade 3, 4 and 5 AEs in the control arm, respectively. Worse toxicities per patient were 12 (11%), 3 (3%) and 2 (2%) late grade 3, 4 and 5 AEs in the AM arm, and 16 (14%), 4 (4%) and 2 (2%) in the control arm, respectively. There were two late treatment-related deaths in each group, all of which were related to pneumonitis. The incidence of late esophageal and late cardiac radiation-related AEs was similar between the two arms: esophageal AEs were 4%, and 3% for AM and no-AM arms, respectively, and cardiac AEs were 6% and 4% for the AM and no-AM arms, respectively. Non-radiation related AEs were assessed separately. No one toxicity type predominated. The incidence of worse overall grade 3, 4 and 5 non-radiation AEs was 9%, 2% and 0% for AM arm vs. 11%, 4% and 1% for the control arm. There was no difference in the cumulative incidence rates of any grade 3+ late toxicity overall or grade 3+ late lung toxicity between the two arms.
Table II. Late adverse events ≥ grade 3 possibly related to radiation.
These are raw patient numbers, unless otherwise indicated.
| Amifostine n=108 | No Amifostine n=114 | |||
|---|---|---|---|---|
|
| ||||
| Grade 3,4 | Grade 5 | Grade 3,4 | Grade 5 | |
| Bone | 1 (1%) | 0 | 1 (1%) | 0 |
| Esophagus | 4 (4%) | 0 | 3 (3%) | 0 |
| Heart | 6 (6%) | 0 | 4 (4%) | 0 |
| Lung | 12 (11%) | 2 (2%) | 12(11%) | 2 (2%) |
| Skin | 0 | 0 | 0 | 0 |
| Spinal cord | 1 (1%) | 0 | 0 | 0 |
| Subcutaneous tissue | 0 | 0 | 0 | 0 |
| Other | 0 | 0 | 2 (2%) | 0 |
|
| ||||
| Worst Toxicity per Patient | 15 (14%) | 2 (2%) | 20 (18%) | 2 (2%) |
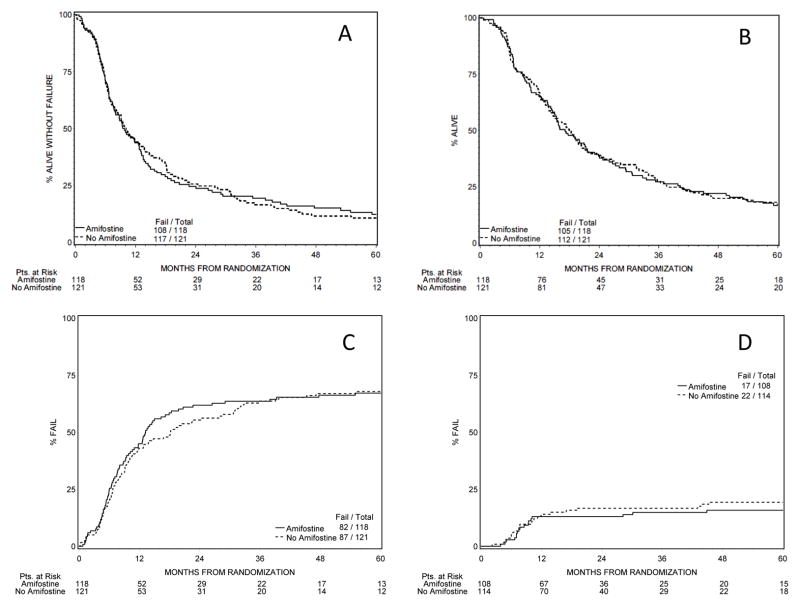
At five years, 18 patients were alive in the AM arm and 20 in the no-AM arm; of these 13 and 12, respectively, were disease-free. There was no significant difference between the arms regarding overall or disease free survival. One, two, four and five year overall-survivals were 65%, 38%, 22% and 17% in the AM arm and 67%, 39%, 20% and 17% in the no-AM arm respectively; hazard ratio 1.03 (0.79, 1.34) p=0.85. Median disease free survival was 9.7 months in the AM arm and 10.1 months in the control arm (Fig. 2), hazard ratio 1.07 (0.82, 1.38), p=0.64. Response rates also did not differ between the arms. At time of final analysis (June 2010), 11% and 7% of patients were alive in the AM and no-AM arms (Table III); median survival times were 17.1 and 17.9 months respectively (p=0.85). Cause of death was assessed by each institution individually.
Figure 2.
(a) Disease-Free Survival, hazard ratio between the arms: 1.07, confidence interval (0.82, 1.38) p=0.64
(b) Overall Survival, hazard ratio between the arms: 1.03, confidence interval (0.79, 1.34) p=0.85
(c) Time to Progression, hazard ratio between the arms: 0.98, confidence interval (0.72, 1.32) p=0.88
(d) Cumulative Severe Toxicity (>= grade 3), hazard ratio between the arms: 1.24, confidence interval (0.66, 2.32) p=0.51.
