Summary
Purpose
Prolonged exposure of cancer cells to triapine, an inhibitor of ribonucleotide reductase, followed by gemcitabine enhances gemcitabine activity in vitro. Fixed-dose-rate gemcitabine (FDR-G) has improved efficacy compared to standard-dose. We conducted a phase I trial to determine the maximum tolerated dose (MTD), safety, pharmacokinetics (PK), pharmacodynamics (PD), and preliminary efficacy of prolonged triapine infusion followed by FDR-G.
Experimental Design
Triapine was given as a 24-hour infusion, immediately followed by FDR-G (1000 mg/m2 over 100-minute). Initially, this combination was administered days 1 and 8 of a 21-day cycle (Arm A, triapine starting dose 120 mg); but because of myelosuppression, it was changed to days 1 and 15 of a 28-day cycle (Arm B, starting dose of triapine 75 mg). Triapine steady-state concentrations (Css) and circulating ribonucleotide reductase M2-subunit (RRM2) were measured.
Results
Thirty-six patients were enrolled. The MTD was determined to be triapine 90 mg (24-hour infusion) immediately followed by gemcitabine 1000 mg/m2 (100-minute infusion), every 2 weeks of a 4-week cycle. DLTs included grade 4 thrombocytopenia, leukopenia and neutropenia. The treatment was well tolerated with fatigue, nausea/vomiting, fever, transaminitis, and cytopenias being the most common toxicities. Among 30 evaluable patients, 1 had a partial response and 15 had stable disease. Triapine PK was similar, although more variable, compared to previous studies using doses normalized to body-surface-area. Steady decline in circulating levels of RRM2 may correlate with outcome.
Conclusions
This combination was well tolerated and showed evidence of preliminary activity in this heavily pretreated patient population, including prior gemcitabine failure.
Keywords: Triapine, Gemcitabine, Phase I, Clinical Trial
Introduction
Treatment for most advanced solid tumors with the currently available antineoplastic agents, such as the broadly active gemcitabine [1–7], is not satisfactory; and there is a clear need for new agents to improve the efficacy of these drugs. The mechanisms responsible for gemcitabine’s anti-tumor effects, and for resistance to gemcitabine, have been investigated extensively [8–11]. Gemcitabine, a deoxycytidine analogue, requires uptake via a nucleoside transporter and is then converted intracellularly to the active metabolites difluorodeoxycytidine di- and tri-phosphate (dFdCDP, dFdCTP), by the enzyme deoxycytidine kinase (dCK). dFdCDP inhibits the M1 subunit of ribonucleotide reductase (RR), an enzyme that converts ribonucleotides to deoxyribonucleotides, thereby decreasing deoxynucleotides (such as deoxycytidine triphosphate, dCTP) available for deoxyribonucleic acid (DNA) synthesis [12]. dFdCTP competes with dCTP for incorporation into DNA, resulting in DNA strand termination and apoptosis.
Based on current understanding of its mechanism of action, the anti-cancer effects of gemcitabine could be enhanced by approaches leading to increased intracellular accumulation and incorporation into DNA. Early preclinical and phase I studies suggest that the accumulation of dFdCTP in peripheral blood mononuclear cells and circulating leukemic blasts was saturated when plasma gemcitabine concentrations exceeded 15–20 µmol/L [13]. Based on that knowledge, improvements of gemcitabine efficacy could be achieved by optimizing its uptake and subsequent activation in tumor cells. Administration of gemcitabine at a fixed-dose-rate (FDR) of 10 mg/m2/min rather than 30-minute bolus infusion typically achieves steady-state plasma levels of 15–20 µmol/L [14]. Tempero et al. conducted a randomized phase II study comparing the activity of FDR gemcitabine to standard 30-minute infusion in patients with advanced pancreatic cancer [15]. The FDR group had a higher response rate, improved survival and time to tumor progression, when compared to the standard group. Hematologic toxicity was the most frequently reported adverse event and occurred more frequently in the FDR group. The median intracellular concentration of dFdCTP in circulating mononuclear cells at the end of each infusion was measured to be 114 µmol/L in standard group vs. 336 µmol/L in the FDR group [15]. Of note, the results of a follow up 3-arm phase III trial suggested only a trend towards a survival benefit for FDR gemcitabine over standard gemcitabine, however the study was possibly underpowered [16].
The activity of the ribonucleotide reductase (RR) enzyme correlates with tumor growth rate [17] and overexpression of RR has been implicated as a mechanism of resistance to gemcitabine [18, 19]. In a number of preclinical experiments, it has been demonstrated that inhibition of cellular RR could enhance the anti-tumor activity of subsequently administered nucleoside analogues [20–24]. Triapine (3-Aminopyridine-2-carboxaldehyde thiosemicarbazone, 3-AP, triapine is a registered trademark of Vion Pharmaceuticals) is a new inhibitor of RR M2-subunit [25], which is significantly more potent than hydroxyurea, the currently available M2-subunit RR inhibitor, in vitro [9, 26] and shows antitumoral effects in hydroxyurea- and gemcitabine-resistant cell lines [9, 26, 27]. In addition, in vitro studies demonstrated that sequential administration of triapine followed by gemcitabine resulted in synergistic or additive antitumoral effects, and this synergism was most evident with prolongation of pre-exposure time to triapine, perhaps resulting in increased cellular and DNA uptake of gemcitabine [28]. Increase in pre-exposure to triapine from 4 to 8 and then 12 h, before administration of gemcitabine, resulted in increasing antiproliferative effects, in vitro. Few phase I clinical trials were conducted in advanced solid tumor malignancies to evaluate the pharmacokinetics and toxicities of triapine, as single-agent or in combination with standard-dose gemcitabine [29–32]. A study that looked at 96-hour triapine infusion defined the MTD to be 120 mg/m2/day every 2 weeks [31]. Triapine was shown to reach its steady state concentration (Css) within the first few hours of the infusion and this Css was maintained through the remainder of the infusion. In addition, at 80–160 mg/m2 dose, the Css was within the range of the in vitro IC50 for tumor cell lines (~ 1–2 µM). The phase I study of combined triapine and standard-dose gemcitabine established that triapine at a dose of 105 mg/m2 (2–4 hour infusion) does not appear to alter the toxicity of gemcitabine at 1000 mg/m2, when administrated on days 1, 8 and 15 of a 28-day cycle [32].
Clinical data support improved efficacy of gemcitabine at FDR as compared to standard-dose. Preclinical evidence also demonstrates synergistic effects for triapine followed by gemcitabine administration, which is most evident with prolonged (at least 12 h) pre-exposure time to triapine. Triapine clearance has not been associated with body surface area (BSA) in previous studies using BSA normalized triapine dosing. Therefore, the current phase I study was initiated to study the tolerability of prolonged, non-BSA normalized, infusion of triapine over 24 h, immediately followed by FDR gemcitabine, in patients with advanced solid tumors.
Patients and methods
The study was conducted at the Ohio State University and approved by the Ohio State University institutional review board (IRB).
