Abstract
In this pilot study, we hypothesize that dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) has the potential to evaluate differences in atherosclerosis profiles in patients subjected to high (initial dust cloud) and low (after 13 September 2001) particulate matter (PM) exposure. Exposure to PM may be associated with adverse health effects leading to increased morbidity. Law enforcement workers were exposed to high levels of particulate pollution after working at “Ground Zero” and may exhibit accelerated atherosclerosis. 31 subjects (28 male) with high (n = 19) or low (n = 12) exposure to PM underwent DCE-MRI. Demographics (age, gender, family history, hypertension, diabetes, BMI, and smoking status), biomarkers (lipid profiles, hs-CRP, BP) and ankle-brachial index (ABI) measures (left and right) were obtained from all subjects. Differences between the high and low exposures were compared using independent samples t test. Using linear forward stepwise regression with information criteria model, independent predictors of increased area under curve (AUC) from DCE-MRI were determined using all variables as input. Confidence interval of 95 % was used and variables with p > 0.1 were eliminated. p < 0.05 was considered significant. Subjects with high exposure (HE) had significantly higher DCE-MRI AUC uptake (increased neovascularization) compared to subjects with lower exposure (LE). (AUC: 2.65 ± 0.63 HE vs. 1.88 ± 0.69 LE, p = 0.016). Except for right leg ABI, none of the other parameters were significantly different between the two groups. Regression model indicated that only HE to PM, CRP > 3.0 and total cholesterol were independently associated with increased neovascularization (in decreasing order of importance, all p < 0.026). HE to PM may increase plaque neovascularization, and thereby potentially indicate worsening atherogenic profile of “Ground Zero” workers.
Keywords: DCE-MRI, Particulate matter, Atherosclerosis, Plaque neovascularization
Introduction
Research has demonstrated a relationship between exposure to ambient air pollutants and cardiovascular disease (CVD) [1]. Studies in cohorts suggest links between pollution exposure and cardiovascular mortality, coronary heart disease events [2], and stroke [3]. Many studies have shown associations between short-term exposures to increased levels of air pollutants and CVD events [4]. This association is further increased in populations with risk factors for cardiovascular disease such as high cholesterol levels, diabetes mellitus, hypertension, and heart failure [5]. The biologic mechanisms for these associations are not entirely clear but are thought to be related to systemic inflammation, autonomic nervous system imbalance, changes in vascular compliance, altered cardiac structure, and development of atherosclerosis [6]. As CVD remains one of the leading causes of mortality and morbidity in the industrialized world, it is important to examine its relationship with exposure to air pollution.
Airborne particulate matter consists of particles that are of different sizes. Particles of sizes down to 0.5 μM demonstrate that they can penetrate the most distal airway units and potentially the systemic circulation and cause a variety of pulmonary effects [7]. Particles less than 2.5 μm (PM2.5) have been more strongly linked with cardiovascular disease [8]. Different components of PM may affect the risk for cardiovascular disease by different mechanisms including electrophysiologic changes, inflammation, coagulation, endothelial cell function effects and increased atherogenesis. It is speculated that changes in atherosclerotic burden are due to changes in endothelial function and consequent inflammatory response brought about by exposure to PM. The endothelium is one of the major regulators of vascular homeostasis, and exerts a number of vasoprotective effects. Thus, measuring endothelial dysfunction and its subsequent consequences such as inflammation and plaque angiogenesis/neovascularization would be of critical clinical importance.
Plaque angiogenesis may therefore be an attractive target to identify asymptomatic high-risk lesions or evaluate propensity for atherosclerotic disease progression. Dynamic contrast enhanced (DCE) MRI has been successfully used as a non-invasive tool to study the extent of plaque neovascularization in animals and patients with atherosclerosis [9–12]. The reproducibility and reliability of DCE-MRI has also been previously shown in several studies [10]. It has also been recently used as an evaluation metric in multi-center clinical trials with imaging that use as an endpoint [13].
An estimated 60,000 men and women worked at “Ground Zero” and Staten Island Landfill (wreckage depository site) after 11 September 2001 and were exposed to thousands of tons of fine particulate matter, cement dust, glass fibers, asbestos, lead, polychlorinated biphenyls (PCBs), and other pollutants [14, 15]. This cohort presents a unique opportunity to examine the effect of an acute particulate matter exposure event on the atherosclerosis cascade, which in turn may offer some insight into cardiovascular prognosis of individuals exposed to PM.
The non-invasive in vivo evaluation of atherosclerotic disease therefore presents an ideal mechanism to evaluate the effect of particulate matter exposure on the atherosclerosis cascade that is primarily responsible for cardiovascular events. It also presents itself as a well-validated and accurate tool for examining subclinical effects of cardiovascular disease. In this small pilot study, we plan to use DCE-MRI to evaluate the relationship between levels of particulate matter exposure after 11 September 2001 and atherosclerotic disease progression as evaluated by changes in plaque neovascularization.
