Abstract
Background
Preterm birth impairs the infant’s stress response due to interruption of autonomic nervous system (ANS) development. Preterm infants demonstrate a prolonged and aberrant sympathetic response to stressors. ANS development may be promoted by massage therapy (MT), which has been shown to improve stress response in preterm infants.
Aims
To compare preterm infant ANS function and stress response during sleep and caregiving epochs, as measured by heart rate variability (HRV), after two weeks of twice-daily MT.
Study Design
A subset of participants from a larger randomized, masked, controlled trial was used.
Subjects
Twenty-one infants (8 males, 13 females) from a larger study of 37 medically stable preterm infants were studied. Infants were receiving full volume enteral feedings with a mean post-menstrual age of 31.4 (MT) and 30.9 (Control) weeks.
Outcome Measures
Low to High frequency (LF:HF) ratio of HRV was the outcome of interest.
Results
There was a significant group x time x sex interaction effect (p <.05). Male Control infants demonstrated a significant decline in LF:HF ratio from baseline to the second caregiving epoch, suggesting decreased mobilization of sympathetic nervous system response when exposed to stressors. Male MT infants demonstrated increased LF:HF ratio during caregiving and decreased LF:HF ratio during sleep epochs, suggesting improved ANS function, although this was not statistically significant. LF:HF ratio was similar in female MT and female Control infants during caregiving and sleep.
Conclusions
Control males had decreased HRV compared to MT males. There was no difference in HRV between MT and Control females.
1. Introduction
Preterm infants in the newborn intensive care unit (NICU) experience chronic exposure to stressors such as mechanical ventilation, medical procedures, care giving activities, and maternal separation. Chronic exposure to stressors while in the NICU is linked to altered regional brain structure and function as well as abnormal motor behavior.1 The stress response and recovery are controlled by the autonomic nervous system (ANS) and ANS development is interrupted by preterm birth. This, in turn, impairs the preterm infant’s stress response and recovery. Interventions are needed to enhance maturation of the ANS, which will enhance appropriate responses to stressors and effective recovery when the stressors are removed in preterm infants while in the NICU.
Massage therapy (MT) is advocated for stress attenuation in preterm infants (<37 weeks post-menstrual age PMA) and incorporates compression and stroking of the soft tissues with kinesthetic movements of the extremities.2–4 Massage therapy improves vagal tone and gastric motility in preterm infants.2,3,5,6 Vagal tone, a measure of ANS function, is quantified by heart rate variability.7 Heart rate variability increased weekly, following 4-weeks of MT in our study of medically stable, preterm infants suggesting an improved ability to manage NICU related stressors.4 We also found sex modified the effect of MT on HRV; male MT infants demonstrated increased HRV from baseline to week 4 compared to females.4 The carry-over effect immediately following MT on HRV during consecutive periods of caregiving and sleep is unknown and has not been previously reported. Should an immediate carry-over effect of MT on HRV exist, preterm infants may develop improved resiliency to stressful events in the NICU.
The purpose of this study was to test the immediate carry-over effect of MT on ANS function as measured by HRV in medically stable preterm infants mid-way through the 4-week study. The specific aim was to compare preterm infants’ ANS function and stress response as measured by HRV during sleep and caregiving epochs immediately after MT at the conclusion of Week 2 of the 4-week MT study. We hypothesized that immediately following MT preterm infants would have improved ANS function, as demonstrated by increased HRV. Four consecutive periods immediately following MT were partitioned for analysis, which included two caregiving and two sleep epochs.
Heart rate variability (HRV) consists of specific defined measureable indicators of ANS activity during short-term events.8 Use of spectral density analysis breaks down components of HRV into regions specific to sympathetic (low-frequency region) and parasympathetic (high-frequency region) branches of the ANS. The amount of variance or power within each of these regions is not fixed; rather the variance in each region changes as the sympathetic and parasympathetic activity changes.8 Adults demonstrate higher values in the low-frequency (LF) region during wake periods and higher values in the high-frequency (HF) region during sleep.8 This finding in adults demonstrates that the sympathetic branch predominates during wakeful periods, where resiliency to daily stressful events is warranted, and a predominance of the parasympathetic branch during sleep, when restoration of systems occurs.