Eligibility criteria
Patients were required to be ≥18 years old with histologically or cytologically confirmed advanced solid tumor with no curable treatment option available. The lapse time between the last dose of prior treatment and enrolment on the study was 3 weeks for radiation therapy and 4 weeks for chemotherapy (6 weeks for nitrosoureas or mitomycin C). Prior treatment with gemcitabine was allowed, if given as a standard (30 min) infusion. Prior exposure to triapine or gemcitabine given at a fixed-dose-rate was not allowed. Patients had Eastern Cooperative Oncology Group performance status (ECOG PS) ≤2; life expectancy >12 weeks; leukocytes >3,000/µl; absolute neutrophil count >1,500/µl; platelets >100,000/µl; total bilirubin < institutional upper limit of normal (ULN); AST(SGOT)/ALT(SGPT) <2.5×ULN; serum creatinine within normal institutional limits, or creatinine clearance >60 mL/min, for patients with creatinine levels above institutional normal. Patients with high risk for glucose-6-phosphate dehydrogenase (G6PD) deficiency were screened and those with this diagnosis were excluded, because of the potential risk for severe methemoglobinemia with triapine. Initially, there were no limitations for number of prior treatments (Arm A), but because of higher than expected myelosuppression, the protocol was amended (Arm B) and no more than 2 lines of systemic chemotherapy (not hormonal or targeted therapies), in the metastatic setting, was allowed. Patients with uncontrolled intercurrent illness, pregnant or breast-feeding females and HIV-positive patients receiving combination anti-retroviral therapy were excluded from the study.
Study design and treatment plan
This was a standard method phase I dose-escalation trial. Treatment was administered on an inpatient basis. Triapine was administered intravenously (i.v.) over 24 h, immediately (0–2 h) followed by gemcitabine i.v. infusion at a fixed rate of 10 mg/m2/min (100 min for 1000 mg/m2). Initially, the 24-hour triapine infusion, which was followed by gemcitabine, was started on days 1 and 8 of a 21-day cycle regimen (Arm A). The starting dose for triapine was 120 mg (dose level 1A), but after dose reductions to dose level -2A (75 mg) because of dose-limiting hematologic toxicities, the protocol was amended and the schedule was changed to a 28-day cycle regimen with treatment started on days 1 and 15 (Arm B). In addition, we limited the number of prior systemic chemotherapies (except for hormonal or targeted therapies) in the metastatic setting to ≤2, to minimize the risk of myelotoxicity. The starting triapine dose for Arm B was 75 mg (dose level 1B).
We followed a standard 3+3 phase I dose escalation trial. For each new dose level, up to three patients were entered. If none of the first three patients at a dose level developed a dose-limiting toxicity (DLT), patients were enrolled at the next higher dose level. If one of the first three patients developed a DLT, up to three more patients were enrolled at that same dose level. If two or more developed a DLT at the same dose, no more patients were enrolled at that dose level and dose escalation was halted, and the previous dose level was expanded to a total of six patients. The maximum tolerated dose (MTD) was defined as the maximum dose level with fewer than 2 of 6 patients experiencing DLT.
Only adverse events occurring in the first cycle of treatment were considered for evaluation of DLT. The National Cancer Institute Common Toxicity Criteria version 3.0 (CTCAE v3.0) was used to grade toxicities. DLT was defined as ≥ Grade 3 non-hematologic or ≥ Grade 4 hematologic adverse event with the following exceptions, which was not considered DLT: 1) ≥ Grade 3 nausea and ≥ Grade 3 vomiting that improved with antiemetic therapy; 2) ≥ Grade 3 diarrhea that improved with lomotil; 3) Grade 4 neutropenia that recovered to ≤Grade 3 within 7 days of first identification. Colony-Stimulating-Factors use was allowed according to American Society of Clinical Oncology guidelines, after cycle 1.
Study monitoring and measurement of effect
Before study entry, patients were assessed with a complete history and physical examination (H&P), vital signs, scoring of ECOG PS, complete blood count with platelets and differential, complete serum chemistries including electrolytes, lactate dehydrogenase and liver function tests, serum pregnancy test in women of child-bearing potential, electrocardiogram (ECG), tumor staging studies including computed tomography (CT) scans as appropriate to the disease, G6PD deficiency screening by using standard clinical assays in high risk patients; i.e. patients with African, Asian or Mediterranean origin/ancestry. Considering the risk of acute reactions to triapine, including the risk of methemoglobinemia, patients were closely monitored (vital signs and pulse oximetry) during triapine infusion (every 30 min) and for 2 h (4 h for cycle one) after completion of infusion (every 60 min). A complete H&P and routine laboratory tests were performed on days 1 and 15 of each cycle. Toxicities were monitored and recorded continuously. Patients were evaluated for tumor response every 8 weeks (2 cycles), with full staging studies. The criteria for response evaluation were based on Response Evaluation Criteria In Solid Tumors (RECIST) version 1.0 [33]. A complete or partial response was confirmed by repeat staging scans in 4 weeks. Patients with partial response and those with stable disease were eligible to continue treatment for 12 cycles, as long as they were tolerating treatment and no evidence of progression. Patients with complete response were to receive one cycle past the cycle where complete response had been documented.
Drug supply and administration
Triapine was supplied by Vion Pharmaceuticals, Inc. and distributed by the Division of Cancer Treatment and Diagnosis (DCTD, CTEP, NCI) to the investigators, in 10 mL amber vials, containing 50 mg of the drug. Prior to administration, triapine was diluted in 500 mL of 0.9 % sodium chloride to a final concentration of 0.01–1 mg/mL. This admixture has been found to be stable for 96 h, when protected from light and stored at room temperature or 2–8°C. Gemcitabine was obtained commercially and reconstituted in 0.9 % sodium chloride. Triapine was administered i.v. over 24 h, immediately (0–2 h) followed by gemcitabine i.v. infusion at a fixed rate of 10 mg/m2/min (100 min for 1000 mg/m2).
Pharmacokinetic studies
Whole blood was collected from each patient within 4 h prior to the end of the 24-hour triapine infusion on day 1 of the first cycle. Plasma was separated and stored at −70°C until analysis. Steady-state triapine levels post-infusion were measured by a validated liquid chromatography-mass spectrometry (LC-MS) assay. The LC-MS system consisted of a Thermo TSQ Quantum Ultra EMR triple quadrupole mass spectrometer and a Shimadzu Prominence HPLC system. Both triapine and internal standard 2-pyridinecarboxaldehyde thiosemicarbazone (2-PAT) were separated on a reverse phase C18 column by isocratic elution with 5 % acetonitrile containing 0.2 % acetic acid. Two parent and product ion pairs 196.2>121.2 and 181.2>106.2 were selected to monitor triapine and 2-PAT in multiple reaction monitoring mode, respectively. Due to strong hydrogen bonding and chelating capabilities of triapine with proteins and metal ions in human plasma [34, 35], EDTA (3 mM) was added to each sample to chelate metal ions prior to sample cleaning by cation exchange solid phase extraction. Plasma concentrations of triapine in patient samples were calculated using a calibration curve of triapine spiked into human plasma prior to extraction. Triapine concentrations were quantifiable between 1 ng/mL to 1000 ng/mL with acceptable precision and accuracy according to FDA guidelines [36]. Clearance (CL) of triapine was calculated as the ratio of infusion rate (dose/infusion time) to steady state concentration.
Quantification of serum human ribonucleotide reductase M2 subunit (hRRM2)
Circulating hRRM2 was evaluated as potential predictive biomarkers for triapine. Patients underwent blood draw immediately before triapine administration on day 1 and immediately after triapine infusion, but prior to gemcitabine infusion, on day 2 of cycles 1 and 2. Peripheral blood mononuclear cell (PBMC) and serum samples were separated and preserved at −70°C. Quantitative assessment of hRRM2 in patients’ serum was performed by ELISA [37]. Polystyrene 96-well plates were coated with anti-hRRM2 antibody; at 2 µg/mL concentration, overnight at 4 °C. Unbound sites were blocked during overnight incubation with 2 % bovine serum albumin. Serum or pure RRM2 protein 10 µg/mL (positive control) were added and left to incubate at room temperature for 2 h. After several washes, these plates were incubated with Biotin labeled RRM2 10 ng/mL at room temperature for 1 h. After several washes, these plates were incubated with horseradish peroxidase-conjugated with streptevidine antibody for 0.5 h at room temperature. Plates were then washed and incubated with the substrate solution and spectrophotometric measurements were taken read at 450/630. All samples were measured in duplicate in 2 separate studies. Mean values for the 2 studies were used in the correlation. All washings were performed 3 times with PBS using a Nunc Immunowasher (Thermo Scientific). All procedures were performed at room temperature.