Methods
The institutional review board approved this study and all subjects provided informed consent prior to participating in this study.
Study population
Subjects were recruited from the Law Enforcement Cardiovascular Screening Program (LECS) subset of the World Trade Center Medical Monitoring and Treatment Program. 31 law enforcement personnel (28 male) with high (n = 19) or low (n = 12) exposure were recruited. Exposure was classified as early, high-intensity exposure if the individual arrived during the morning of 11 September 2001 (Day 1) and was present during the collapse of North or South tower and the initial dust cloud; and as low-intensity exposure if they arrived on or after 13 September 2001 (Day 3) when PM was present but reduced.
Demographics (age, gender, family history, hypertension, diabetes, BMI, and smoking status), biomarkers (lipid profiles, hs-CRP, blood pressure) and ankle-brachial index (ABI) measures (left and right) were obtained from all subjects.
Multi contrast MRI
The bilateral carotid arteries of subjects were imaged on a 3T whole body MRI systems (Philips Achieva, The Netherlands). An 8-channel phased array coil (Philips Medical Systems, Shanghai, China) was used for carotid imaging.
Acquisition
After localization with fast gradient echo sequences, multi-contrast MR images were obtained using double inversion recovery (DIR) turbo spin echo (TSE) technique. Carotid images were acquired without cardiac triggering and free breathing. Proton density (PDW), T1 and T2 weighted images were acquired [16, 17]. A total of 6 transverse images starting and extending below the carotid bifurcation were obtained. Imaging parameters were as follows: repetition time, 2,000/2,000/1,000 ms (PDW/T2/T1 images); echo time 10/40/10 ms (PDW/T2/T1 images); field of view 14 cm; slice thickness 3 mm; 10 % inter-slice gap; Pixel size 0.5 mm × 0.5 mm; no phase wrap; number of signal averages 2/4/3 (PDW/T2/T1 images); turbo factor (echo train length), 11/11/7 (PDW/T2/T1 images); no zero filling. Inversion time, 157 ms. SPAIR was used for fat suppression [16, 17]. Images were obtained without cardiac or respiratory gating.
Image analysis
Inner and outer wall boundaries were manually traced by a trained observer (S.K.W.) on T2W images. A software program (Vessel Mass, Leiden, The Netherlands) was then used to determine the lumen area, wall area, total vessel area, normalized wall index and wall thickness [13, 18–21]. Plaque characterization was also performed based on multi-contrast images [22].
Dynamic contrast enhanced (DCE) MRI
Acquisition
DCE-MRI of the vessel wall was performed using a multislice T1 W black blood turbo spin echo (TSE) sequence (dose: 0.1 mmol/kg followed by 20 ml saline chase injected at 4 ml/s via an antecubital vein, acquisition time approximately ~12 min) [9].
Image analysis
Dynamic images were processed by applying de-noising and motion correction algorithms [10, 12]. DCE-MRI data were analyzed using non model-based approaches [23]. The area under the signal intensity versus time curves for 2 and 7 min after contrast agent injection were calculated [10]. All DCE-MRI analysiswas done using a custom-built software program in MATLAB (Math Works, Inc, Natick, MA, USA) [9, 10].
Statistical analysis
Statistical analysis was performed using IBM SPSS Version 19 (IBM SPSS Inc., Armonk, NY, USA). Continuous variables were expressed as mean ± SD. Levene's test was used to test for equality of variance. Differences between the high and low exposures in continuous variables were then compared using an independent samples t test. Using linear forward stepwise multivariate regression with Akaike information criteria corrected (AICC) [24], independent predictors of increased area under curve (AUC7) from DCE-MRI were determined using all variables as input. Confidence interval of 95 % was used and variables with p > 0.1 were eliminated. p < 0.05 was considered significant.
Results
Table 1 shows the results of demographics, biomarkers, ankle brachial indices, and MRI measures. Average across all patients, as well as distribution across the high and low exposure groups are presented. In terms of demographics and biomarkers, the 2 groups were well matched with no significant differences observed between the two groups.
Table 1.