Overall survival and disease-free survival hazard ratios from Cox proportional hazards model; hazard ratios for time to progression and toxicity from Fine-Gray proportional hazards model.
Table III. Patient disposition and cause of death at time of final data analysis June 30, 2010.
These are raw patient numbers, unless otherwise indicated. Difference in overall survival, disease-free survival and time-to-progression between the two treatment-arms are not statistically significant. ‘Cause of Death’ was determined at the institutional level, there are consequently slight discrepancies between these figures and grade 5 toxicity data that was determined centrally.
| Amifostine (n=118) | No Amifostine (n=121) | |
|---|---|---|
| Evidence of disease progression | 82 (69.5%) | 87 (71.9%) |
|
| ||
| Total Alive | 13 (11.0%) | 9 (7.4%) |
| Alive and disease free | 10 (8.5%) | 4 (3.3%) |
|
| ||
| Total Dead | 105 (89.0%) | 112 (92.6%) |
| Dead with disease progression | 79 (66.9%) | 82 (67.8%) |
| Dead without disease progression | 26 (22.0%) | 30 (24.8%) |
|
| ||
| Cause of Death | ||
| Definitely or probably related to lung cancer | 80 (76%) | 82 (73%) |
| Related to or probably related to protocol treatment toxicity | 4 (4%) | 5 (4%) |
| Related to or probably related to non-protocol treatment | 0 | 1 (1%) |
| Related to or probably related to second primary tumor | 0 | 1 (1%) |
| Other cause | 12 (11%) | 11 (10%) |
| Unknown/non reported | 9 (9%) | 12 (10%) |
For 68% of subjects in each arm disease, progression was reported, hazard ratio 0.98 (0.72, 1.32) p=0.88. Patterns of failure were similar between the two arms. The percentage of patients whose first site of failure was local-regional, metastatic or simultaneously local-regional and metastatic was 44%, 39% and 17% in the AM arm, and 37%, 41% and 22% in the no-AM arm respectively.
Discussion
The primary aim of the trial was to test the ability of the cytoprotectant, AM to reduce chemoradiotherapy-induced esophagitis and evaluate its influence on quality of life (QOL) and swallowing symptoms. Amifostine acts as a free-radical scavenger (other mechanisms may also play a role) protecting tissues from the cytotoxic effects of DNA damaging agents such as ionizing radiation and platinum based chemotherapeutics[13]. Its principal activity is to protect normal tissue, but there are concerns that it may possibly protect tumor tissues as well. Previously we reported that although AM did not protect against acute esophagitis, patients receiving AM reported less swallowing symptoms than those receiving control[8]. This update reiterates that there was no difference between the arms regarding progression-free survival, or overall survival. It is important to note that RTOG 9801 was powered to detect differences in severe acute esophagitis, and quality of life. Conversely, the trial was not powered to detect small differences in survival between the arms, a fact reflected in the wide confidence intervals on the hazard ratios for overall- and disease-free survival. Therefore, based upon our data we are unable to state conclusively that amifostine did not affect survival outcomes. Our results demonstrating no difference in survival between arms are in agreement with other randomized studies examining the ability of amifostine to prevent radiation-induced toxicity in lung[14] and head-and-neck[15–17] cancer. Of note, Bourhis performed a meta-analysis of 12 trials and 1,119 patients based upon individual patient data (including RTOG 9801), covering three disease sites including lung cancer[18]. Bourhis concluded that amifostine did not affect tumor control, with a hazard ratio (HR) of death 0.98 (95% CI, 0.84 to 1.14;P = 0.78) and a HR of progression, relapse, or death 1.05 (95% CI, 0.90 to 1.22; P =0.53).
In order to view the results of RTOG 9801 in context we performed a systematic review of randomized clinical trials involving concurrent chemo-radiation in NSCLC. Articles containing the terms “non small cell lung cancer and randomized trial and (concurrent or concurrent) and (radiation and radiotherapy)” were searched for in PubMed. Only clinical trials published in English, randomizing more than 150 patients, involving radiation to the thorax with curative intent were included. This search produced 18 studies, further studies were added through the process of “snow-balling”. Results of the review are presented in Table IV.
Table IV. Historical comparison with other chemotherapeutic regimes.
Survival data for several of the studies were derived from published Kaplan-Meier curves. The selection of trials included in the Table is explained in Discussion.