Statistical considerations
The standard phase I 3+3 design was followed for dose escalation and 3–6 patients were entered per dose level. No intra-patient dose escalation was allowed. Summary statistics (i.e. median and range for continuous variables, and frequency for discrete data) were calculated for patient demographics. The maximum grade for each type of toxicity was recorded for each patient, and frequency tables were provided. Time to progression (TTP) was determined from the date of treatment start to progression. Overall survival (OS) was determined from the date of treatment start to death from any cause or censored at the date of off study. Median TTP and OS was estimated using Kaplan–Meier method. The association between PK parameters (CL or Css) and categorical variables were studied by using ANOVA. Pearson correlation and linear regression model were used to explore the association between PK parameters and continuous variables.
To evaluate the potential predictive or prognostic value of circulating hRRM2, the baseline hRRM2 levels and its changes during cycle 1, cycle 2, and throughout both cycles were correlated with the clinical outcomes of interest, such as TTP, OS, and disease control rate (DCR = = partial response + stable disease). Survival curves were compared using the log-rank test. Fisher’s exact test was used to compare DCR according to categorical baseline and changes in hRRM2 levels. Paired t-test was used to evaluate differences in hRRM2 levels at specific time-points (cycle 1 day 1 vs. cycle 1 day 2, cycle 2 day 1 vs. cycle 2 day 2).
Results
Patient characteristics
Thirty-six patients with a median age of 59 years were enrolled between July 2006 and April 2010 (Table 1). Two patients (5 %) received no prior chemotherapy and 8 (22 %) received 3 or more prior chemotherapies (up to 8). Fourteen patients (39 %) had received prior gemcitabine. Slightly over half of the patients (19 patients) had lung, pancreas or bladder primary cancers; and 14 patients (39 %) had 3 or more metastatic organ sites.
Table 1.
Patient characteristics
| Characteristics | Number of Patients |
|---|---|
| Total of Patients Enrolled | 36 |
| Median Age (range) in Years | 59 (38–81) |
| Gender | |
| Female | 21 |
| Male | 15 |
| ECOG PSa | |
| 0 | 10 |
| 1 | 23 |
| 2 | 3 |
| Primary Tumor Site | |
| Lung | 8 |
| Pancreas | 6 |
| Bladder | 5 |
| Breast | 4 |
| Gallbladder | 3 |
| Othersb | 10 |
| Sites of Metastases | |
| Liver | 21 |
| Lung | 14 |
| Bone | 8 |
| Adrenal Glands | 6 |
| Lymph Nodes | 16 |
| Soft Tissues | 5 |
| Others | 7 |
| Number of Metastatic Sites | |
| 1–2 | 22 |
| 3–4 | 14 |
| Number of Prior Systemic Chemotherapies | |
| 0–2 | 28 |
| >2 (Up to 8) | 8 |
ECOG PS: Eastern Cooperative Group performance status
Others: Colon, Rectum, Anus, Stomach, Gastroesophageal Junction, Gastrointestinal Stroma (GIST), Merkel Cell, Cervix, Uterus Endometrium, Unknown Primary
Adverse events
Table 2 describes dose escalation, DLTs and dose modifications for each dose level. In the initial dose escalation (Arm A), both triapine and gemcitabine were given on days 1 and 8 of a 21-day cycle regimen. No DLTs were observed for the first 3 patients at 120 mg triapine (starting dose level 1A). At 160 mg (dose level 2A), we observed a grade 4 neutropenia and a grade 3 fatigue and dyspnea. Dyspnea was not related to methemoglobinemia and it occurred in a patient with metastatic non-small cell carcinoma of the lung, who had 8 prior systemic chemotherapies, radiation and 2 systemic targeted therapies. Triapine dose was deescalated to 120 mg (level 1A), and one patient experienced grade 4 neutropenia and another patient had grade 4 leukopenia, neutropenia and thrombocytopenia. Therefore, triapine dose was again deescalated to 90 mg (dose level -1A). At this dose level, 2 out of 5 patient experienced DLTs, which were grade 4 leukopenia, neutropenia and thrombocytopenia in one patient and grade 4 neutropenia in the other. Another deescalation to 75 mg triapine was made (level -2A), and the first enrolled patient experienced the same DLTs of grade 4 neutropenia and thrombocytopenia. This patient, who had a diagnosis of metastatic small cell lung cancer with 3 prior systemic chemotherapies, also experienced somnolence, confusion, slurred speech, and tachycardia, after completion of triapine and gemcitabine infusion. Her ECG showed regular sinus tachycardia and her pulse oximetry was at 92 %. With a clinical suspicion of methemoglobinemia, she was started on i.v. methylene blue. Other causative factors include recent change in her narcotic dosage. Her symptoms eventually resolved. Her head CT scan was non-specific and her methemoglobin measured 1.3 % (normal <1.3 %), which was not elevated at a clinically meaningful level (usually 10–15 %).
Table 2.
Treatment by dose level
| Dose Level | Triapine 24-hours i.v. Infusion (mg) |
Gemcitabine i.v., Fixed-Dose-Rate (mg/m2) |
No. of Patients |
No. with DLTa |
No. with DRb or DDc |
Description of DLTs |
|---|---|---|---|---|---|---|
| −2A | 75 | 1000 | 1 | 1 | 1 | Neutropenia, Thrombocytopenia |
| −1A | 90 | 1000 | 5 | 2 | 4 | Neutropenia, Leukopenia, Thrombocytopenia |
| 1A | 120 | 1000 | 5 | 2 | 4 | Neutropenia, Leukopenia, Thrombocytopenia |
| 2A | 160 | 1000 | 2 | 2 | 2 | Neutropenia, Fatigue & Dyspnea (Grade 3) |
| 1B | 75 | 1000 | 6 | 1 | 4 | Neutropenia |
| 2Bd | 90 | 1000 | 15 | 1 | 8 | Thrombocytopenia |
| 3B | 120 | 1000 | 2 | 2 | 1 | Neutropenia, Leukopenia, Infection (Grade 3) |
DLT: Dose limiting toxicity
DR: Dose reduction
DD: Dose delay
Including expansion cohort of 6 patients at this dose level
A: Arm A of the study, during which, both drugs were given on days 1 and 8 of a 21-day cycle regimen
B: Arm B of the study, during which, both drugs were given on days 1 and 15 of a 28-day cycle regimen
At this point, considering the significant hematologic toxicities that we observed with this schedule and knowing that a biweekly gemcitabine schedule is similar in efficacy and less myelosuppressive than weekly [38–40], we amended the trial and the schedule was changed to a 28-day cycle regimen with both drugs given on days 1 and 15 (Arm B). In addition, we limited the number of prior systemic chemotherapies (not hormonal or targeted therapies), in the metastatic setting, to ≤2 to minimize the risk of myelotoxicity. The starting dose for triapine in Arm B was 75 mg, the same as dose level -2A. At this dose level (1B), 1 out of 6 patients experienced DLT, which was grade 4 neutropenia. We enrolled 4 patients at dose level 2B (triapine 90 mg). One patient was replaced because of rapid progression of disease after day 1 of the first cycle and the other 3 did not experience DLT. At the next dose level (3B, 120 mg triapine), 2 patients were enrolled and both experienced DLTs. One patient had grade 4 leukopenia and neutropenia and the other one had grade 4 neutropenia and grade 3 infection (i.v. catheter-related cellulitis and pneumonia). Deescalation to dose level 2B was made and 3 additional patients were enrolled. One patient experienced grade 4 thrombocytopenia. The other 2 patients mistakenly received treatment at dose level 3B for day1/cycle1, and then received appropriate treatment at dose level 2B. Although, none experienced a DLT, both continued treatment on the trial, but were replaced. No further DLTs were noted, defining dose level 2B with 90 mg triapine as the MTD. At this point, we expanded this dose level and enrolled 6 more patients to further define the preliminary activity and safety of this dose level.
