Abstract
Expression of the hominoid-specific oncoprotein TBC1D3 promotes enhanced cell growth and proliferation by increased activation of signal transduction through several growth factors. Recently we documented the role of CUL7 E3 ligase in growth factors-induced ubiquitination and degradation of TBC1D3. Here we expanded our study to discover additional molecular mechanisms that control TBC1D3 protein turnover. We report that TBC1D3 is palmitoylated on two cysteine residues: 318 and 325. The expression of double palmitoylation mutant TBC1D3:C318/325S resulted in protein mislocalization and enhanced growth factors-induced TBC1D3 degradation. Moreover, ubiquitination of TBC1D3 via CUL7 E3 ligase complex was increased by mutating the palmitoylation sites, suggesting that depalmitoylation of TBC1D3 makes the protein more available for ubiquitination and degradation. The results reported here provide novel insights into the molecular mechanisms that govern TBC1D3 protein degradation. Dysregulation of these mechanisms in-vivo could potentially result in aberrant TBC1D3 expression and promote oncogenesis.
Keywords: TBC1D3, hominoid-specific proteins, protein palmitoylation, protein ubiquitination, protein degradation, cancer, oncogene, CUL7, growth factor receptor signaling
INTRODUCTION
TBC1D3, a hominoid-specific gene, was identified some twenty-five years ago in pioneering cross-species transfection experiments [1]. Some years later, Pei et al [2] showed that TBC1D3 (aka PRC17) is an oncogene, with variable copy number, that is over-expressed in prostate and breast cancer. This has been extended in recent years to include bladder cancer [3] as well as MDS (myelodysplastic syndrome) [4]. Pei et al [2] also showed that TBC1D3 encodes a protein with a TBC domain that binds the low molecular weight GTPase Rab5 suggesting that the TBC1D3 TBC domain may serve as a Rab5 GAP. More recent work [5] indicates that the TBC domain of TBC1D3 lacks an arginine finger motif and has very low Rab GAP activity. Paulding et al [6] carried out a comparative study on the origin of TBC1D3 and reported that it is hominoid-specific and widely expressed in human tissues. More recent work [7]demonstrated that TBC1D3 is encoded by a collection of very similar paralogs with multiple copies of each paralog. Using the database made available by the 1000 genome project, Eichler and colleagues [8] showed that TBC1D3 is amongst the highest multi-copied genes in the human genome with some human genomes encoding well over 50 copies depending on ethnic origin of the donor. TBC1D3 operates as a regulator of growth factor signaling by enhancing the signaling of the EGF receptor [9] and the insulin/IGF receptors [10]. As part of an apparent activation-deactivation cycle, TBC1D3 degradation is regulated by the E3 ligase CUL7 [11]. In this paper we show that TBC1D3 is palmitoylated and localized to lipid rafts. Moreover, we show that palmitoylation influences TBC1D3 ubiquitination and degradation.
MATERIALS AND METHODS
Plasmids and reagents
The cDNAs for full-length TBC1D3 and domains were amplified by PCR and ligated into EcoRI/NotI sites of a pCMV-Myc vector (Invitrogen). Cysteine mutants were generated with a QuikChange Site-Directed Mutagenesis Kit (Agilent). Anti - Myc antibody was from Santa Cruz; Anti-Tubulin, GAPDH and CUL7 (monoclonal) were from Sigma; Anti-ubiquitin was from Invitrogen; 2C7 monoclonal anti-TBC1D3 antibody was generated by the Washington University Hybridoma Center with the last 50 amino acids of TBC1D3 as antigen.
Cell culture and transfections
HeLa cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone) supplemented with 10% fetal bovine serum. Transfections were carried out using Lipofectamine 2000 (Invitrogen) and experiments were carried out approximately 18 h after transfection.
Palmitoylation assays
Metabolic labeling
HeLa cells, transiently transfected with Myc-TBC1D3 or cysteine mutants- Myc-TBC1D3:C318S, Myc-TBC1D3:C325S, Myc-TBC1D3:C383S, Myc-TBC1D3:C318/325S, and Myc-TBC1D3:C318/325/383S were pre-incubated for 30 min in serum-free DMEM with fatty acid-free bovine serum albumin and then labeled with 0.5 mCi/ml [3H] palmitate (PerkinElmer) for 3–4 h. Cell lysates made in the 1% Triton X-100 lysis buffer were immunoprecipitated with anti-Myc monoclonal antibody and separated by SDS-PAGE. The gel was fixed, dried under vacuum and exposed to film at −80°C for 3–4 weeks.
Fatty acyl exchange labeling
Palmitoylation was detected as described in [12], TBC1D3 was isolated [11] from whole cell lysates (Cell Signaling Technology), and free protein thiol groups in the lysate were blocked with 1mM N-ethylmaleimide. Cys-palmitoyl thioester linkages were cleaved with hydroxylamine (HA) and subsequently labeled with 4mM biotin–HPDP (Biotin-HPDP-N-[6-(Biotinamido)hexyl]-3’-(2’-pyridyldithio)propionamide (Sigma). Biotinylated proteins were then affinity-purified using neutravidin–agarose. Bound proteins were released from the affinity resin through reduction of the protein–biotin disulfide linkages by the addition of 1% β-mercaptoethanol. Palmitoylated TBC1D3 was detected by western blot with anti-Myc antibody. Samples cleaved using Tris-HCl, instead of HA, served as negative controls
Lipid Raft Fractionation
Detergent-free lipid rafts were prepared using published protocols with some modifications [13,14]. Briefly, HeLa cell pellets were resuspended in 1 ml of non-detergent lysis buffer (25 mM Hepes-HCl, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, pH 7.4) with a protease inhibitors cocktail supplemented with 10 mM NaF and 1 mM Na3VO4 and homogenized by 20 strokes through a 23-gauge needle. Lysates were obtained by centrifugation at 1,000 g for 10 min at 4°C. A step gradient was prepared by overlaying the sample in 40% sucrose (2 ml) with 30% sucrose (6.5 ml) and 5% sucrose (3.5 ml) on top. Gradients were centrifuged at 39,000 RPM (180,000 g) for 20 hr at 4°C. 1 ml fractions were collected from the top of the gradient. Samples were analyzed by immunoblot after SDS-PAGE.