Unlike adults, preterm infants have an immature ANS, demonstrated by a significantly higher variance in the LF region and a lower variance in the HF region of HRV compared to term infants.9–11 Many investigators found that the variance in the HF region increased in term and preterm infants between 31 and 35 weeks gestational age, but none reported significant differences by sex.11–14 The higher variance in the LF region and diminished variance in the HF region predisposes the preterm infant to excessive sympathetic response to even the most minor care activities such as a diaper change. The stress response is meant to be limited and of short duration, yet preterm infants with their prolonged dysfunctional ANS are unable to mitigate the stress response when the stressor is removed.9,10
The use of LF and HF values to identify sympathetic and parasympathetic activity is one method to evaluate HRV; however, because the LF region is influenced somewhat by parasympathetic activity, it is not a pure measure of sympathetic activity.8,10,13 The HF region is specific to vagal tone, thus it is an appropriate measure of parasympathetic activity. Because of the parasympathetic influences on the LF region, the LF:HF ratio is used as the ANS balance measure.10,13 A higher LF:HF ratio indicates increased sympathetic activity and a lower LF:HF ratio indicates increased parasympathetic activity.8
2. Method
The data for this study of 21 infants were collected as part of a larger randomized, masked, controlled trial (N = 37).4 The larger trial was a longitudinal 4-week trial. We collected HRV data for this study at the conclusion of study Week 2, immediately after MT for 8 contiguous hours.
2.1. Participants
Medically stable preterm infants were eligible if they were: (1) born between 28 4/7 weeks and 32 3/7 weeks post menstrual age, (2) appropriately grown for gestation, and (3) receiving enteral feedings of 100 mL/kg/day at day of life 14. Infants were ineligible if they had congenital anomalies, complex cardiac disease, birth injury, hypothyroidism, inborn errors of metabolism or intraventricular hemorrhage > grade 2.4 After parents gave informed consent, infants were randomly assigned to MT or Control by random draw of group membership from an envelope. Infants entered the study between day of life 10 and 14; they had a mean gestational age of 31.4 (+/−0.8) weeks (MT) and 30.9 (+/−0.7) weeks (Control). At the end of study Week 2, mean gestational age of MT infants was 33.4 weeks and of Control infants was 32.9 weeks. Eight hours of HRV data on 8 males (4 MT, 4 Control) and 13 females (6 MT, 7 Control) at the end of study Week 2 were analyzed. Mean gestational age of infants at the end of study Week 2 were 31
2.2 Measures
Data were acquired from medical records, Mortara H12+™ Holter monitors (Mortara Instrument, Milwaukee, WI), and infant observation. Demographic data including anthropometric measures, diagnoses, feeding type, and gestational age were obtained via medical record review.
2.3. HRV
Electrocardiograph (ECG) data collection began prior to MT or Control at the end of study Week 2 and continued such that successive epochs of sleep and caregiving post MT or Control were captured. The ECG data acquisition continued for 8 hours so that two sleep epochs and two caregiving epochs were captured from each infant. Mortara H12+ Holter monitors (Mortara Instrument, Milwaukee, WI) were calibrated prior to data acquisition and acquired ECG data at a sampling rate of 1000 Hz. HRV measures, i.e., the variance in the HF and LF regions, were extracted from the ECG data.
2.4. Caregiving and Sleep
A trained research assistant observed and recorded caregiving and infant sleep data using an investigator developed paper and pencil form during the 8 hours of HRV data collection. The research assistant was monitored by the project coordinator every 4th infant to ensure data collection fidelity and comparability. The research assistant sat by the infant’s bedside observing and documenting the initiation and completion of caregiving and periods of sleep. Caregiving was documented by type and included vital signs, diaper change, gavage tube feeding, and infant position change by caregiver. Sleep was defined as eyes closed, regular or irregular breathing, no movement or startles and small limb movements, and rapid eye movement or no rapid eye movement.15 Due to the prolonged dysfunction of ANS in preterm infants as reported by Patural and Longin, differentiation between sleep states was considered unnecessary in this exploratory study; therefore, differentiation between quiet sleep and active sleep as described by Holditch-Davis was not recorded.10,13,15 During sleep, infants were in the incubator, undisturbed and no caregiver or parent intervention occurred.
2.5. Procedures
After approval from the Institutional Review Board, mothers of medically stable preterm infants born between 28 4/7 weeks and 32 3/7 weeks gestation at two Level IIIA NICUs in the Intermountain West were informed about the nature of the study. After informed consent was obtained, infant medical records were reviewed and the project coordinator collected demographic data.