Treatment-related toxicities are summarized in Table 3. Nausea, vomiting, fatigue, transaminitis, fever and bone marrow suppression, resulting in anemia, thrombocytopenia, leukopenia, neutropenia and lymphopenia were the most common toxicities. A 74 year-old male with a diagnosis of metastatic gastroesophageal junction carcinoma, who had 3 cycles of chemotherapy on this trial, experienced sudden death, 7 days after the last treatment, thought to be possibly related to the treatment, although no autopsy was performed and this was not confirmed. Twenty-four patients (67 %) required dose delay or dose reduction throughout their treatment on this trial (Table 2).
Table 3.
Adverse events
| Adverse events | Grade 1/2 N (%) |
Grade 3 N (%) |
Grade 4 N (%) |
|---|---|---|---|
| Anorexia | 5 (14) | 2 (6) | 0 |
| Fatigue | 23 (64) | 10 (28) | 0 |
| Skin/Rash | 9 (25) | 1 (3) | 0 |
| Nausea/Vomiting | 24 (67) | 1 (3) | 0 |
| Myalgia | 7 (19) | 2 (6) | 0 |
| Infectiona | 2 (6) | 3 (8) | 0 |
| Dyspnea | 2 (6) | 3 (8) | 1 (3) |
| Altered Taste | 5 (14) | 0 | 0 |
| Pruritus | 7 (19) | 0 | 0 |
| Diarrhea | 6 (17) | 1 (3) | 0 |
| Fever | 15 (42) | 0 | 0 |
| Febrile Neutropenia | 0 | 2 (6) | 0 |
| Chills | 8 (22) | 0 | 0 |
| Flu-Like Syndrome | 4 (11) | 0 | 0 |
| Leukopenia | 15 (42) | 13 (36) | 5 (14) |
| Neutropenia | 9 (25) | 7 (19) | 14 (39) |
| Thrombocytopenia | 17 (47) | 3 (8) | 6 (17) |
| Anemia | 17 (47) | 18 (50) | 0 |
| Lymphopenia | 14 (39) | 10 (28) | 3 (8) |
| Transaminitis | 15 (42) | 1 (3) | 0 |
Three cellulitis, one pneumonia, and one urinary tract infection One sudden death (possibly treatment-related), on cycle 3 of treatment at dose level 1B
Response to treatment
Out of 36 enrolled patients, 30 patients were evaluable for response and 6 patients were non-evaluable, mostly due to early removal from the study, secondary to toxicity. Out of 30 evaluable patients, 14 patients (47 %) had progressive disease, 15 (50 %) had stable disease and 1 (3 %) had partial response. The patient with partial response was a 62 year old male with metastatic cholangiocarcinoma of the gallbladder to the liver, who had no prior systemic chemotherapy. He was receiving triapine at the MTD (90 mg) and his partial response lasted for 6 months. The 15 patients with stable disease had a median time to progression of 111 days (95 % CI 84–115 days) and 7 of them had previously progressed on gemcitabine (single agent or combination) chemotherapy. In addition, most (12 patients) had prolonged stable disease more than 3 months. The most common primary sites with stable disease were pancreas (4 patients), lung (3 patients) and bladder (2 patients). One patient with metastatic endometrial adenocarcinoma, previously treated with paclitaxel and carboplatin, showed a 23 % tumor reduction, received 4 cycles and had a time to tumor progression of 202 days. Table 1S (supplement) shows the response to treatment data, number of received cycles, and prior treatment with gemcitabine for each patient. The median number of received cycles was 2, with a range of 1–8 cycles.
Pharmacokinetic analysis
End of infusion steady-state triapine concentrations (Css) were measured in 31 of the 36 patients enrolled in the study, including 13 and 23 patients treated in Arms A and B, respectively. The administered dose levels are described in Table 2. Plasma steady-state concentrations (Css) and calculated clearance (CL) of triapine are presented in Table 4. For Arm A, with triapine doses ranging from 75 to 160 mg, mean Css and CL were 0.43±0.14 µM (rang, 0.24–0.82 µM) and 34.82±10.78 L/hr (range, 13.54–48.23 L/hr), respectively. For Arm B, with triapine doses ranging from 75 to 120 mg, the mean Css and CL were 0.38±0.14 µM (rang, 0.19–0.72 µM) and 33.64±11.52 L/hr (range, 15.90–53.96 L/hr), respectively. Triapine Css and CL were comparable between Arms A and B, which was expected since triapine concentrations were determined at 24 h on day 1 prior to the time when treatment differed between the two Arms. With Arm A and B combined, the Css and CL were 0.40±0.14 (0.21–0.82) µMand 33.72±10.94 (13.54–53.96) L/hr, respectively, for 31 patients receiving 75 to 160 mg triapine over 24-hour i.v. infusion on Day 1.
Table 4.