Immunoblot analysis
Whole cell lysates (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 1% Triton X-100, 10% glycerol, 1 mM EDTA, and protease inhibitor cocktail supplemented with 10 mM NaF and 1 mM Na3VO4) were separated by SDS-PAGE and transferred to nitrocellulose membranes (Schleicher & Schuell). The membranes were blocked in TBST (100 mM NaCl, 10 mM Tris-HCl, pH 7.5, 0.1% Tween 20) containing 5% non-fat milk and incubated with primary antibodies in 2% BSA/TBST overnight at 4°C or 2 h at room temperature, followed by incubation with HRP-conjugated goat anti-rabbit or anti-mouse IgG (Jackson ImmunoResearch) and detected by chemiluminescence (Pierce). Immunoblot data were quantified by AlphaEaseFC 4.0 software (Alpha Innotech Corp.).
TBC1D3 degradation assay
Cells in 12-well plates were transfected with Myc-TBC1D3. At 18 h posttransfection, the cells were starved in serum-free medium for 3 h and either un-treated as a control or incubated with 10% fetal calf serum (FCS). Cell lysates were subjected to SDS-PAGE and immunoblotting with specific antibodies.
Immunofluorescence Microscopy
HeLa cells, plated onto the coverslips overnight, were transfected with TBC1D3 constructs and fixed with 3% paraformaldehyde (Electron Microscope Sciences) for 20 min, quenched for 10 min with 50 mM ammonium chloride, permeabilized with 0.1% Triton X-100 for 10 min, blocked with 2% goat serum and 1% BSA for 1 h, and incubated with primary antibodies for 1 h, followed by a secondary antibody, Alexa-Fluor 568 goat anti-mouse IgG (Invitrogen) for 30 min at room temperature. All solutions were made in phosphate buffered saline. The coverslips were mounted with Fluorescent Mounting Medium (DakoCytomation) and examined under a MRC1024 confocal microscopy (Bio-Rad) using a 63× objective lens.
In Vitro Ubiquitination Assay
HeLa cells seeded in 6-well plates were transfected with HA-CUL7, Myc-TBC1D3 or various TBC1D3 mutant constructs described above. The cells were washed and homogenized in cold sucrose buffer (0.25 M sucrose, 5 mM Tris-HCl, pH 7.4, 2 mM EDTA) supplemented with protease inhibitor cocktail and 10 mM Nethylmaleimide by 10 strokes through a 25-gauge needle. A post-nuclear supernatant (PNS) was prepared by centrifugation (2,000 g) for 10 min. Cytosol and membrane fractions were derived from the PNS fraction by centrifugation for 20 min at 100,000 g. The membrane fractions were solubilized in 1% TritonX-100/ lysis buffer. The insoluble material was pelleted by centrifugation for 15 min at 10,000 rpm. The supernatant was saved as the “membrane fraction”. The assay was initiated by incubating control or HA-CUL7-enriched cytosol with control or TBC1D3-enriched “membrane fractions” in buffer (5 mM MgCl2, 20 mM Tris-HCl pH 7.5, 2 mM ATP) at room temperature for 45 min. TBC1D3 was then immunoprecipitated with polyclonal Myc antibody, resolved by SDS-PAGE, and analyzed by immunoblot with anti-ubiquitin antibody.