Characteristics of study population in the two exposure groups
| Variable | Total, n (%) | High exposure, n (%) | Low exposure, n (%) | p value |
|---|---|---|---|---|
| N = 31 | N = 19 | N = 12 | ||
| Demographics | ||||
| Gender-male | 28 (90.3) | 18 (94.7) | 10 (83.3) | |
| Gender-female | 3 (9.7) | 1 (5.3) | 2 (16.7) | |
| Smoker (current) | 1 (3.2) | 1 (5.3) | 0 (0.0) | |
| Smoker (past) | 4 (12.9) | 3 (15.8) | 1 (8.3) | |
| Diabetes | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Hypertension | 5 (16.1) | 3 (15.8) | 2 (16.7) | |
| Family history of CVD | 15 (48.4) | 8 (42.1) | 7 (58.3) | |
| Calcium score >75th percentile | 7 (22.6) | 4 (57.1) | 3 (42.9) |
| N = 31 | N = 19 | N = 12 | ||
|---|---|---|---|---|
| Measured variables | ||||
| Age (years) | 45.1 ± 4.4 | 44.6 ± 4.1 | 45.8 ± 4.8 | 0.497 |
| Total cholesterol (mg/dl) | 200 ± 43 | 205 ± 43 | 190 ± 43 | 0.338 |
| HDL (mg/dl) | 52 ± 19 | 55 ± 20 | 47 ± 17 | 0.298 |
| LDL (mg/dl) | 121 ± 34 | 123 ± 36 | 117 ± 30 | 0.657 |
| Fasting blood glucose | 88 ± 24 | 89 ± 30 | 85 ± 8 | 0.662 |
| Heart rate (bpm) | 68 ± 11 | 70 ± 12 | 64 ± 7 | 0.134 |
| SBP (mm Hg) | 121 ± 9 | 120 ± 9 | 121 ± 10 | 0.930 |
| DBP (mm Hg) | 76 ± 7 | 76 ± 8 | 77 ± 7 | 0.667 |
| BMI | 27 ± 2.7 | 27 ± 2.6 | 28 ± 2.7 | 0.206 |
| Waist: hip ratio | 0.91 ± 0.05 | 0.92 ± 0.05 | 0.90 ± 0.04 | 0.202 |
| ABI-left | 1.23 ± 0.11 | 1.21 ± 0.10 | 1.26 ± 0.12 | 0.215 |
| ABI-right | 1.21 ± 0.09 | 1.18 ± 0.08 | 1.26 ± 0.08 | 0.017 |
| N = 31 | N = 19 | N = 12 | ||
|---|---|---|---|---|
| MRI morphometrics | ||||
| Wall area (cm2) | 0.26 ± 0.08 | 0.26 ± 0.07 | 0.27 ± 0.10 | 0.882 |
| Total vessel area (cm2) | 0.61 ± 0.12 | 0.61 ± 0.11 | 0.61 ± 0.14 | 0.999 |
| Lumen area (cm2) | 0.35 ± 0.05 | 0.35 ± 0.05 | 0.35 ± 0.05 | 0.801 |
| Normalized wall index | 0.42 ± 0.05 | 0.41 ± 0.04 | 0.42 ± 0.06 | 0.702 |
| Wall thickness (mm) | 1.06 ± 0.24 | 1.05 ± 0.20 | 1.07 ± 0.30 | 0.869 |
| N = 23 | N = 16 | N = 7 | ||
|---|---|---|---|---|
| DCE-MRI measures | ||||
| Norm. AUC-2 min | 2.76 ± 1.61 | 3.14 ± 1.6 | 1.88 ± 1.37 | 0.084 |
| Norm. AUC-7 min | 2.42 ± 0.73 | 2.66 ± 0.63 | 1.88 ± 0.69 | 0.016 |
Values are indicated as mean ± SD and categorical data is indicated as absolute numbers and percentages
Morphometric MRI did not show any significant differences between the exposure groups. None of the individuals in either exposure group had complex carotid artery plaques that could be classified [22] by multi contrast MRI. Figure 1 shows MR images from sample patients with high exposure (HE) and low exposure. Subjects with HE had significantly higher DCE-MRI AUC uptake (increased neovascularization) compared to subjects with lower exposure (LE). (AUC7: 2.65 ± 0.63 HE vs. 1.88 ± 0.69 LE, p = 0.016). Figure 2 shows sample DCE-MRI AUC maps from sample patients with high and low exposures.
Fig. 1.
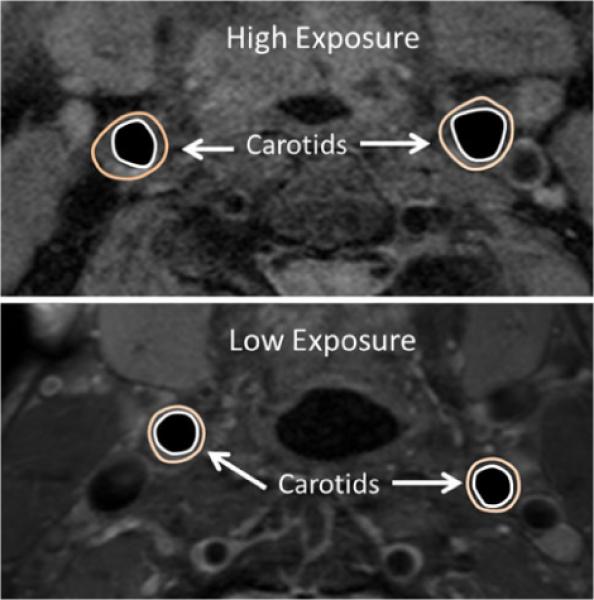
Sample carotid images from patients with high (top) and low (bottom) PM exposure
Fig. 2.