| Trial | n | Non-concomitant tx Induction (I)/consolidative (C) | Concomitant tx | Radiation dose (Gy) | Median PFS* (months) | Median OS (months) | 2 year survival | 4 year survival | 5 yr survival | Late tox >=Gr 3 | ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | RTOG 9801 | 118 | I: Ca+P | Ca+P± Amifostine | 69.6 bid | 9.7 | 17.1 | 38% | 22% | 17% | 15.8% | |
| 121 | I: Ca+P | Ca+P | 69.6 bid | 10.1 | 17.9 | 39% | 20% | 18% | 19.3% | |||
| 1995 | Yugoslovia 1995 | 61 | - | none | 64.8 BID | 6 | 8 | 25 | 4.9 | 4.9 | 3.3% | [37] |
| 52 | - | Ca + vp16 | 64.8 BID | 16 | 18 | 35 | 21 | 21 | 15.8% | |||
| 56 | - | Ca + vp16 | 64.8 BID | 11 | 13 | 27 | 16 | 16 | 14.3% | |||
| 1997 | RTOG 92-04 | 81 | I: Vib + Cppt | Cppt | 63 | 9.4 | 16.4 | 39 | 16 | 13 | 30% | [38] |
| 82 | Cppt + VP16 | 69.6 BID | 8.2 | 15.5 | 32 | 17 | 16 | 43% | ||||
| 1999 | CALGB/ECOG | 137 | I: Vib + Cppt | 60 | 8.2 | 13.5 | 26% | 10% | 10% | NR | [39] | |
| 146 | I: Vib + Cppt | Ca | 60 | 8.6 | 13.4 | 29% | 13% | 10% | NR | |||
| 1999n | West Japan | 156 | Mmc + Vnd + Cppt | 56 | 10 | 16.5 | 22.2% | 16.9% | 15.8% | NR | [6] | |
| 158 | I: Mmc + Vnd + Cppt | 56 | 13.3 | 27.4% | 10.1% | 8.9% | NR | |||||
| 2001 | Yugoslovia 2001 | 98 | Ca + vp16 | 69.6 BID | NR | 20 | 47% | 20% | 20% | 21% | [40] | |
| 97 | Ca + vp16 | 69.6 BID | NR | 22 | 49% | 23% | 23% | 20% | ||||
| 2002 | NCCTG | 117 | Cppt + VP16 | 60 | 9.4 | 14 | 37% | 21% | 13% | 5% | [41] | |
| 117 | Cppt + VP16 | 60 split BID | 9.6 | 15 | 40% | 26% | 20% | 5% | ||||
| 2002 | CALGB 9431 | 62 | I: Cppt + Gem | Cppt + Gem | 66 | 8.4 | 18.3 | 37% | NR | NR | NR | [30] |
| 58 | I: Cppt + P | Cppt + P | 66 | 9.1 | 14.8 | 29% | NR | NR | NR | |||
| 55 | I: Cppt + Vin | Cppt + Vin | 66 | 11.5 | 17.7 | 40% | NR | NR | NR | |||
| 2005 | GLOT-GFPC NPC 95-01 | 101 | I: Cppt + Vin | 66 | NR | 14.5 | 26.5% | 14.2% | 10% | NR | [42] | |
| 100 | C: Cppt + Vin | Cppt + Vp16 | NR | 16.3 | 39.3% | 20.7% | 18% | NR | ||||
| 2005 | LAMP | 91 | I: Ca + P | - | 63 | 9 | 13 | 30% | NR | NR | NR | [43] |
| 74 | I: Ca + P | Ca+P | 63 | 6.7 | 12.7 | 25% | NR | NR | NR | |||
| 92 | C: Ca + P | Ca+P | 63 | 8.7 | 16.3 | 31% | NR | NR | NR | |||
| 2007 | EORTC 08972-22973 | 78 | Cppt | 66 | 8.5 | 16.5 | 39% | 17% | NR | NR | [44] | |
| 80 | I: Gem + Cppt | 66 | 10.8 | 16.2 | 34% | 15% | NR | NR | ||||
| 2007 | China | 98 | I: Cppt + VP16 | Cppt + VP16 | ENI 60-64 | 11.5 | 15 | 25.6% | 20% | 18.3% | NR | [45] |
| 98 | I: Cppt + VP16 | Cppt + VP16 | IFI 68-74 | 17 | 20 | 39.4% | 28% | 25.1% | NR | |||
| 2007 | CALGB 39801 | 182 | - | Ca+P | 66 | 7 | 12 | 29% | 14% | 11% | NR | [7] |
| 184 | I: Ca+P | Ca+P | 66 | 8 | 14 | 31% | 17% | 14% | NR | |||
| 2008 | GLCCG | 142 | I: Cppt + VP16, resection following RT | Ca + Vnd | 45 BID pre-op | 9.5 | 15.7 | 34% | 25% | 21% | NR | [46] |
| 154 | I: Cppt + VP16, resection following chemo | 54 post-op | 10 | 17.6 | 38% | 23% | 18% | NR | ||||
| 2008 | SWOG S0023 | 125 | C: Doc | Cppt + Vp16 | 61 | 11.7** | 35** | 60% | 40% | NR | NR | [47] |
| 118 | C: Doc + gefitinib | Cppt + Vp16 | 61 | 8.3** | 23** | 46% | 20% | NR | NR | |||
| 2010 | WJTOG0105 | 153 | C: Cppt + Vnd + Mmc | Cppt + Vnd + Mmc | 60 split | 8.2 | 20.5 | 45% | 28% | 17.5% | NR | [26] |
| 152 | C: Ca + Cpt11 | Ca + Cpt11 | 60 | 8.0 | 19.8 | 40% | 20% | 17.8% | NR | |||
| 156 | C: Ca + P | Ca + P | 60 | 9.5 | 22.0 | 45% | 22% | 19.5% | NR | |||
| 2010 | OLCSG 0007 | 99 | Cppt + Doc | 60 | 13.4 | 26.8 | 60.3% | 30% | 23% | NR | [27] | |
| 101 | Mmc + Vnd + Cppt | 60 | 10.5 | 23.7 | 48.1% | 23% | 16% | NR | ||||
| 2011 | RTOG 9410 | 195 | I: Cppt + Vib | - | 63 | NR | 14.6 | 31% | 12% | 10% | 19% | [5] |
| 195 | Cppt + Vib | 63 | NR | 17.0 | 37% | 21% | 16% | 21% | ||||
| 187 | VP16 + Cppt | 69.6 bid | NR | 15.2 | 32% | 17% | 13% | 23% | ||||
| 2012 | ECOG 3598 | 275 | I: Ca + P + | Ca + P*** | 60 | 7.4 | 15.3 | 34 | 18 | 16 | NR | [48] |
| 271 | I: T+Ca + P C: T | Ca + P*** + T | 60 | 7.8 | 16 | 34 | 20 | 15 | NR |
Abbreviations: Adj adjuvant, bid twice-daily radiation, C consolidative/adjuvant chemotherapy, Ca carboplatin, C225 cetuximab, Cppt Cisplatin, Cpt11 irinotecan, DFS disease free survival, Doc Docetaxel, ENI Elective Nodal Irradiation, Gem Gemcitabine, I induction/neoadjuvant chemotherapy, IFI Involved-Field Irradiation, Mmc Mitomycin, NR not reported, P paclitaxel, Px Pemetrexed, T thalidomide, tox toxicity, tx treatment, Vib vinblastine, Vin Vinorelbine, Vnd Vindesine, VP16 Etoposide