RESULTS
TBC1D3 is palmitoylated and localized to the plasma membrane
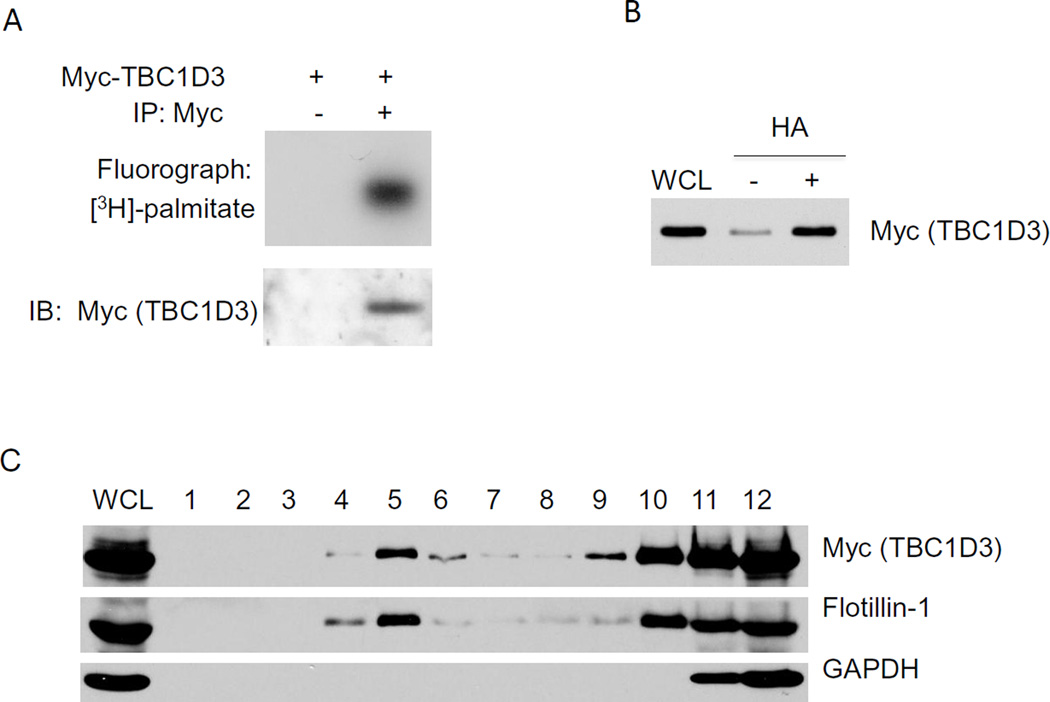
Earlier work demonstrated that TBC1D3 was preferentially localized to the plasma membrane [5,9]. Since TBC1D3 lacks an obvious transmembrane domain we entertained the possibility that palmitoylation could be involved in membrane targeting. HeLa cells, transiently expressing Myc-TBC1D3, were labeled with [3H] palmitate and TBC1D3 was immunoprecipitated with anti-Myc monoclonal antibody. The samples were separated by SDS-PAGE and incorporation of radioactive palmitate by TBC1D3 was measured by fluorography (Figure 1A). Expressed TBC1D3 protein efficiently and specifically incorporated radioactive palmitate, suggesting that TBC1D3 protein is palmitoylated in HeLa cells.
Figure 1. TBC1D3 is palmitoylated and localized to lipid rafts.
(A) HeLa cells were transiently transfected with Myc-TBC1D3 and metabolically labeled with [3H] palmitate. Cell lysates were immunoprecipitated with or without anti-Myc antibody. [3H] palmitate incorporation (upper panel) and immunoblot (IB) (lower panel) are shown. (B) Biotin-labeled palmitoylation assay was performed in Myc-TBC1D3 transfected HeLa cells. Palmitoylated TBC1D3 was detected after the treatment of hydroxylamine (HA) by western blot with anti-Myc antibody. (C) HeLa cell lysates were fractionated by ultracentrifugation on a discontinuous sucrose gradient as described in Experimental Methods. A total of 12 fractions were collected from top to bottom of the gradient. An aliquot from each fraction and total lysate were resolved by SDS-PAGE and then immunoblotted with TBC1D3, Flotillin-1, a lipid raft marker and GAPDH antibodies.
To confirm our findings, TBC1D3 palmitoylation was assayed by fatty acyl exchange labeling[12]. HeLa cells were transiently transfected with Myc-TBC1D3. Cys-palmitoyl thioester linkages were cleaved with hydroxylamine (HA) as described in Methods. The data in Figure 2B show that immunoprecipitated TBC1D3 has readily detectable palmitate groups.
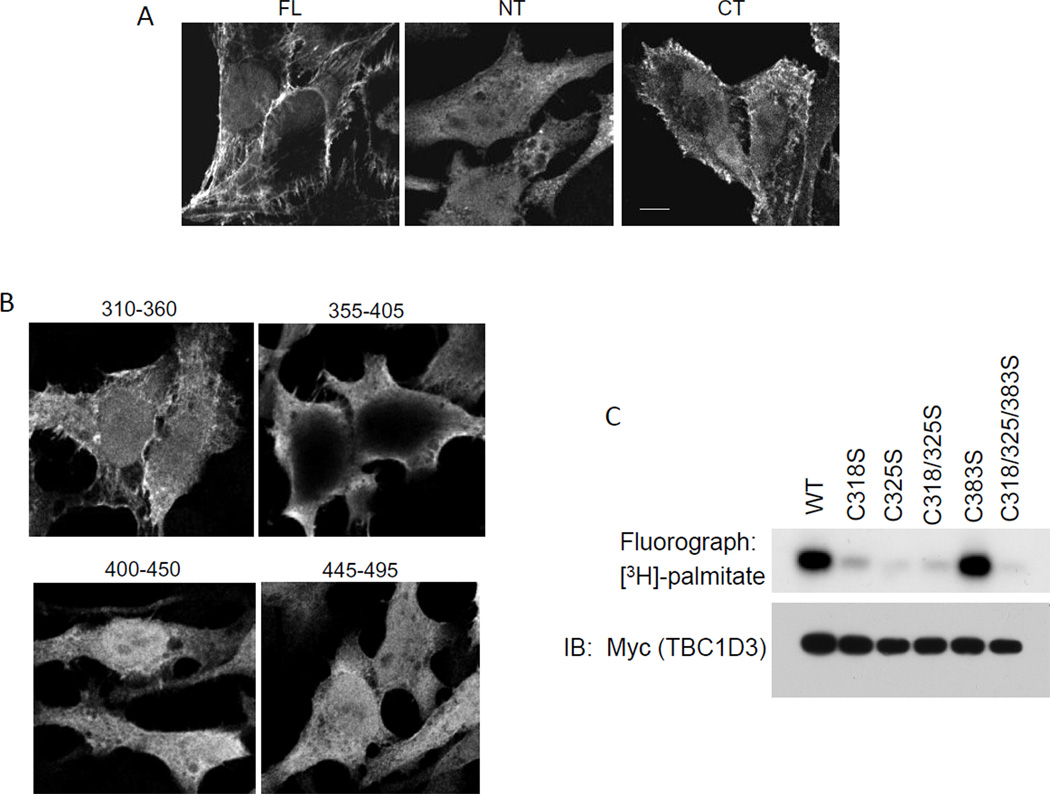
Figure 2.