Steady-state triapine levels and calculated triapine clearance
| Arm A Scheme | |||
|---|---|---|---|
| Dose (mg/m2) | N | Cssa(µM)b | CLc(L/hr)b |
| 75 | 1 | 0.41 | 22.42 |
| 90 | 5 | 0.45±0.23 | 29.82±13.19 |
| 120 | 5 | 0.39±0.09 | 39.18±8.33 |
| 160 | 2 | 0.52 and 0.46 | 38.09 and 42.80 |
| Mean ± SD | 0.43±0.14 | 34.82±10.78 | |
| Arm B Scheme | |||
| Dose (mg/m2) | N | Css (µM) | CL (L/hr) |
| 75 | 4 | 0.36±0.05 | 25.88±4.28 |
| 90 | 10 | 0.35±0.15 | 36.76±13.24 |
| 120 | 4 | 0.47±0.17 | 33.62±9.77 |
| Mean ± SD | 0.38±0.14 | 33.64±11.52 | |
| Arm A and B Combined Dose (mg/m2) | N | Css (µM) | CL (L/hr) |
| 75 | 5 | 0.37±0.05 | 25.19±4.01 |
| 90 | 15 | 0.38±0.18 | 34.45±13.18 |
| 120 | 9 | 0.44±0.12 | 35.77±8.53 |
| 160 | 2 | 0.52 and 0.46 | 38.09 and 42.80 |
| Mean ± SD (Range) | 0.40±0.14 (0.21 →0.82) | 33.72±10.94 (13.54→ 53.96) |
Css: Serum steady-state concentrations
Values are mean ± SD
CL: Calculated triapine clearance
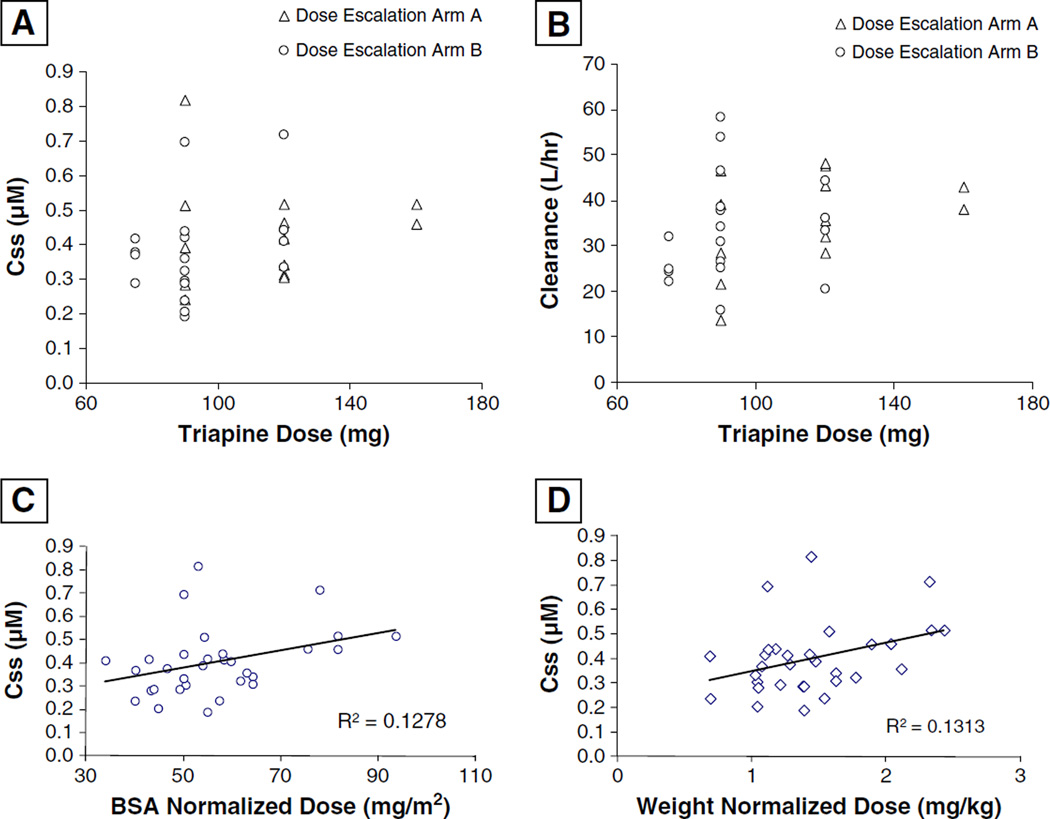
Performance status (PS) was not significantly associated with either PK parameter, and Sex was found to significantly associate with Css (p = 0.03) but not with CL (p = 0.14). Females had higher mean Css (0.44±0.16 µM, n = 18) compared to males (0.33±0.08 µM, n = 13). But, when we controlled for the BSA, there was no significant association of Css and Sex (p = 0.12), considering that female patients had a significantly lower mean BSA (p = 0.03). We also evaluated Dose level as a categorical variable and found no significant associations with either Css or CL (p-values 0.69 and 0.21, respectively). Scatter plots for PK parameters vs. Dose are displayed in Fig. 1 (a and b). However, we did observe significant associations with CL versus both Weight and BSA when evaluated with linear regression (p-values 0.05 and 0.04, respectively). Css was not directly associated with either Weight or BSA (p-values 0.20 and 0.14, respectively), however, it was significantly associated with Dose normalized to both Weight and BSA (p-values 0.04 and 0.05, respectively) (Fig. 1c and d). Normalized doses were not associated with triapine CL (p-values 0.63 and 0.69, respectively). Neither Css nor CL were significantly associated with Age (p-values 0.16 and 0.06, respectively), although data suggested increasing Age may trend with decreasing CL.
Fig. 1.
Plots of triapine steady-state serum concentrations (Css) (a) and calculated clearance (b) against dose. Correlative plots of Css with normalized dose based on body surface area (BSA) (c) and weight (d)
We also evaluated pharmacokinetics against Cycle 1 pretreatment values for serum creatinine, creatinine clearance, total bilirubin, albumin, total protein, and hemoglobin using linear regression. Among all lab variables evaluated, only one significant association was identified, which was a positive correlation between Css and hemoglobin (p = 0.02).
Pharmacodynamic correlatives
Circulating hRRM2 data were obtained from 28 patients with complete data from both cycles 1 and 2 obtained for 19 patients. Twenty one patients (75 %) had decline in hRRM2 levels during treatment cycles 1 and 2, with a significant decrease in hRRM2 following treatment on cycle 1 for all patients (p = 0.0003), regardless of their RECIST response; but no significant change with cycle 2 (p = 0.06). Data regarding the relationship between circulating hRRM2 and clinical outcomes are summarized in Table 5. There was no association between baseline hRRM2 level and DCR, TTP, or OS. Decline in hRRM2 level during cycle 1 was significantly predictive of improved OS (p = 0.0002) but not TTP or DCR. Continuous decline in hRRM2 during the first 2 treatment cycles was a significant predictor of DCR (p = 0.03), and median TTP (p = 0.02), but not median OS (p = 0.21). There was no significant correlation between Css and changes in RRM2 levels for cycle 1 (p = 0.30) or maximum changes of hRRM2 levels across cycles 1 and 2 (p = 0.73). Table 2S (supplement) shows the details of hRRM2 expression levels and their changes at different time-points throughout the treatment, for each patient.
Table 5.
Relationship between circulating hRRM2 and clinical outcomes
| hRRM2a Levels | DCRb% (N=25) |
p-value | mTTPc(m) (N=28) |
95 % CId | p-value | mOSe(m) (N=28) |
95 % CI | p-value |
|---|---|---|---|---|---|---|---|---|
| Baseline level | ||||||||
| >2.41f | 50 | 1.0 | 4.3 | (1.6, 5.0) | 0.68 | 7.9 | (6.3, 9.3) | 0.94 |
| <2.41 | 50 | 3.1 | (1.7, 4.6) | 8.5 | (1.4, 17.2) | |||
| ↓with cycle 1 | ||||||||
| Yes | 60 | 0.24 | 3.8 | (1.9, 5.0) | 0.21 | 8.5 | (6.3, 10.7) | 0.0002 |
| No | 25 | 2.4 | (1.0, 4.8) | 2.1 | (1.1, 7.3) | |||
| Continued↓ g | ||||||||
| Yes | 100 | 0.031 | 8.1 | (2.9, 8.1) | 0.021 | 9.3 | (8.5, 9.3) | 0.21 |
| No | 37.5 | 3.0 | (1.7, 3.9) | 7.3 | (3.7, 8.2) |
hRRM2 0 Human ribonucleotide reductase M2 subunit
DCR 0 Disease control rate, which is partial response + stable disease
mTTP 0 Median time to progression (months)
95% CI 0 95 % confidence interval
mOS = Median overall survival (months)
2.41 is the median value for hRRM2 levels
Denotes continued decline from first measurement on cycle 1 day 1 to last measurement on cycle 2 day 2
Discussion
This phase I study was designed to establish the MTD for prolonged (24-hour) infusion of triapine followed by fixeddose-rate gemcitabine; to determine the safety and tolerability of this combination; to examine the preliminary antitumoral activity of this combination; and to perform limited pharmacokinetic and correlative pharmacodynamic analysis.