For some studies ‘disease free survival’ is listed
Although there is a consensus regarding the need to combine chemotherapy with radiation therapy concomitantly for locally advanced NSCLC in fit patients, considerable debate persists concerning the choice of chemotherapeutic agents [19]. Although there are no clinical trials directly comparing the radiosensitizing properties of cisplatin versus carboplatin in the absence of additional chemotherapy agents, a meta-analysis concluded that there was no evidence of superiority for one platinum over the other in the context of chemoradiation[20]. An additional consideration is toxicity: a more tolerable agent may be associated with fewer treatment breaks and a shorter overall treatment time – a factor associated with improved outcome[21]. Compared to cisplatin, carboplatin is more convenient to administer, it causes less nephrotoxicity, emesis, and neurotoxicity; although it is more myelotoxic, this is rarely symptomatic. In a randomized phase III trial comparing chemotherapeutic doublets in advanced NSCLC (without radiation), cisplatin-paclitaxel yielded 73% grade 4–5 toxicity compared to 57% for carboplatin-paclitaxel (p<0.05)[22]. In NSCLC, concurrent chemoradiation increases the risk of severe esophagitis by a factor of 1.8–4.9[1, 23, 24] over radiation alone; however, it is not known whether there is a difference between cisplatin and carboplatin in this regard. Because of its more favorable toxicity profile, the pool of patients with locally advanced NSCLC eligible to receive carboplatin is potentially larger than the pool eligible for cisplatin. Hence, in lieu of a direct prospective comparison, all historic comparisons are potentially subject to bias favoring cisplatin-based strategy. It is perhaps surprising that late-toxicity in RTOG 9802 was significantly higher than in some other trials (not all trials have reported late-toxicities). On inspection of Table IV it is evident that differences in late-toxicity between trials are much greater than the differences between arms; suggesting that technical factors (completeness of follow-up, toxicity scale) may play a large role in determining numeric late toxicity rates.
A Chinese trial compared cisplatin etoposide concomitant with thoracic radiation to carboplatin paclitaxel with concomitant radiation, although the former combination appeared more potent, the trial was underpowered with less than 35 patients per arm[25]. Two Japanese randomized trials comparing different chemo-radiation regimes were recently published. WJTOG0105 randomized 456 patients with unresectable NSCLC to thoracic radiation plus either (A) cisplatin, vindesine, mitomycin; (B) carboplatin, irinotecan or (C) carboplatin, paclitaxel[26]. Subjects received concurrent chemo-radiation (60Gy) followed by two additional chemotherapy cycles. Cisplatin was only administered once every 4 weeks in arm A, whereas carboplatin (AUC 2) was administered weekly at radiosensitizing doses in arms B and C. Radiation therapy was split course in arm A, but continuous in arms B and C. There were no significant differences in response rates, progression-free or overall-survival between the three arms. However, the toxicity profiles were different: Arm A was associated with worse hematological and gastro-intestinal toxicity, whereas treatment interruptions were more common in arm B. Arm C was associated with slightly more grade 3 or 4 esophagitis (not significant). The authors concluded that carboplatin and paclitaxel was equally efficacious and had the most favorable toxicity profile of the three different regimens. OLCSG 0007 compared two cisplatin-based regimens: mitomycin, vindesine, cisplatin (MVP) vs. docetaxel, cisplatin (DP), both of which were given concurrently with 60 Gy in unresectable stage III NSCLC. Hematologic toxicity was worse in the MVP arm, while esophagitis was worse in the DP arm. There was no statistically significant difference in the anti-tumor efficacy of the two arms, although there was a trend to better outcomes in the DP arm compared with the MVP arm (median survival 26.8 vs. 23.7 months) [27]. These results suggest that third generation taxane-based regimens in combination with platinum and radiation may be superior to older, second-generation regimens featuring a vinca and mitomycin as platinum partners.