The C-terminal region of TBC1D3 is necessary but not sufficient for its plasma membrane localization. Confocal images of HeLa cells expressing various Myc-TBC1D3 constructs: TBC1D3:1–549 (full length), TBC1D3:1–313, TBC1D3:313–549 (A) and C-terminal region was further investigated by TBC1D3:310–360, 355–405, 400–450 and 445–495 (B). 24 h after transfection, cells were fixed and stained with monoclonal anti-Myc antibody followed by an Alexa 488-conjugated anti-mouse IgG secondary antibody. Scale bar represents 5µm. (C) HeLa cells were transiently transfected with Myc-TBC1D3 wild-type or various mutants. Cells were metabolically labeled with [3H] palmitate. Cell lysates were prepared and immunoprecipitated with anti-Myc antibody. [3H] palmitate incorporation (upper panel) and immunoblot (IB) with Myc antibody (lower panel) are shown.
TBC1D3 is localized to lipid rafts
Lipid rafts operate as a platforms that recruit and retain a diverse collection of signaling molecules that promote interactions with signal transducing receptors [15]. Proteins lacking trans-membrane domains are targeted to lipid rafts by post-translational modifications including palmitoylation [16]. A series of cell fractionation experiments were carried out to examine TBC1D3 localization in lipid rafts using both a detergent-free and a detergent-based method that yielded similar results [17]. HeLa cells were transfected with Myc-TBC1D3 and at 24 h post-transfection, the cells were lysed and a post-nuclear supernatant (PNS) was prepared. The PNS was loaded on the bottom of a sucrose gradient and after centrifugation, fractions were collected, proteins were resolved by SDS-PAGE and analyzed by Western blot. Figure 1C shows the distribution of markers across the gradient fractions. Flotillin-1, a lipid raft marker [18], and Myc-TBC1D3 both floated in the lipid raft fraction. GAPDH, a soluble protein, did not float and was found at the bottom of the gradient. These results indicated that TBC1D3 is localized to lipid rafts.
TBC1D3 is targeted to the plasma membrane by protein palmitoylation
To identify the regions of TBC1D3 that mediate membrane targeting, we over-expressed various truncated fragments of TBC1D3 into HeLa cells and observed their intracellular localization (Figure 2A). Both the full-length TBC1D3 (FL, 1–549) and a C-terminal fragment (CT, 313–549) localized mainly to the plasma membrane while an N-terminal fragment (NT, 1–313) was diffusely distributed throughout the cytoplasm. The localization of several smaller epitope-tagged truncations of the C-terminal fragment was examined including TBC1D3:310–360, TBC1D3:350–400, TBC1D3:395–450 and TBC1D3:445–500 (Figure 2B). Among these only the TBC1D3:310–360 fragment was preferentially localized to the membrane suggesting that this fragment determines the plasma membrane targeting of TBC1D3.
The sequence of the membrane-targeted TBC1D3:310–360 fragment revealed two cysteine residues at position 318 and 325 as potential palmitoylation sites. Cysteine 383, although outside the targeting fragment, was used as a control. TBC1D3 mutants were constructed in which each of these three cysteine residues was mutated to serine. HeLa cells were transfected with the wild type TBC1D3 or the following mutant constructs - TBC1D3:C318S, TBC1D3:C325S, TBC1D3:C383S, TBC1D3:C318/325S and TBC1D3:C318/325/383S. The cells were radiolabeled with [3H] palmitate and TBC1D3 was immunoprecipitated with anti-Myc monoclonal antibody. Western blots (Figure 2C) showed that wild type TBC1D3 and mutants were all expressed at comparable levels. Fluorography of the filter showed that while wild type TBC1D3 had incorporated [3H] palmitate, the deletion of either cysteine 318 or cysteine 325 or both almost completely abrogated palmitoylation of TBC1D3. Deletion of cysteine 383 did not alter the extent of TBC1D3 palmitoylation (Figure 2C). These findings indicate that cysteine 318 and cysteine 325 within the membrane-targeting fragment of TBC1D3 are palmitoylated, suggesting a mechanism for membrane insertion.