Licensed massage therapists delivered MT, a 20-minute intervention consisting of moderate pressure and stroking of the soft tissues followed by kinesthetic movement of the extremities as described in our previous studies.4,16,17 The Control condition consisted of the licensed massage therapist standing at the infant’s incubator for 20 minutes without manipulating the infant. Infants received MT or Control twice daily Monday through Saturday for four consecutive weeks. At the end of study Week 2, during the 8-hours of ECG data collection, the research assistant, seated at the infant’s incubator with direct visualization of the infant observed, recorded, and time-stamped the beginning and end of caregiver activities and of infant sleep. MT and Control were delivered behind a privacy screen to maintain masking of assignment from parents and health care providers. Nine massage therapists were trained in MT and Control with treatment fidelity ensured by random observations of MT or Control by the lead massage therapist (SH) every 20 treatments.4
2.6. Statistical Analysis
Demographic characteristics of MT and Control infants were compared using independent samples t-tests for continuous variables and chi-square for nominal and ordinal variables.
ECG data from with HRV measures were analyzed was a multi-step process as follows. HRV files were partitioned into caregiving and sleep epochs according to the time stamp recorded by the research assistant. Five consecutive epochs were partitioned for HRV analysis: baseline (pre-MT or Control), caregiving epoch 1, sleep epoch 1, caregiving epoch 2, and sleep epoch 2. ECG analogue data were digitized so that each r-wave was tagged for HRV frequency domain analysis. Power spectral density analysis was used to determine the variances within each frequency region of HRV (LF and HF) with SPLUS 7.0 for Windows (TIBCO Software, Palo Alto, CA, USA) for each epoch. The LF and HF ranges were based upon the work of Chatow et al.18 The LF:HF ratio for each epoch was entered into data files for subsequent analysis with SPSS® version 20.0 (IBM SPSS, Armonk, NY, USA).
The SPSS® generalized estimating equations (GEE) procedure was used to test for a treatment effect, a treatment x time interaction effect, and a treatment x time x sex interaction effect on LF:HF ratio. Generalized estimating equations (GEE) procedure is specifically used for within-subjects designs and statistically controls for dependent data collected at multiple time points and differences in group size.19,20 The Wald X2 statistic is used to test the value of the parameter of interest against the chi square distribution. The LF:HF variable was positively skewed, thus the Gamma distribution was used to achieve goodness of fit.
3. Results
3.1. Description of the Sample
Infants were medically stable and receiving full enteral feedings of human milk or preterm formula via nasogastric tube. MT and Control infants were similar on postmenstrual age, ethnicity, sex, and birth weight (Table 1). Three Control and 4 MT infants received caffeine for apnea of prematurity. Given the potential stimulatory effect of caffeine on LF:HF ratio, caffeine use was included as a covariate in the statistical analysis; however, caffeine had no statistically significant effect on LF:HF ratio.
Table 1.
Demographic characteristics of infants in the massage therapy study
| Characteristic | MT
|
Control*
|
|---|---|---|
| n (%) | n (%) | |
| Sex | ||
| Female | 6 (46) | 7 (54) |
| Male | 4 (50) | 4 (50) |
| Ethnicity | ||
| Hispanic | 6 (40) | 4 (60) |
| White non-Hispanic | 4 (36) | 7 (64) |
|
| ||
| Group Mean (SD) | Group Mean (SD) | |
|
| ||
| Postmenstrual Age (weeks) | 31.4 (0.8) | 30.9 (0.7) |
| Birth Weight (g) | 1588.7 (149.4) | 1553.2 (332.8) |
Note: MT = massage therapy.
no significant differences between groups on demographic variables by t-test for continuous variables and chi square for nominal variables.
3.2 Heart Rate Variability
Significant interaction effects of group x time (Wald X2 38.52, df 4, p <.001) and group x time x sex (Wald X2 46.46, df 8, p <.001) on LF:HF ratio were found. The changes in LF:HF ratio were sex-specific. Female MT and Control infants demonstrated similar LF:HF ratio values between groups and over time (Figure 1). The LF:HF ratio was similar during caregiving epochs and sleep epochs in female MT and Control infants.
Figure 1.