Some clinicians have suggested that carboplatin / paclitaxel based chemoradiation is not optimal in NSCLC, based upon results from CALGB 39801. In this randomized phase III trial, induction chemotherapy followed by chemoradiation with carboplatin and paclitaxel was compared to chemoradiation alone (Table IV). Median survival was low in both arms, 14 and 12 months, respectively, compared to 16–18 months in contemporaneous studies using cisplatin-based chemoradiation[28–31]. These survival data are lower than those observed in RTOG 9801, despite use of similar chemotherapy regimens, suggesting that there may be another explanation for the results. In contrast to many studies, the CALGB trial allowed entry of patients with weight loss of up to 10% (rather than 5%). When patients with a 5–10% weight loss were excluded, the median survival increased to 16 and 14 months in the two arms. A re-analysis of the CALGB 39801 trial demonstrated that weight-loss (≥5%), older age, poor performance status and anemia, each had a major deleterious impact on survival. Patients with none of these factors had a median survival of 24 months, as opposed to 8 months in subjects with 3 or 4 factors[32]. Alternatively, it might be that the relatively improved results in RTOG 9801 were due to the use of twice daily radiation; according to Mauguen s meta-analysis modified fractionation improves overall-survival in NSCLC, producing an absolute benefit of 2.5% at 5 years [33]. The results of RTOG 9801 (closed to accrual in 2002) compare favorably with more contemporary studies despite the fact that the RTOG 9801 subjects did not benefit from baseline PET-CT scans (associated with more accurate staging[34]), which was not standard at that time, and that 3-dimensional radiation planning (associated with improved prognosis[35]) was not mandatory Trials in NSCLC are difficult to compare for many reasons: differences in study populations, thoroughness of staging, and choice and intensity of chemotherapy and radiation doses. Indeed, a recent analysis of RTOG 9801 demonstrated that the patient’s baseline quality of life was a more important predictor of outcome than traditional prognosticators[36]. Despite these limitations, the long-term survival outcomes in RTOG 9801 are comparable to those observed with standard cisplatin-based chemotherapeutic regimens (Table IV) used in other multi-institutional randomized studies.
Conclusion
The long-term results of RTOG 9801 demonstrate the efficacy of radiation therapy when combined with carboplatin and paclitaxel, with long-term survival rates similar to those obtained in contemporary trials. They provide further evidence that amifostine probably does not protect tumor from the cytotoxic activity of chemoradiation, but neither does it have much impact on toxicity outcomes. Better data is required regarding the comparative long-term toxicity of different chemoradiation regimens.
Acknowledgments
We appreciate Dr Mohan Suntharalingam s careful reading of the manuscript and his erudite comments.
Sources of funding:
This trial was conducted by the RTOG and was supported by RTOG grant U10 CA21661, CCOP grant U10 CA37422, and Stat grant U10 CA32115 from the National Cancer Institute (NCI) and by Medimmune Oncology. This manuscript s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCI. Medimmune played no role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; nor in the decision to submit the manuscript for publication.
Footnotes
Conflicts of interest:
Past advisory activity with Medimmune and BMS [CJ Langer].