Palmitoylation modulates TBC1D3 degradation
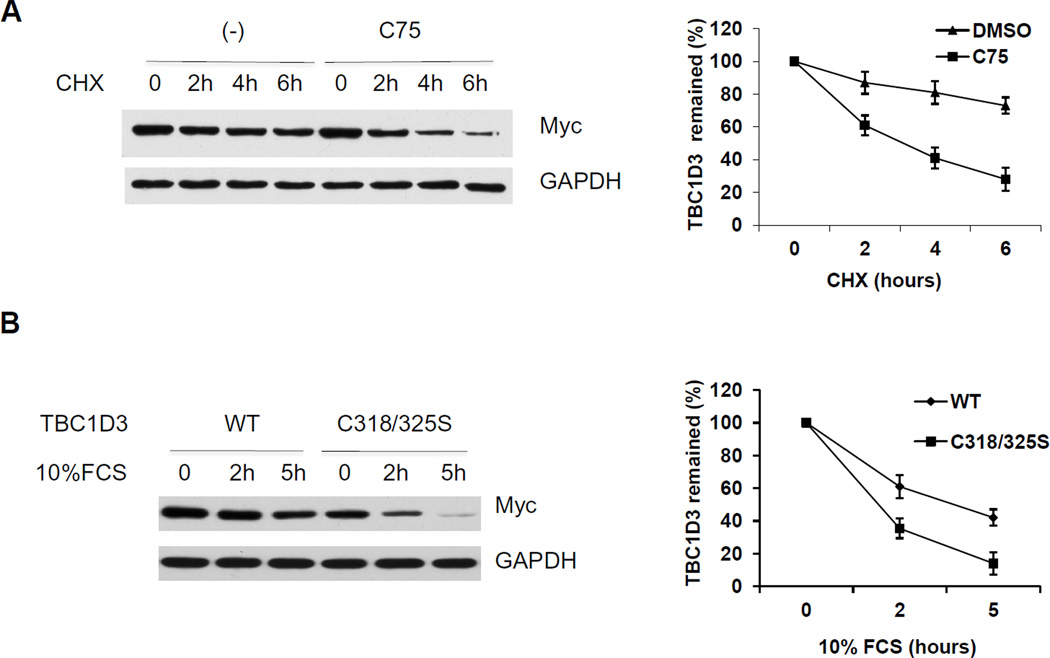
To explore the role of palmitoylation in TBC1D3 turnover, we limited endogenous palmitate synthesis using the FA synthase inhibitor C75 [19]. Palmitoylation is known to be a dynamic process, with palmitate residues removed and replaced over time. Cells were transfected with Myc-TBC1D3 and incubated with or without C75 in presence of cycloheximide (CHX). TBC1D3 protein levels were monitored by immunoblot analysis over a 6 h period. As shown in Figure 3A, TBC1D3 decayed over the course of the experiment; however C75 accelerated the rate of loss of TBC1D3. These results suggested that the palmitate residues on TBC1D3 are continually replenished by palmitoyl-transferase and that their absence enhanced TBC1D3 degradation. To confirm the effect of palmitoylation on growth factor-induced degradation, wild type TBC1D3 and TBC1D3:C318/325S degradation was examined in cells that were starved and treated with 10% FCS. Whole cell lysates were prepared, resolved by SDS-PAGE and analyzed by immunoblotting. Both wild type TBC1D3 and TBC1D3: C318/325S mutants experienced enhanced degradation following FCS stimulation. However, the TBC1D3: C318/325S mutant exhibited a significantly higher rate of TBC1D3 degradation compared with wild type TBC1D3 (Figure 3B) further highlighting the importance of palmitoylation in TBC1D3 degradation.
Figure 3. Palmitoylation influences TBC1D3 degradation.
(A) HeLa cells were transfected with Myc-TBC1D3. At 18 h after transfection, cells were treated with CHX (25µg/ml) with or without the fatty acid synthase inhibitor C75 (50µM). Cell extracts (10µg) were separated by SDS-PAGE followed by immunoblot analysis to monitor the level of TBC1D3. The graph shows the quantification from three experiments. (B) HeLa cells were transfected with wild-type and the palmitoylation-deficient TBC1D3 mutant -TBC1D3:C318/325S. The cells were starved for 4 h and stimulated with 10% FCS for the times indicated. Lysates were separated by SDS-PAGE. TBC1D3 levels were measured by immunoblotting with an anti-Myc antibody. The graph shows the quantification from three experiments.
Palmitoylation of TBC1D3 suppresses CUL7-induced TBC1D3 ubiquitination
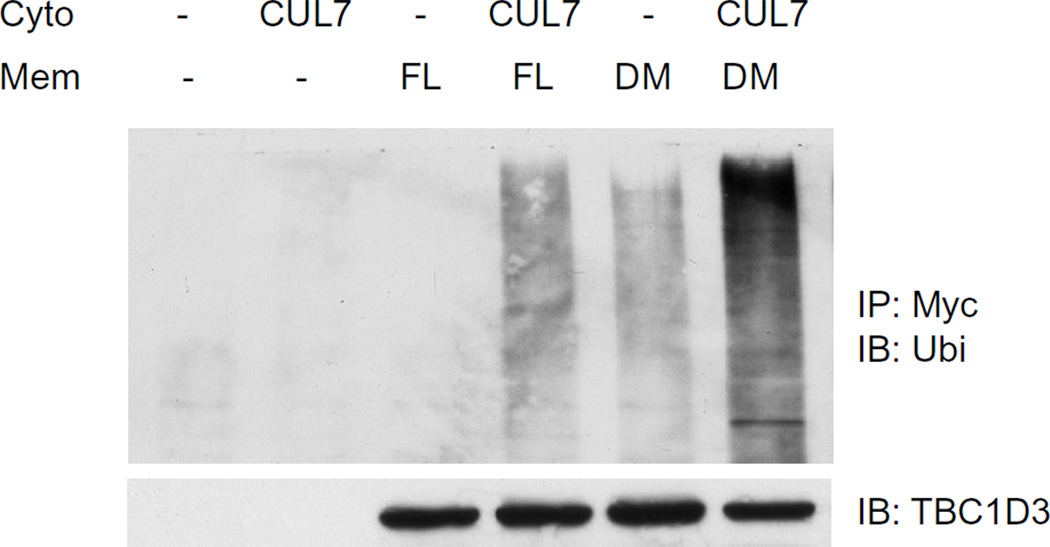
Our previous study has shown that ubiquitination and degradation of TBC1D3 is regulated by CUL7 E3 ligase[11]. To investigate whether palmitoylation of TBC1D3 is also important for CUL7-mediated ubiquitination, a CUL7-enriched cytosolic fraction was prepared from HeLa cells transfected with CUL7. TBC1D3 full length (FL) or palmitoylation-deficient TBC1D3:C318/325S (double mutant or DM) constructs were expressed in a second set of HeLa cells. TritonX-100-extracted membrane fractions from these cells served as a source of TBC1D3 (FL) or TBC1D3 (DM). CUL7-enriched cytosol and TBC1D3-enriched membrane fractions were incubated together with ATP and MgCl2. Polyubiquitination of TBC1D3 and the TBC1D3 double mutant were analyzed, following SDS-PAGE, by immunoblot using anti-ubiquitin antibody. As shown in Figure 4, TBC1D3 ubiquitination was substantially increased compared to the absence of cytosol. However, the palmitoylation-deficient TBC1D3 construct appeared to be a superior substrate for ubiquitination by CUL7-enriched cytosol (Figure 4). Consistent with the data presented in Figure 3, these data strongly suggest that TBC1D3 palmitoylation suppresses TBC1D3 ubiquitination.