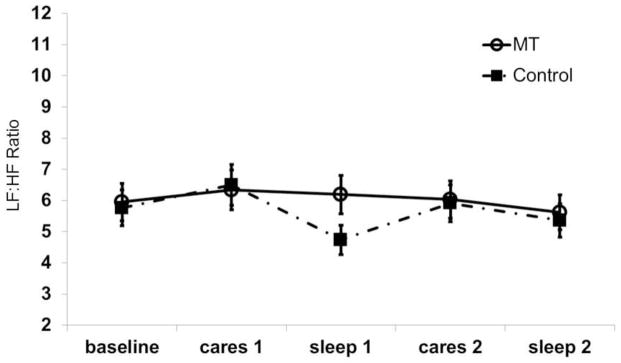
LF:HF ratio during successive epochs of nursing care activity and sleep in female infants receiving massage therapy (MT) or Control. No significant differences were found over time or between groups.
In contrast, Male Control infants had a significantly higher LF:HF ratio at baseline compared to male MT infants (Figure 2). Male Control infants exhibited a significant decline in LF:HF ratio from baseline to the second caregiving epoch, suggesting decreased HRV and a diminished sympathetic response to caregiving events. During the second sleep epoch, male Control infants had an increase in LF:HF ratio, suggesting increased sympathetic activity during a period when parasympathetic activity should increase. Male MT infants demonstrated increased LF:HF ratio during caregiving epochs with a decrease in LF:HF ratio during sleep epochs (Figure 2). Although these findings were not significant, likely due to the small sample size, the increase in LF:HF ratio during caregiving is an expected sympathetic response to stressors. During sleep, the LF:HF ratio decreased, suggesting increased parasympathetic activity.
Figure 2.
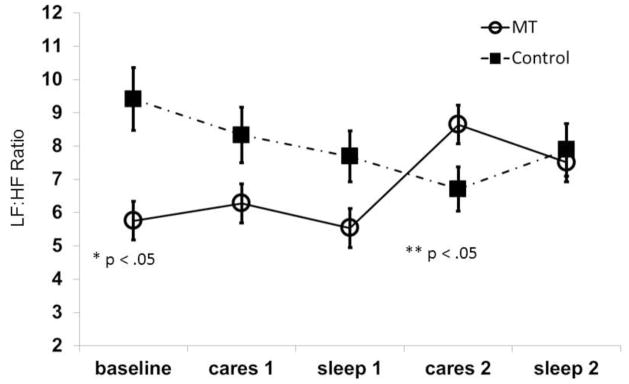
LF:HF ratio during successive epochs of nursing care activity and sleep in male infants receiving massage therapy (MT) or Control. *LF:HF ratio significantly higher at baseline in the control group. **LF:HF ratio significantly decreased from baseline to the second caregiving epoch (cares 2) in Controls.
4. Discussion
We demonstrated an immediate carry-over effect of MT on HRV in preterm male infants. The LF:HF ratio increased during periods of caregiving, suggesting an increase in sympathetic activity during a physiologically demanding time period. During sleep, a period of rest and restoration when the parasympathetic branch of the ANS predominates, the LF:HF ratio decreased in the male MT infants. These findings suggest that preterm male infants have a differential response to MT compared to preterm females. The decrease in LF:HF ratio during sleep in our infants is similar to that seen in term infants.21 Thus, MT may promote maturation of the parasympathetic branch of the ANS in preterm male infants. Notably, the male MT infants had a significantly lower LF:HF ratio at baseline. This lower baseline measure is likely the effect of receiving two weeks of MT as part of the larger randomized trial. In the randomized trial, we reported improved LF:HF ratio in male infants after 4 weeks of MT; however, from these findings, this effect is likely evident at two weeks.4 To our knowledge, we are the first to show this type of sex-specific carry-over effect on the relationship between MT and LF:HF ratio in a group of preterm infants during contiguous caregiving and sleep epochs. This sustained effect of MT on increased HRV and improved vagal tone may promote improved stress response and recovery, thus diminishing the effects of prolonged stressor exposure preterm infants.