None of the other co-authors have conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus R, Yamanaka T, Bozonnat MC, Uitterhoeve A, Wang X, Stewart L, Arriagada R, Burdett S, Pignon JP. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 2.Komaki R, Scott CB, Sause WT, Johnson DH, Taylor SGt, Lee JS, Emami B, Byhardt RW, Curran WJ, Jr, Dar AR, Cox JD. Induction cisplatin/vinblastine and irradiation vs. irradiation in unresectable squamous cell lung cancer: failure patterns by cell type in RTOG 88-08/ECOG 4588. Radiation Therapy Oncology Group. Eastern Cooperative Oncology Group. Int J Radiat Oncol Biol Phys. 1997;39:537–544. doi: 10.1016/s0360-3016(97)00365-9. [DOI] [PubMed] [Google Scholar]
- 3.Stewart L. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 4.Rowell NP, O’rourke NP. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2004:CD002140. doi: 10.1002/14651858.CD002140.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Curran WJ, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, Movsas B, Wasserman T, Rosenthal SA, Gore E, Machtay M, Sause W, Cox JD. Sequential vs Concurrent Chemoradiation for Stage III Non Small Cell Lung Cancer: Randomized Phase III Trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, Katagami N, Ariyoshi Y. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 7.Vokes EE, Herndon JE, 2nd, Kelley MJ, Cicchetti MG, Ramnath N, Neill H, Atkins JN, Watson DM, Akerley W, Green MR. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol. 2007;25:1698–1704. doi: 10.1200/JCO.2006.07.3569. [DOI] [PubMed] [Google Scholar]
- 8.Movsas B. Randomized Trial of Amifostine in Locally Advanced Non-Small-Cell Lung Cancer Patients Receiving Chemotherapy and Hyperfractionated Radiation: Radiation Therapy Oncology Group Trial 98-01. J Clin Oncol. 2005;23:2145–2154. doi: 10.1200/JCO.2005.07.167. [DOI] [PubMed] [Google Scholar]
- 9.Sarna L, Swann S, Langer C, Wernerwasik M, Nicolaou N, Komaki R, Machtay M, Byhardt R, Wasserman T, Movsas B. Clinically Meaningful Differences in Patient-Reported Outcomes With Amifostine in Combination With Chemoradiation for Locally Advanced Non-Small-Cell Lung Cancer: An Analysis of RTOG 9801. Int J Radiat Oncol Biol Phys. 2008;72:1378–1384. doi: 10.1016/j.ijrobp.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958:457–481. [Google Scholar]
- 11.Pike MC. Asymptomatically efficient rank invariant procedures. JR Stat Soc Series A. 1972;135:201–203. [Google Scholar]
- 12.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 13.Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: The First Selective-Target and Broad-Spectrum Radioprotector. The Oncologist. 2007;12:738–747. doi: 10.1634/theoncologist.12-6-738. [DOI] [PubMed] [Google Scholar]
- 14.Antonadou D, Coliarakis N, Synodinou M, Athanassiou H, Kouveli A, Verigos C, Georgakopoulos G, Panoussaki K, Karageorgis P, Throuvalas N. Randomized phase III trial of radiation treatment +/− amifostine in patients with advanced-stage lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:915–922. doi: 10.1016/s0360-3016(01)01713-8. [DOI] [PubMed] [Google Scholar]
- 15.Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, Eschwege F, Zhang J, Russell L, Oster W, Sauer R. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 16.Buentzel J, Micke O, Adamietz IA, Monnier A, Glatzel M, de Vries A. Intravenous amifostine during chemoradiotherapy for head-and-neck cancer: a randomized placebo-controlled phase III study. Int J Radiat Oncol Biol Phys. 2006;64:684–691. doi: 10.1016/j.ijrobp.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Wasserman TH, Brizel DM, Henke M, Monnier A, Eschwege F, Sauer R, Strnad V. Influence of intravenous amifostine on xerostomia, tumor control, and survival after radiotherapy for head-and- neck cancer: 2-year follow-up of a prospective, randomized, phase III trial. Int J Radiat Oncol Biol Phys. 2005;63:985–990. doi: 10.1016/j.ijrobp.2005.07.966. [DOI] [PubMed] [Google Scholar]
- 18.Bourhis J, Blanchard P, Maillard E, Brizel DM, Movsas B, Buentzel J, Langendijk JA, Komaki R, Swan Leong S, Levendag P, Pignon JP. Effect of amifostine on survival among patients treated with radiotherapy: a meta-analysis of individual patient data. J Clin Oncol. 2011;29:2590–2597. doi: 10.1200/JCO.2010.33.1454. [DOI] [PubMed] [Google Scholar]