Figure 4. Palmitoylation of TBC1D3 regulates CUL7-induced TBC1D3 ubiquitination.
HeLa cells were transfected with HA-CUL7, Myc-TBC1D3 full length (FL) or Myc-TBC1D3:C318/325S (double mutant, DM). Cytosol fractions (cyto) were prepared from CUL7-expressing cells and membrane fractions (mem) were prepared from TBC1D3-expressing cells, respectively. The cytosol and membrane fractions were incubated together at room temperature for 45 min. TBC1D3 was immunoprecipitated with anti-Myc antibody. Polyubiquitination of TBC1D3 was analyzed by immunoblot using anti-ubiquitin antibody. The experiment was repeated three times.
DISCUSSION
TBC1D3 is one of a small number of hominoid-specific genes. Although few in number and poorly understood [20], the human genome encodes scores of human-specific genes. The pool of hominoid-specific genes that are shared between humans and other hominoid species may be larger yet. Perry et al [21] have proposed that recently evolved multi-copied genes may play a role in cell proliferation and cell signaling which is consonant with the concept of more highly evolved signal regulation. TBC1D3 appears to fit into this category although the physiological function remains unclear.
TBC1D3 expression enhances cell proliferation. This observation led several groups to examine the EGF receptor, prototypical for the study of cell proliferation [5,9]. TBC1D3 expression enhances EGFR signaling by sparing the receptor from ubiquitination and degradation [9]. The mechanisms are still unclear but the data point to an effect of TBC1D3 on EGFR phosphorylation and the recruitment of CBL, an E3 ligase known to ubiquitinate EGFR. Fritolli et al [5] examined the effect of TBC1D3 on EGF stimulated pinocytosis and concluded that TBC1D3 enhanced EGFR signaling.
A yeast two-hybrid screen identified the E3 ligase CUL7 as a TBC1D3 interacting protein [11]. CUL7 is a key enzyme in the insulin/IGF1 signaling pathway where it is responsible for the ubiquitination and subsequent degradation of IRS1, an important insulin/IGF1 mediator[22]. Indeed, we showed that TBC1D3 expression led to a selective suppression of the phosphorylation of IRS1 accompanied by delayed ubiquitination and degradation [10]. Key phosphorylation sites on IRS1 that control ubiquitination are regulated by the mTOR-regulated kinase, S6 kinase. In turn, S6 kinase is regulated (i.e., deactivated) by the phosphatase PP2A [23]. Initial results indicate that TBC1D3 interacts with B56γ, one of the regulatory subunits of PP2A [11]. The story is incomplete but the effect of TBC1D3 expression on the phosphorylation/dephosphorylation of key intermediaries in signaling pathways, such as the EGFR and the insulin/IGF1 pathways, emerges as a likely possibility.
We examined the role of CUL7 in TBC1D3 ubiquitination and degradation and observed that TBC1D3 is ubiquitinated directly by CUL7 by interacting with the recruitment arm of CUL7, FBox8. Moreover, TBC1D3 ubiquitination is a process that is enhanced by growth factor stimulation. We proposed that TBC1D3 ubiquitination and degradation completes an activation/deactivation cycle whereby TBC1D3 is activated to assist signaling mechanisms and subsequently ubiquitinated and degraded to reverse or abrogate receptor signaling. The activation/deactivation cycle of TBC1D3 must involve targeting information that allows TBC1D3 to interact with its physiological partners.