This combination was generally well tolerated at the MTD with myelosuppression being the main DLT, as expected in this heavily pretreated patient population. The other common toxicities, such as nausea, vomiting, fatigue, and transaminitis were similar in incidence to the reported toxicities expected with gemcitabine alone. Interestingly, we did not observe any methemoglobinemia or cardiopulmonary toxicity, such as hypotension, hypoxia, and respiratory distress. The MTD on this study was determined to be triapine 90 mg (24-hour infusion) immediately followed by gemcitabine 1000 mg/m2 (100-minute infusion), every 2 weeks of a 4-week cycle. Thirty patients were evaluable for response on this study. We observed a disease control rate of 53 % (50 % SD and 3 % PR) and a nearly 4 months median time to tumor progression for patients with SD. About half of these patients had prior disease progression on standard gemcitabine, suggesting preliminary evidence of synergism between fixed-dose-rate gemcitabine and 24-hour infusion triapine that may overcome gemcitabine resistance.
We initially observed significant hematologic toxicities on the Arm A of the protocol, when there was no limitations on prior number of chemotherapy regimens and patients were receiving treatment on days 1 and 8 of a 21-day cycle regimen. After the protocol was amended and the number of prior cytotoxic therapies was limited to 2 or less and the regimen was changed to days 1 and 15 of a 28-day cycle regimen, this combination was much better tolerated with significantly less myelosuppression. Out of 15 patients, who were treated at MTD, 8 required a dose delay or dose reduction at some point throughout their treatment and none of them came off treatment because of toxicity. Single agent triapine, like other ribonucleotide reductase inhibitors, can result in reversible myelosuppression [41], which can explain the higher rate of myelosuppression observed with this combination, as compared to gemcitabine alone. We need to mention that there was a sudden death on this trial, which occurred 7 days after the last treatment on cycle 3 in a 74 year-old male with a diagnosis of metastatic gastroesophageal junction carcinoma. Although no autopsy was performed and given its timing, this was thought to be “possibly” related to both the treatment and the disease.
In contrast, prior to our study other phase I/II trials of this combination with different dose/schedule [32, 42–44], reported more significant toxicities including hypoxia, methemoglobinemia and significant cardiopulmonary toxicity. The initial phase I trial defined the MTD as triapine at 105 mg/m2 infused over 2–4 h followed by gemcitabine at 1000 mg/m2 over 30 min (standard-dose), on days 1, 8, and 15, for a 28-day cycle [32]. This recommended phase II dose was tested in 4 different phase II solid tumor trials including 2 in non-small cell lung cancer, 1 in advanced pancreas cancer and 1 in biliary cancer [42–45]. Taken together, the only observed responses were in 3 patients with biliary cancers. Of note, in our trial, the only patient with a meaningful PR had metastatic biliary tract carcinoma.
Previous PK studies in humans indicated triapine had linear PK behavior when doses were normalized to BSA [30, 31, 46]. However, significant inter-patient variability and lack of associations between weight or BSA and triapine CL suggested dose normalization may not be required and may contribute to inter-patient variability. One aim of our study was to evaluate the triapine Css and clearance using a dosing regimen without normalization to body size. As with the previous studies, we observed no significant association of triapine dose with CL (Fig. 1b). However, we did not observe a clear linear trend for triapine dose and Css (Fig. 1a). Variability observed for Css and CL was approximately 30 % coefficient of variation, both within and between dose groups (Table 4). This is not drastically different than the variability reported in the previous studies. However, linearity across dose groups was evident, especially in the report by Murren et al. [30]. Furthermore, in our study, triapine CL was found to be significantly associated with patient weight and BSA, and Css was significantly associated with dose normalized to weight or BSA. Also, we observed gender was associated with Css, but this is likely due to female patients having smaller body habitus (BSA) compared to male patients in our study; considering that this correlation was no longer significant when we control for BSA.
The total body CL of 33.72±10.94 L/hr was higher relative to the population estimated CL of 25 L/hr in patients with advanced primary or metastatic tumors, published by Kolesar et al. [47]. The steady-state triapine serum concentrations measured in our study were below mean in vitro tumor growth inhibitory concentrations across multiple cell types (mean GI50 1.6 µM in a 48-h exposure assay), as previously reported [26]. This may suggest systemic concentrations of triapine were not sufficient to produce the desired hRRM2 inhibitory effects. In fact, cycle 1 hRRM2 levels were inhibited only by an average of 4 % relative to baseline in our study. More investigation will be required to understand why triapine clearance in our study was higher compared to the previous reports in solid tumor patients. This could be related to the differences in the respective assays used for triapine quantification (LC-MS in our study vs. LC-UV in the previous study) [46]. Alternatively, it may reflect differences in triapine volume of distribution or clearance in the respective patient populations. Interestingly, we observed Css was significantly associated with patients’ hemoglobin. As triapine is a metal chelator and has been clearly shown to bind iron, hemoglobin may be an important variable in triapine disposition.
Additionally, we evaluated circulating hRRM2 as a potential predictive biomarker for the clinical efficacy of the combination of triapine and gemcitabine. We show that baseline hRRM2 levels do not appear to predict clinical outcomes. Study treatment resulted in a significant decline in hRRM2 levels with cycle 1. This decline did not predict the response but did correlate with survival. Additionally, our data indicates that continuous decline in circulating hRRM2 during cycles 1 and 2 of treatment correlates with disease control. These results suggest that steady decline in circulating hRRM2 levels may correlate with outcome. These interesting findings need to be explored in future trials to confirm the potential use of circulating hRRM2 on clinical outcomes with the combination of triapine and gemcitabine.
In conclusion, our study defined a safe and tolerable recommended phase II dose (RP2D) of triapine 90 mg (24-hour infusion) immediately followed by gemcitabine 1000 mg/m2 (100-minute infusion), every 2 weeks of a 4-week cycle. This RP2D should be tested in future prospective trials with gemcitabine and triapine in various solid tumors, more specifically in patients with biliary cancers.
Supplementary Material
Acknowledgements
We thank all the patients who participated in this trial, as well as the Clinical Trials Office personnel, James Cancer Hospital inpatient nurses and enrolling physicians, for their help in completion of this study. This study was supported by the National Institutes of Health, National Cancer Institute, Bethesda, U.S.A. (NCI Protocol # 7043).
Grant Support This study was supported by the U01 Grant through National Institutes of Health (Grant # U01 CA076576).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10637-012-9863-1) contains supplementary material, which is available to authorized users.
Disclosure of Potential Conflict of Interest There are no potential conflicts of interest among the authors of this article. This article has been seen, read, and agreed on in its content by all designated authors. This article has not been submitted or published elsewhere.
Contributor Information
Amir Mortazavi, Division of Medical Oncology, Department of Internal Medicine, College of Medicine, The Ohio State University and The Comprehensive Cancer Center, A454 Starling-Loving Hall, 320 West 10th Ave, Columbus, OH 43210, USA.
Yonghua Ling, Pharmacoanalytical Shared Resources, The Ohio State University and The Comprehensive Cancer Center, Columbus, OH 43210, USA.
Ludmila Katherine Martin, Division of Medical Oncology, Department of Internal Medicine, College of Medicine, The Ohio State University and The Comprehensive Cancer Center, A454 Starling-Loving Hall, 320 West 10th Ave, Columbus, OH 43210, USA.
Lai Wei, Center for Biostatistics, The Ohio State University and The Comprehensive Cancer Center, Columbus, OH 43210, USA.
Mitch A. Phelps, Pharmacoanalytical Shared Resources, The Ohio State University and The Comprehensive Cancer Center, Columbus, OH 43210, USA Division of Pharmaceutics, College of Pharmacy, The Ohio State University and The Comprehensive Cancer Center, Columbus, OH 43210, USA.