- 19.Movsas B, Moughan J, Komaki R, Choy H, Byhardt R, Langer C, Goldberg M, Graham M, Ettinger D, Johnstone D, Abrams R, Munden R, Starkschall G, Owen J. Radiotherapy Patterns of Care Study in Lung Carcinoma. J Clin Oncol. 2003;21:4553–4559. doi: 10.1200/JCO.2003.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Auperin A. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): A meta-analysis of individual data from 1764 patients. Ann Oncol. 2006;17:473–483. doi: 10.1093/annonc/mdj117. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell M, Chuanchieh H, Ritsuko K, William TS, Swann RS, Corey JL, Roger WB, Walter JC. Effect of overall treatment time on outcomes after concurrent chemoradiation for locally advanced non-small-cell lung carcinoma: Analysis of the Radiation Therapy Oncology Group (RTOG) experience. Int J Radiat Oncol Biol Phys. 2005;63:667–671. doi: 10.1016/j.ijrobp.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH the Eastern Cooperative Oncology G. Comparison of Four Chemotherapy Regimens for Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 23.Eileen R, May T, Yee U, Jean-Philippe P, Patrick C, Edward C. Comparison of the efficacy and acute toxicity of weekly versus daily chemoradiotherapy for non small-cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys. 2004;58:196–203. doi: 10.1016/s0360-3016(03)01447-0. [DOI] [PubMed] [Google Scholar]
- 24.O’Rourke N, Roque i Figuls M, Farre Bernado N, Macbeth F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2010:6. doi: 10.1002/14651858.CD002140.pub3. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Wu S, Ou G, Bi N, Li W, Ren H, Cao J, Liang J, Li J, Zhou Z, Lv J, Zhang X. Randomized phase II study of concurrent cisplatin/etoposide or paclitaxel/carboplatin and thoracic radiotherapy in patients with stage III non-small cell lung cancer. Lung Cancer. 2012;77:89–96. doi: 10.1016/j.lungcan.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto N, Nakagawa K, Nishimura Y, Tsujino K, Satouchi M, Kudo S, Hida T, Kawahara M, Takeda K, Katakami N, Sawa T, Yokota S, Seto T, Imamura F, Saka H, Iwamoto Y, Semba H, Chiba Y, Uejima H, Fukuoka M. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol. 2010;28:3739–3745. doi: 10.1200/JCO.2009.24.5050. [DOI] [PubMed] [Google Scholar]
- 27.Segawa Y, Kiura K, Takigawa N, Kamei H, Harita S, Hiraki S, Watanabe Y, Sugimoto K, Shibayama T, Yonei T, Ueoka H, Takemoto M, Kanazawa S, Takata I, Nogami N, Hotta K, Hiraki A, Tabata M, Matsuo K, Tanimoto M. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J Clin Oncol. 2010;28:3299–3306. doi: 10.1200/JCO.2009.24.7577. [DOI] [PubMed] [Google Scholar]
- 28.Curran W, Scott C, Langer C, Komaki R, Lee J, Hauser S, Movsas B, Wasserman T, Sause W, Cox J. Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with unresected stage III nsclc: RTOG 9410 (abstract 2499) Proc Am Soc Clin Oncol. 2003:22. [Google Scholar]
- 29.Hanna N, Neubauer M, Yiannoutsos C, Mcgarry R, Arseneau J, Ansari R, Reynolds C, Govindan R, Melnyk A, Fisher W, Richards D, Bruetman D, Anderson T, Chowhan N, Nattam S, Mantravadi P, Johnson C, Breen T, White A, Einhorn L. Phase III Study of Cisplatin, Etoposide, and Concurrent Chest Radiation With or Without Consolidation Docetaxel in Patients With Inoperable Stage III Non-Small-Cell Lung Cancer: The Hoosier Oncology Group and U.S. Oncology J Clin Oncol. 2008;26:5755–5760. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 30.Vokes EE, Herndon JE, 2nd, Crawford J, Leopold KA, Perry MC, Miller AA, MRG Randomized Phase II Study of Cisplatin With Gemcitabine or Paclitaxel or Vinorelbine as Induction Chemotherapy Followed by Concomitant Chemoradiotherapy for Stage IIIB Non-Small-Cell Lung Cancer: Cancer and Leukemia Group B Study 9431. J Clin Oncol. 2002;20:4191–4198. doi: 10.1200/JCO.2002.03.054. [DOI] [PubMed] [Google Scholar]
- 31.Zatloukal P, Petruzelka L, Zemanova M, Havel L, Janku F, Judas L, Kubik A, Krepela E, Fiala P, Pecen L. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer. 2004;46:87–98. doi: 10.1016/j.lungcan.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Stinchcombe TE, Hodgson L, Herndon JE, II, Kelley MJ, Cicchetti MG, Ramnath N, Niell HB, Atkins JN, Akerley W, Green MR, Vokes EE for C, Leukemia Group B. Treatment Outcomes of Different Prognostic Groups of Patients on Cancer and Leukemia Group B Trial 39801: Induction Chemotherapy Followed by Chemoradiotherapy Compared with Chemoradiotherapy Alone for Unresectable Stage III Non-small Cell Lung Cancer. J Thorac Oncol. 2009:4. doi: 10.1097/JTO.0b013e3181b27b33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mauguen A, Le Pechoux C, Saunders MI, Schild SE, Turrisi AT, Baumann M, Sause WT, Ball D, Belani CP, Bonner JA, Zajusz A, Dahlberg SE, Nankivell M, Mandrekar SJ, Paulus R, Behrendt K, Koch R, Bishop JF, Dische S, Arriagada R, De Ruysscher D, Pignon J-P. Hyperfractionated or Accelerated Radiotherapy in Lung Cancer: An Individual Patient Data Meta-Analysis. J Clin Oncol. 2012;30:2788–2797. doi: 10.1200/JCO.2012.41.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer B, Lassen U, Mortensen J, Larsen S, Loft A, Bertelsen A, Ravn J, Clementsen P, Hogholm A, Larsen K, Rasmussen T, Keiding S, Dirksen A, Gerke O, Skov B, Steffensen I, Hansen H, Vilmann P, Jacobsen G, Backer V, Maltbaek N, Pedersen J, Madsen H, Nielsen H, Hojgaard L. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med. 2009;361:32–39. doi: 10.1056/NEJMoa0900043. [DOI] [PubMed] [Google Scholar]