Epitope-tagged constructs of TBC1D3 localize to the plasma membrane and gradient sedimentation indicates that TBC1D3 is a lipid raft protein. We examined truncated constructs of TBC1D3 to identify regions associated with the plasma membrane. Inspection of TBC1D3:310–360 revealed two adjacent cysteine residues that might serve as targets for palmitoylation. Adjacent palmitoylation sites are not uncommon [24,25] and indeed, we showed that both intact TBC1D3 and the TBC1D3:310–360 fragment incorporated radiolabeled palmitate which was reversed by deleting two cysteine residues (C318 and C325). Deletion of either C318 or C325 results in a nearly complete loss of palmitate labeling suggesting either that palmitoylation of these two nearby sites is linked (e.g., palmitoylation of one is required for palmitoylation of the other) or that one site is palmitoylated and the other site is a regulatory site. It is noted that palmitoylation of cyteines in proteins often occurs in multiple, closely associated cysteine residues. In addition to palmitoylation, there are other possible post translational modifications, such as tyrosine nitration [26], reported for TBC1D3, that could promote plasma membrane and lipid raft association.
Because TBC1D3 is degraded by proteasomes [11], we speculated that TBC1D3 is likely de-palmitoylated prior to ubiquitination and proteasomal degradation. Indeed, palmitoylation-deficient TBC1D3 mutants were more extensively ubiquitinated by CUL7 and more rapidly degraded in response to a growth factor challenge than intact TBC1D3. It is possible that palmitoylated TBC1D3 is inaccessible to modifying E3 ligases because of its localization in lipid rafts or that palmitoylation modifies the folding of TBC1D3 such that the interaction between TBC1D3 and Fbw8, the recognition arm of CUL7, is impeded, perhaps for steric reasons.
We conclude that TBC1D3 is palmitoylated, targeted to lipid rafts and degraded following ubiquitination by CUL7. These findings may be important in developing an understanding of the pathobiology of TBC1D3, a breast and prostate cancer oncogene that has been linked to a variety of other cancers including myelodysplastic syndrome, as the impact of TBC1D3 expression on cell proliferation becomes more clearly resolved.
Highlights.
Hominoid-specific oncogene TBC1D3 is targeted to plasma membrane by palmitoylation.
TBC1D3 is palmitoylated on two cysteine residues: 318 and 325.
TBC1D3 palmitoylation governs growth factors-induced TBC1D3 degradation.
Post-translational modifications may regulate oncogenic properties of TBC1D3.
Acknowledgments
We very much appreciate the advice on palmitoylation provided by Maurine Linder (Cornell University) and reagents provided by Jim DeCaprio (Harvard Medical School) and Zhen-Qiang Pan (The Mount Sinai Hospital). This work was supported by a grant from the NIH and from the McDonnell Center for Neurobiology at Washington University.
Abbreviations
- GAP
GTPase activating protein
- EGFR
epidermal growth factor receptor
- IR
insulin receptor
- EGF
epidermal growth factor
- IGF-1
insulin-like growth factor1
- FCS
fetal calf serum
- PP2A
protein phosphatase 2A
- IRS-1
insulin receptor substrate1
- Fbw8
F-box and WD repeat domain containing 8
- PBS
phosphate-buffered saline
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Huebner K, Cannizzaro LA, Nakamura T, Hillova J, Mariage-Samson R, Hecht F, Hill M, Croce CM. A rearranged transforming gene, tre, is made up of human sequences derived from chromosome regions 5q, 17q and 18q. Oncogene. 1988;3:449–455. [PubMed] [Google Scholar]
- 2.Pei L, Peng Y, Yang Y, Ling XB, Van Eyndhoven WG, Nguyen KC, Rubin M, Hoey T, Powers S, Li J. PRC17, a novel oncogene encoding a Rab GTPase-activating protein, is amplified in prostate cancer. Cancer Res. 2002;62:5420–5424. [PubMed] [Google Scholar]
- 3.Hatanaka H, Tsukui M, Takada S, Kurashina K, Choi YL, Soda M, Yamashita Y, Haruta H, Hamada T, Ueno T, Tamada K, Hosoya Y, Sata N, Yasuda Y, Nagai H, Sugano K, Mano H. Identification of transforming activity of free fatty acid receptor 2 by retroviral expression screening. Cancer science. 2010;101:54–59. doi: 10.1111/j.1349-7006.2009.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starczynowski DT, Vercauteren S, Telenius A, Sung S, Tohyama K, Brooks-Wilson A, Spinelli JJ, Eaves CJ, Eaves AC, Horsman DE, Lam WL, Karsan A. High-resolution whole genome tiling path array CGH analysis of CD34+ cells from patients with low-risk myelodysplastic syndromes reveals cryptic copy number alterations and predicts overall and leukemia-free survival. Blood. 2008;112:3412–3424. doi: 10.1182/blood-2007-11-122028. [DOI] [PubMed] [Google Scholar]