Zhongfa Liu, Pharmacoanalytical Shared Resources, The Ohio State University and The Comprehensive Cancer Center, Columbus, OH 43210, USA; Division of Pharmaceutics, College of Pharmacy, The Ohio State University and The Comprehensive Cancer Center, Columbus, OH 43210, USA.
Erica J. Harper, Division of Medical Oncology, Department of Internal Medicine, College of Medicine, The Ohio State University and The Comprehensive Cancer Center, A454 Starling-Loving Hall, 320 West 10th Ave, Columbus, OH 43210, USA
S. Percy Ivy, Cancer Therapeutics Evaluation Program, National Cancer Institute, Rockville, MD 20852, USA.
Xin Wu, Division of Medical Oncology, Department of Internal Medicine, College of Medicine, The Ohio State University and The Comprehensive Cancer Center, A454 Starling-Loving Hall, 320 West 10th Ave, Columbus, OH 43210, USA.
Bing-Sen Zhou, City of Hope Comprehensive Cancer Center, Duarte, CA 91010, USA.
Xiyong Liu, City of Hope Comprehensive Cancer Center, Duarte, CA 91010, USA.
Deidre Deam, Division of Medical Oncology, Department of Internal Medicine, College of Medicine, The Ohio State University and The Comprehensive Cancer Center, A454 Starling-Loving Hall, 320 West 10th Ave, Columbus, OH 43210, USA.
J. Paul Monk, Division of Medical Oncology, Department of Internal Medicine, College of Medicine, The Ohio State University and The Comprehensive Cancer Center, A454 Starling-Loving Hall, 320 West 10th Ave, Columbus, OH 43210, USA.
William J. Hicks, Division of Medical Oncology, Department of Internal Medicine, College of Medicine, The Ohio State University and The Comprehensive Cancer Center, A454 Starling-Loving Hall, 320 West 10th Ave, Columbus, OH 43210, USA
Yun Yen, City of Hope Comprehensive Cancer Center, Duarte, CA 91010, USA.
Gregory A. Otterson, Division of Medical Oncology, Department of Internal Medicine, College of Medicine, The Ohio State University and The Comprehensive Cancer Center, A454 Starling-Loving Hall, 320 West 10th Ave, Columbus, OH 43210, USA
Michael R. Grever, Division of Hematology, Department of Internal Medicine, The Ohio State University and The Comprehensive Cancer Center, Columbus, OH 43210, USA
Tanios Bekaii-Saab, Email: Tanios.Saab@osumc.edu, Division of Medical Oncology, Department of Internal Medicine, College of Medicine, The Ohio State University and The Comprehensive Cancer Center, A454 Starling-Loving Hall, 320 West 10th Ave, Columbus, OH 43210, USA.
References
- 1.Bokemeyer C, et al. Gemcitabine in patients with relapsed or cisplatin-refractory testicular cancer. J Clin Oncol. 1999;17(2):512–516. doi: 10.1200/JCO.1999.17.2.512. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA, 3rd, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.Cardenal F, et al. Randomized phase III study of gemcitabinecisplatin versus etoposide-cisplatin in the treatment of locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 1999;17(1):12–18. doi: 10.1200/JCO.1999.17.1.12. [DOI] [PubMed] [Google Scholar]
- 4.Heinemann V. Role of gemcitabine in the treatment of advanced and metastatic breast cancer. Oncology. 2003;64(3):191–206. doi: 10.1159/000069315. [DOI] [PubMed] [Google Scholar]
- 5.Markman M. Second-line treatment of ovarian cancer with single-agent gemcitabine. Semin Oncol. 2002;29(1 Suppl 1):9–10. doi: 10.1053/sonc.2002.31588. [DOI] [PubMed] [Google Scholar]
- 6.Scheithauer W. Review of gemcitabine in biliary tract carcinoma. Semin Oncol. 2002;29(6 Suppl 20):40–45. doi: 10.1053/sonc.2002.37380. [DOI] [PubMed] [Google Scholar]
- 7.von der Maase H. Gemcitabine in transitional cell carcinoma of the urothelium. Expert Rev Anticancer Ther. 2003;3(1):11–19. doi: 10.1586/14737140.3.1.11. [DOI] [PubMed] [Google Scholar]
- 8.Bjorklund S, et al. S-phase-specific expression of mammalian ribonucleotide reductase R1 and R2 subunit mRNAs. Biochemistry. 1990;29(23):5452–5458. doi: 10.1021/bi00475a007. [DOI] [PubMed] [Google Scholar]
- 9.Cory JG, et al. Inhibitors of ribonucleotide reductase. Comparative effects of amino- and hydroxy-substituted pyridine-2- carboxaldehyde thiosemicarbazones. Biochem Pharmacol. 1994;48(2):335–344. doi: 10.1016/0006-2952(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 10.Duxbury MS, et al. RNA interference targeting the M2 subunit of ribonucleotide reductase enhances pancreatic adenocarcinoma chemosensitivity to gemcitabine. Oncogene. 2004;23(8):1539–1548. doi: 10.1038/sj.onc.1207272. [DOI] [PubMed] [Google Scholar]
- 11.Jung CP, Motwani MV, Schwartz GK. Flavopiridol increases sensitization to gemcitabine in human gastrointestinal cancer cell lines and correlates with down-regulation of ribonucleotide reductase M2 subunit. Clin Cancer Res. 2001;7(8):2527–2536. [PubMed] [Google Scholar]
- 12.Heinemann V, et al. Inhibition of ribonucleotide reduction in CCRF-CEM cells by 2′,2′-difluorodeoxycytidine. Mol Pharmacol. 1990;38(4):567–572. [PubMed] [Google Scholar]
- 13.Gandhi V, et al. Prolonged infusion of gemcitabine: Clinical and pharmacodynamic studies during a phase I trial in relapsed acute myelogenous leukemia. J Clin Oncol. 2002;20(3):665–673. doi: 10.1200/JCO.2002.20.3.665. [DOI] [PubMed] [Google Scholar]
- 14.Grunewald R, et al. Gemcitabine in leukemia: A phase I clinical, plasma, and cellular pharmacology study. J Clin Oncol. 1992;10(3):406–413. doi: 10.1200/JCO.1992.10.3.406. [DOI] [PubMed] [Google Scholar]
- 15.Tempero M, et al. Randomized phase II comparison of doseintense gemcitabine: Thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21(18):3402–3408. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 16.Poplin E, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: A trial of the eastern cooperative oncology group. J Clin Oncol. 2009;27(23):3778–3785. doi: 10.1200/JCO.2008.20.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elford HL, et al. Ribonucleotide reductase and cell proliferationIVariations of ribonucleotide reductase activity with tumor growth rate in a series of rat hepatomas. J Biol Chem. 1970;245(20):5228–5233. [PubMed] [Google Scholar]
- 18.Bergman AM, Pinedo HM, Peters GJ. Determinants of resistance to 2′,2′-difluorodeoxycytidine (gemcitabine) Drug Resist Updat. 2002;5(1):19–33. doi: 10.1016/s1368-7646(02)00002-x. [DOI] [PubMed] [Google Scholar]
- 19.Goan YG, et al. Overexpression of ribonucleotide reductase as a mechanism of resistance to 2,2-difluorodeoxycytidine in the human KB cancer cell line. Cancer Res. 1999;59(17):4204–4207. [PubMed] [Google Scholar]