- 35.Chen AB, Neville BA, Sher DJ, Chen K, Schrag D. Survival outcomes after radiation therapy for stage III non-small-cell lung cancer after adoption of computed tomography-based simulation. J Clin Oncol. 2011;29:2305–2311. doi: 10.1200/JCO.2010.33.4466. [DOI] [PubMed] [Google Scholar]
- 36.Movsas B, Moughan J, Sarna L, Langer C, Werner-Wasik M, Nicolaou N, Komaki R, Machtay M, Wasserman T, Bruner DW. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: an analysis of RTOG 9801. J Clin Oncol. 2009;27:5816–5822. doi: 10.1200/JCO.2009.23.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeremic B, Shibamoto Y, Acimovic L, Djuric L. Randomized trial of hyperfractionated radiation therapy with or without concurrent chemotherapy for stage III non-small-cell lung cancer. J Clin Oncol. 1995;13:452–458. doi: 10.1200/JCO.1995.13.2.452. [DOI] [PubMed] [Google Scholar]
- 38.Komaki R, Seiferheld W, Ettinger D, Lee JS, Movsas B, Sause W. Randomized phase II chemotherapy and radiotherapy trial for patients with locally advanced inoperable non-small-cell lung cancer: long-term follow-up of RTOG 92-04. Int J Radiat Oncol Biol Phys. 2002;53:548–557. doi: 10.1016/s0360-3016(02)02793-1. [DOI] [PubMed] [Google Scholar]
- 39.Clamon G, Herndon J, Cooper R, Chang AY, Rosenman J, Green MR. Radiosensitization with carboplatin for patients with unresectable stage III non-small-cell lung cancer: a phase III trial of the Cancer and Leukemia Group B and the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:4–11. doi: 10.1200/JCO.1999.17.1.4. [DOI] [PubMed] [Google Scholar]
- 40.Jeremic B, Shibamoto Y, Acimovic L, Milicic B, Milisavljevic S, Nikolic N, Dagovic A, Aleksandrovic J, Radosavljevic-Asic G. Hyperfractionated radiation therapy and concurrent low-dose, daily carboplatin/etoposide with or without weekend carboplatin/etoposide chemotherapy in stage III non-small-cell lung cancer: a randomized trial. Int J Radiat Oncol Biol Phys. 2001;50:19–25. doi: 10.1016/s0360-3016(00)01546-7. [DOI] [PubMed] [Google Scholar]
- 41.Schild SE, Stella PJ, Geyer SM, Bonner JA, Marks RS, McGinnis WL, Goetz SP, Kuross SA, Mailliard JA, Kugler JW, Schaefer PL, Jett JR. Phase III trial comparing chemotherapy plus once-daily or twice-daily radiotherapy in Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;54:370–378. doi: 10.1016/s0360-3016(02)02930-9. [DOI] [PubMed] [Google Scholar]
- 42.Fournel P. Randomized Phase III Trial of Sequential Chemoradiotherapy Compared With Concurrent Chemoradiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol. 2005;23:5910–5917. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 43.Belani CP, Choy H, Bonomi P, Scott C, Travis P, Haluschak J, Curran WJ. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23:5883–5891. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 44.Belderbos J, Uitterhoeve L, van Zandwijk N, Belderbos H, Rodrigus P, van de Vaart P, Price A, van Walree N, Legrand C, Dussenne S, Bartelink H, Giaccone G, Koning C. Randomised trial of sequential versus concurrent chemo-radiotherapy in patients with inoperable non-small cell lung cancer (EORTC 08972-22973) Eur J Cancer. 2007;43:114–121. doi: 10.1016/j.ejca.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Yuan S, Sun X, Li M, Yu J, Ren R, Yu Y, Li J, Liu X, Wang R, Li B, Kong L, Yin Y. A Randomized Study of Involved-Field Irradiation Versus Elective Nodal Irradiation in Combination With Concurrent Chemotherapy for Inoperable Stage III Nonsmall Cell Lung Cancer. Am J Clin Oncol. 2007;30:239–244. doi: 10.1097/01.coc.0000256691.27796.24. [DOI] [PubMed] [Google Scholar]
- 46.Thomas M, Rübe C, Hoffknecht P, Macha HN, Freitag L, Linder A, Willich N, Hamm M, Sybrecht GW, Ukena D, Deppermann K-M, Droge C, Riesenbeck D, Heinecke A, Sauerland C, Junker K, Berdel WE, Semik M German Lung Cancer Cooperative G. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol. 2008;9:636–648. doi: 10.1016/S1470-2045(08)70156-6. [DOI] [PubMed] [Google Scholar]
- 47.Kelly K, Chansky K, Gaspar LE, Albain KS, Jett J, Ung YC, Lau DH, Crowley JJ, Gandara DR. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26:2450–2456. doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]
- 48.Hoang T, Dahlberg SE, Schiller JH, Mehta MP, Fitzgerald TJ, Belinsky SA, Johnson DH. Randomized phase III study of thoracic radiation in combination with paclitaxel and carboplatin with or without thalidomide in patients with stage III non-small-cell lung cancer: the ECOG 3598 study. J Clin Oncol. 2012;30:616–622. doi: 10.1200/JCO.2011.36.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]