- 5.Frittoli E, Palamidessi A, Pizzigoni A, Lanzetti L, Garre M, Troglio F, Troilo A, Fukuda M, Di Fiore PP, Scita G, Confalonieri S. The primatespecific protein TBC1D3 is required for optimal macropinocytosis in a novel ARF6-dependent pathway. Mol Biol Cell. 2008;19:1304–1316. doi: 10.1091/mbc.E07-06-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulding CA, Ruvolo M, Haber DA. The Tre2 (USP6) oncogene is a hominoid-specific gene. Proc Natl Acad Sci U S A. 2003;100:2507–2511. doi: 10.1073/pnas.0437015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodzic D, Kong C, Wainszelbaum MJ, Charron AJ, Su X, Stahl PD. TBC1D3, a hominoid oncoprotein, is encoded by a cluster of paralogues located on chromosome 17q12. Genomics. 2006;88:731–736. doi: 10.1016/j.ygeno.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Sudmant PH, Kitzman JO, Antonacci F, Alkan C, Malig M, Tsalenko A, Sampas N, Bruhn L, Shendure J, Eichler EE. Diversity of human copy number variation and multicopy genes. Science. 2010;330:641–646. doi: 10.1126/science.1197005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wainszelbaum MJ, Charron AJ, Kong C, Kirkpatrick DS, Srikanth P, Barbieri MA, Gygi SP, Stahl PD. The hominoid-specific oncogene TBC1D3 activates Ras and modulates epidermal growth factor receptor signaling and trafficking. J Biol Chem. 2008;283:13233–13242. doi: 10.1074/jbc.M800234200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wainszelbaum MJ, Liu J, Kong C, Srikanth P, Samovski D, Su X, Stahl PD. TBC1D3, a Hominoid-Specific Gene, Delays IRS-1 Degradation and Promotes Insulin Signaling by Modulating p70 S6 Kinase Activity. PloS one. 2012;7:e31225. doi: 10.1371/journal.pone.0031225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong C, Samovski D, Srikanth P, Wainszelbaum MJ, Charron AJ, Liu J, Lange JJ, Chen PI, Pan ZQ, Su X, Stahl PD. Ubiquitination and degradation of the hominoid-specific oncoprotein TBC1D3 is mediated by CUL7 E3 ligase. PloS one. 2012;7:e46485. doi: 10.1371/journal.pone.0046485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan J, Roth AF, Bailey AO, Davis NG. Palmitoylated proteins: purification and identification. Nature protocols. 2007;2:1573–1584. doi: 10.1038/nprot.2007.225. [DOI] [PubMed] [Google Scholar]
- 13.Macdonald JL, Pike LJ. A simplified method for the preparation of detergent-free lipid rafts. Journal of lipid research. 2005;46:1061–1067. doi: 10.1194/jlr.D400041-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Persaud-Sawin DA, Lightcap S, Harry GJ. Isolation of rafts from mouse brain tissue by a detergent-free method. Journal of lipid research. 2009;50:759–767. doi: 10.1194/jlr.D800037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levental I, Grzybek M, Simons K. Greasing their way: lipid modifications determine protein association with membrane rafts. Biochemistry. 2010;49:6305–6316. doi: 10.1021/bi100882y. [DOI] [PubMed] [Google Scholar]
- 16.Levental I, Lingwood D, Grzybek M, Coskun U, Simons K. Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22050–22054. doi: 10.1073/pnas.1016184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanson D, Murray PG, O'Sullivan J, Urquhart J, Daly S, Bhaskar SS, Biesecker LG, Skae M, Smith C, Cole T, Kirk J, Chandler K, Kingston H, Donnai D, Clayton PE, Black GC. Exome sequencing identifies CCDC8 mutations in 3-M syndrome, suggesting that CCDC8 contributes in a pathway with CUL7 and OBSL1 to control human growth. Am J Hum Genet. 2011;89:148–153. doi: 10.1016/j.ajhg.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuermer CA. Microdomain-forming proteins and the role of the reggies/flotillins during axon regeneration in zebrafish. Biochimica et biophysica acta. 2011;1812:415–422. doi: 10.1016/j.bbadis.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Wei X, Schneider JG, Shenouda SM, Lee A, Towler DA, Chakravarthy MV, Vita JA, Semenkovich CF. De novo lipogenesis maintains vascular homeostasis through endothelial nitric-oxide synthase (eNOS) palmitoylation. The Journal of biological chemistry. 2011;286:2933–2945. doi: 10.1074/jbc.M110.193037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clamp M, Fry B, Kamal M, Xie X, Cuff J, Lin MF, Kellis M, Lindblad-Toh K, Lander ES. Distinguishing protein-coding and noncoding genes in the human genome. Proc Natl Acad Sci U S A. 2007;104:19428–19433. doi: 10.1073/pnas.0709013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry GH, Yang F, Marques-Bonet T, Murphy C, Fitzgerald T, Lee AS, Hyland C, Stone AC, Hurles ME, Tyler-Smith C, Eichler EE, Carter NP, Lee C, Redon R. Copy number variation and evolution in humans and chimpanzees. Genome Res. 2008;18:1698–1710. doi: 10.1101/gr.082016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X, Sarikas A, Dias-Santagata DC, Dolios G, Lafontant PJ, Tsai SC, Zhu W, Nakajima H, Nakajima HO, Field LJ, Wang R, Pan ZQ. The CUL7 E3 ubiquitin ligase targets insulin receptor substrate 1 for ubiquitindependent degradation. Mol Cell. 2008;30:403–414. doi: 10.1016/j.molcel.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn K, Miranda M, Francis VA, Vendrell J, Zorzano A, Teleman AA. PP2A regulatory subunit PP2A-B' counteracts S6K phosphorylation. Cell Metab. 2010;11:438–444. doi: 10.1016/j.cmet.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Robinson LJ, Michel T. Mutagenesis of palmitoylation sites in endothelial nitric oxide synthase identifies a novel motif for dual acylation and subcellular targeting. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:11776–11780. doi: 10.1073/pnas.92.25.11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi T, Thomas GM, Huganir RL. Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron. 2009;64:213–226. doi: 10.1016/j.neuron.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia M, Mateoiu C, Souchelnytskyi S. Protein tyrosine nitration in the cell cycle. Biochemical and biophysical research communications. 2011;413:270–276. doi: 10.1016/j.bbrc.2011.08.084. [DOI] [PubMed] [Google Scholar]