This sex difference suggests that males may be less resilient to stressors and require a prolonged time to recover compared to females. Although no significant differences were found between female MT and female Control infants, the pattern of change in LF:HF ratio during caregiving and during sleep was similar to the pattern seen in male MT infants, with the LF:HF ratio increasing during caregiving and decreasing during sleep. This finding provides evidence of increased sympathetic activity during exposure to stressors and increased parasympathetic activity during sleep when restoration occurs. Male infants appeared more sensitive to caregiving as a stressor with increased LF:HF ratio, suggesting sympathetic activation which may be related to higher testosterone levels as reported by Cho et al.22 Sex differences in the response to stressful events were reported in rat pups. Male rat pups exposed to stress developed visceral adiposity more than female pups, suggesting increased sympathetic activation of the hypothalamic-pituitary-adrenal axis and excessive cortisol release.23 Although we did not measure cortisol, our findings indicate that MT may promote development of the ANS, specifically the parasympathetic branch, in male preterm infants and improve their ability to respond to and recover from stressful events during their NICU hospitalization.
There are limitations to this study. Our sample size is too small to be adequately powered; however, the findings are consistent with the findings in our larger trial, which was sufficiently powered.4 One trained observer carried out the observations for interventions and sleep periods. Inter-observer reliability was not determined as we were only identifying when caregiving events were occurring and when infants were sleeping without any interventions from parents or health care providers. We also had nine LMTs delivering the intervention, which may be a large number of therapists for this sample. Increasing random observations by the lead LMT to every 5 treatments would have enhanced treatment fidelity.
We did not differentiate between types of sleep. Infant behavioral state evaluation using a standard tool as described by others may have strengthened this study; however, the purpose of our study was to evaluate HRV during caregiving and during sleep, regardless of sleep state. In addition, HRV during active vs. quiet sleep states did not differ in prior research.24
5. Conclusion
Preterm infants are exposed to numerous stressors in the neonatal intensive care unit, leading to increased sympathetic responses to caregiving activities. We hypothesized that MT would improve the stress response demonstrating increase HRV during caregiving and sleep immediately following MT. At the end of study Week 2, male MT infants demonstrated increased HRV during caregiving and sleep immediately after MT, although this was not significant, whereas, control males demonstrated significantly decreased HRV. Females did not demonstrate a significant change in HRV. These findings suggest that MT has a positive effect on ANS function in male preterm infants and that ANS function in female infants is not affected by MT. MT interventions may need to be tailored based upon sex of infants. Future research is needed to delineate the mechanisms by which MT affects ANS development, identify epigenetic factors that may influence the stress response, and determine epigenetic areas that may be altered by MT.
Acknowledgments
Supported by Grants from: the National Institutes of Health (NCCAM R21 AT004185-01, Moyer-Mileur, PI); and University of Utah Interdisciplinary Research Committee, College of Nursing Research Committee, and Division of Neonatology Development Fund. Haley S. was funded with an NIH-F32 (1F32AT005568-01)
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest and the study sponsors had no role in the study design, data collection, analysis, interpretation of the data, and the writing of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith GC, Gutovich J, Smyser C, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 2011 Oct;70(4):541–549. doi: 10.1002/ana.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diego MA, Field T, Hernandez-Reif M. Vagal activity, gastric motility, and weight gain in massaged preterm neonates. J Pediatr. 2005 Jul;147(1):50–55. doi: 10.1016/j.jpeds.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Field T, Diego MA, Hernandez-Reif M, Deeds O, Figuereido B. Moderate versus light pressure massage therapy leads to greater weight gain in preterm infants. Infant Behav Dev. 2006 Dec;29(4):574–578. doi: 10.1016/j.infbeh.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith SL, Lux R, Haley S, Slater H, Beechy J, Moyer-Mileur LJ. The effect of massage on heart rate variability in preterm infants. J Perinatol. 2012 Apr 26; doi: 10.1038/jp.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diego MA, Field T, Hernandez-Reif M, Deeds O, Ascencio A, Begert G. Preterm infant massage elicits consistent increases in vagal activity and gastric motility that are associated with greater weight gain. Acta Paediatr. 2007 Nov;96(11):1588–1591. doi: 10.1111/j.1651-2227.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- 6.Field T, Scafidi F, Schanberg S. Massage of preterm newborns to improve growth and development. Pediatr Nurs. 1987;13:385–387. [Google Scholar]