- 20.Gandhi V, et al. Fludarabine infusion potentiates arabinosylcytosine metabolism in lymphocytes of patients with chronic lymphocytic leukemia. Cancer Res. 1992;52(4):897–903. [PubMed] [Google Scholar]
- 21.Iwasaki H, et al. Differential incorporation of ara-C, gemcitabine, and fludarabine into replicating and repairing DNA in proliferating human leukemia cells. Blood. 1997;90(1):270–278. [PubMed] [Google Scholar]
- 22.Kubota M, et al. Differential modulation of 1-beta-Darabinofuranosylcytosine metabolism by hydroxyurea in human leukemic cell lines. Biochem Pharmacol. 1988;37(9):1745–1749. doi: 10.1016/0006-2952(88)90438-8. [DOI] [PubMed] [Google Scholar]
- 23.Walsh CT, Craig RW, Agarwal RP. Increased activation of 1-beta-D-arabinofuranosylcytosine by hydroxyurea in L1210 cells. Cancer Res. 1980;40(9):3286–3292. [PubMed] [Google Scholar]
- 24.Zhou B, et al. Time and sequence dependence of hydroxyurea in combination with gemcitabine in human KB cells. Anticancer Res. 2002;22(3):1369–1377. [PubMed] [Google Scholar]
- 25.Moore EC, Booth BA, Sartorelli AC. Inhibition of deoxyribonucleotide synthesis by pyridine carboxaldehyde thiosemicarbazones. Cancer Res. 1971;31(3):235–238. [PubMed] [Google Scholar]
- 26.Finch RA, et al. Triapine (3-aminopyridine-2-carboxaldehydethiosemicarbazone): A potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem Pharmacol. 2000;59(8):983–991. doi: 10.1016/s0006-2952(99)00419-0. [DOI] [PubMed] [Google Scholar]
- 27.Zhu L, et al. Inhibitory mechanisms of heterocyclic carboxaldehyde thiosemicabazones for two forms of human ribonucleotide reductase. Biochem Pharmacol. 2009;78(9):1178–1185. doi: 10.1016/j.bcp.2009.06.103. [DOI] [PubMed] [Google Scholar]
- 28.Chen C-H, King I, Belcourt M. Triapine, a ribonucleotide reductase inhibitor, enhances incorporation of gemcitabine into DNA and cytotoxicity to KB cells. European journal of cancer (Oxford, England : 1990) 2002;38:S26. [Google Scholar]
- 29.Feun L, et al. Phase I and pharmacokinetic study of 3- aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) using a single intravenous dose schedule. Cancer Chemother Pharmacol. 2002;50(3):223–229. doi: 10.1007/s00280-002-0480-0. [DOI] [PubMed] [Google Scholar]
- 30.Murren J, et al. Phase I and pharmacokinetic study of triapine, a potent ribonucleotide reductase inhibitor, administered daily for 5 days in patients with advanced solid tumors. Clin Cancer Res. 2003;9(11):4092–4100. [PubMed] [Google Scholar]
- 31.Wadler S, et al. Phase I and pharmacokinetic study of the ribonucleotide reductase inhibitor-3-aminopyridine-2-carboxaldehyde thiosemicarbazone, administered by 96-hour intravenous continuous infusion. J Clin Oncol. 2004;22(9):1553–1563. doi: 10.1200/JCO.2004.07.158. [DOI] [PubMed] [Google Scholar]
- 32.Yen Y, et al. A phase I trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone in combination with gemcitabine for patients with advanced cancer. Cancer Chemother Pharmacol. 2004;54(4):331–342. doi: 10.1007/s00280-004-0821-2. [DOI] [PubMed] [Google Scholar]
- 33.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 34.Birch N, Wang X, Chong H-S. Iron chelators as therapeutic iron depletion agents. Expert Opinion on Therapeutic Patents. 2006;16(11):1533–1556. [Google Scholar]
- 35.Shao J, et al. A Ferrous-Triapine complex mediates formation of reactive oxygen species that inactivate human ribonucleotide reductase. Mol Cancer Ther. 2006;5(3):586–592. doi: 10.1158/1535-7163.MCT-05-0384. [DOI] [PubMed] [Google Scholar]
- 36.US Food and Drug Administration Center for Drug Evaluation and Research (CDER) and Center for Veterinary Medicine (CVM) Guidance for Industry on Bioanalytical Method Validation. 2001 [Google Scholar]
- 37.Zhou B, et al. Production of a monoclonal antibody against the hRRM2 subunit of ribonucleotide reductase and immunohistochemistry study of human cancer tissues. Hybridoma (Larchmt) 2006;25(5):264–270. doi: 10.1089/hyb.2006.25.264. [DOI] [PubMed] [Google Scholar]
- 38.Ko AH, et al. Phase II study of fixed dose rate gemcitabine with cisplatin for metastatic adenocarcinoma of the pancreas. J Clin Oncol. 2006;24(3):379–385. doi: 10.1200/JCO.2005.01.8267. [DOI] [PubMed] [Google Scholar]
- 39.Lopes G, et al. Oxaliplatin and fixed-rate infusional gemcitabine in the second-line treatment of patients with metastatic colon cancer: Final results of a phase II trial prematurely closed as a result of poor accrual. Clin Colorectal Cancer. 2007;6(9):641–645. doi: 10.3816/CCC.2007.n.032. [DOI] [PubMed] [Google Scholar]
- 40.Louvet C, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23(15):3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 41.Nutting CM, et al. Phase II study of 3-AP Triapine in patients with recurrent or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2009;20(7):1275–1279. doi: 10.1093/annonc/mdn775. [DOI] [PubMed] [Google Scholar]
- 42.Mackenzie MJ, et al. A Phase II study of 3-aminopyridine-2- carboxaldehyde thiosemicarbazone (3-AP) and gemcitabine in advanced pancreatic carcinoma. A trial of the Princess Margaret hospital Phase II consortium. Invest New Drugs. 2007;25(6):553–558. doi: 10.1007/s10637-007-9066-3. [DOI] [PubMed] [Google Scholar]
- 43.Traynor AM, et al. A phase II trial of triapine (NSC# 663249) and gemcitabine as second line treatment of advanced non-small cell lung cancer: Eastern cooperative oncology group study 1503. Invest New Drugs. 2010;28(1):91–97. doi: 10.1007/s10637-009-9230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma B, et al. A multicenter phase II trial of 3-aminopyridine- 2-carboxaldehyde thiosemicarbazone (3-AP, Triapine) and gemcitabine in advanced non-small-cell lung cancer with pharmacokinetic evaluation using peripheral blood mononuclear cells. Invest New Drugs. 2008;26(2):169–173. doi: 10.1007/s10637-007-9085-0. [DOI] [PubMed] [Google Scholar]
- 45.Ocean AJ, et al. Phase II trial of the ribonucleotide reductase inhibitor 3-aminopyridine-2-carboxaldehydethiosemicarbazone plus gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol. 2011;68(2):379–388. doi: 10.1007/s00280-010-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giles FJ, et al. Phase I and pharmacodynamic study of Triapine, a novel ribonucleotide reductase inhibitor, in patients with advanced leukemia. Leuk Res. 2003;27(12):1077–1083. doi: 10.1016/s0145-2126(03)00118-8. [DOI] [PubMed] [Google Scholar]
- 47.Kolesar J, et al. Population pharmacokinetics of 3- aminopyridine-2-carboxaldehyde thiosemicarbazone (Triapine (R)) in cancer patients. Cancer Chemother Pharmacol. 2011;67(2):393–400. doi: 10.1007/s00280-010-1331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.