- 7.De Rogalski-Landrot I, Roche F, Pichot V, et al. Autonomic nervous system activity in premature and full-term infants from theoretical term to 7 years. Auton Neurosci. 2007 Oct 30;136(1–2):105–109. doi: 10.1016/j.autneu.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996 Mar;17(3):354–381. [PubMed] [Google Scholar]
- 9.Patural H, Barthelemy JC, Pichot V, et al. Birth prematurity determines prolonged autonomic nervous system immaturity. Clin Auton Res. 2004 Dec;14(6):391–395. doi: 10.1007/s10286-004-0216-9. [DOI] [PubMed] [Google Scholar]
- 10.Patural H, Pichot V, Jaziri F, et al. Autonomic cardiac control of very preterm newborns: a prolonged dysfunction. Early Hum Dev. 2008 Oct;84(10):681–687. doi: 10.1016/j.earlhumdev.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Clairambault J, Curzi-Dascalova L, Kauffmann F, Medigue C, Leffler C. Heart rate variability in normal sleeping full-term and preterm neonates. Early Hum Dev. 1992 Feb;28(2):169–183. doi: 10.1016/0378-3782(92)90111-s. [DOI] [PubMed] [Google Scholar]
- 12.Krueger C, van Oostrom JH, Shuster J. A longitudinal description of heart rate variability in 28--34-week-old preterm infants. Biological research for nursing. 2010 Jan;11(3):261–268. doi: 10.1177/1099800409341175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longin E, Gerstner T, Schaible T, Lenz T, Konig S. Maturation of the autonomic nervous system: differences in heart rate variability in premature vs. term infants. J Perinat Med. 2006;34(4):303–308. doi: 10.1515/JPM.2006.058. [DOI] [PubMed] [Google Scholar]
- 14.Longin E, Schaible T, Lenz T, Konig S. Short term heart rate variability in healthy neonates: normative data and physiological observations. Early Hum Dev. 2005 Aug;81(8):663–671. doi: 10.1016/j.earlhumdev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Holditch-Davis D, Scher M, Schwartz T, Hudson-Barr D. Sleeping and waking state development in preterm infants. Early Hum Dev. 2004 Oct;80(1):43–64. doi: 10.1016/j.earlhumdev.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Moyer-Mileur L, Luetkemeier M, Boomer L, Chan GM. Effect of physical activity on bone mineralization in premature infants. J Pediatr. 1995 Oct;127(4):620–625. doi: 10.1016/s0022-3476(95)70127-3. [DOI] [PubMed] [Google Scholar]
- 17.Moyer-Mileur LJ, Brunstetter V, McNaught TP, Gill G, Chan GM. Daily physical activity program increases bone mineralization and growth in preterm very low birth weight infants. Pediatrics. 2000 Nov;106(5):1088–1092. doi: 10.1542/peds.106.5.1088. [DOI] [PubMed] [Google Scholar]
- 18.Chatow U, Davidson S, Reichman BL, Akselrod S. Development and maturation of the autonomic nervous system in premature and full-term infants using spectral analysis of heart rate fluctuations. Pediatr Res. 1995 Mar;37(3):294–302. doi: 10.1203/00006450-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Ghisletta P, Spini D. An introduction to generalized estimating equations and an application to assess selectivity effects in a longitudinal study on very old individuals. J Educ Behav Stat. 2004;29(4):421–437. [Google Scholar]
- 20.Moyer-Mileur LJ, Haley S, Slater H, Beachy J, Smith SL. Massage improves growth quality by decreasing body fat deposition in male preterm infants. J Pediatr. 2012 doi: 10.1016/j.jpeds.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle OM, Korotchikova I, Lightbody G, Marnane W, Kerins D, Boylan GB. Heart rate variability during sleep in healthy term newborns in the early postnatal period. Physiological measurement. 2009 Aug;30(8):847–860. doi: 10.1088/0967-3334/30/8/009. [DOI] [PubMed] [Google Scholar]
- 22.Cho JI, Carlo WA, Su X, McCormick KL. Associations between salivary testosterone and cortisol levels and neonatal health and growth outcomes. Early Hum Dev. 2012 Oct;88(10):789–795. doi: 10.1016/j.earlhumdev.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moyer-Mileur LJ, Haley S, Gulliver K, et al. Mechanical-tactile stimulation (MTS) during neonatal stress prevents hyperinsulinemia despite stress-induced adiposity in weanling rat pups. Early Hum Dev. 2011 Mar;87(3):159–163. doi: 10.1016/j.earlhumdev.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yiallourou SR, Sands SA, Walker AM, Horne RS. Maturation of heart rate and blood pressure variability during sleep in term-born infants. Sleep. 2012 Feb;35(2):177–186. doi: 10.5665/sleep.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]