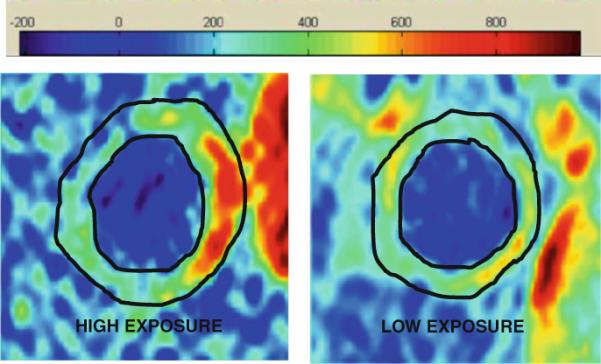
Sample DCE-MRI AUC (7 min) images of a left common carotid artery from high and low PM exposure patients
The ABI of the right side was significantly higher in the low exposure group compared to the HE group. (ABI-r: 1.18 ± 0.08 HE vs. 1.26 ± 0.08 LE, p = 0.017).
Multivariate AICC regression model indicated that only HE to PM, CRP >3.0 and total cholesterol were independently associated with increased neovascularization as defined by DCE-MRI AUC uptake (in decreasing order of importance, all p < 0.026; Adjusted R2 of model = 0.455).
Discussion
This preliminary pilot study hinted that law enforcement personnel exposed to high levels of PM after 11 September 2001 may have increased neovascularization as measured by DCE-MRI compared to those with low exposure. These results also seem to indicate that exposure to PM after 9/11 may potentially be associated with increased progression of atherosclerosis.
Previous studies have shown that high levels of exposure in non-rescue and non-recovery individuals, when compared with low exposed non-rescue and non-recovery individuals, were associated with heart-disease-related mortality [25]. This association was not found in rescue and recovery personnel. To our knowledge, the current study is among the first to use MR imaging to look at cardiovascular complications in law enforcement personnel with WTC exposure. This study seems to indicate that long-term cardiovascular effects may indeed be associated with WTC PM Exposure and that such effects warrant further investigation.
Studies have also shown that acute mental stress can lead to changes in myocardial wall motion or changes to perfusion [26] as shown by imaging. It is speculated that this effect may also be due to arterial vasoconstriction during mental stress, occurring at sites that manifest similar vasoconstriction during acetylcholine infusion. Other studies demonstrate that mental stress also effects endothelial function in the short term [27]. If this endothelial dysfunction caused by stress in the short term is coupled with deleterious effects caused by acute PM exposure over a prolonged period, it could provide a mechanism for advancing the rate of atherosclerosis progression in the exposed population.
Limitations
While the results of this study show some promise, several limitations need to be addressed. Firstly, this study was performed in a very small population and these findings need to be validated in a larger population. The current population could also be affected by selection bias, as the subjects were volunteers that were interested in participating in the study. This study also was limited to law enforcement personnel, who have higher rates of cardiovascular disease, in general, than the general population. The subject exposure groups in this study were also not fully similar in other aspects such as smoking status, gender and cholesterol which may potentially bias the results. Framingham risk scores were also not calculated for these subjects. The number of samples required for performing a robust multivariate regression is also limited. Despite these significant caveats, the multivariate regression identified HE to PM as an independent predictor of DCE uptake lending credence to the possibility that the effect observed in this study merits further investigation.
Inflammation is posited as the mechanism for the increased progression of atherosclerosis. 18-FDG-PET imaging [28, 29] may provide more direct evidence of increased inflammation in vasculature. Because of radiation exposure and cost issues, this was not done as part of this pilot study, but is being considered as a possible avenue for future work.
The use of DCE-MRI for evaluating atherosclerosis is a relatively new field and the optimal models for analysis are still under development. Previous studies have established a relationship between neovascularization on histology and DCE-MRI measures, and have used DCE-MRI to evaluate treatment effects in both humans and animal models [9–11, 30]. The reproducibility and robustness of the DCE-MRI approaches used in this study have also been previously evaluated [10]. Nevertheless, there was no histological verification possible in this study as none of the patients fit the criteria for a carotid endarterectomy.
Finally, the Akaike information criterion model has limitations as it only measures a relative goodness of fit and does not reveal how well a model fits the data in an absolute sense. The model also assumes a linear model with normally-distributed errors (conditional upon regressors).
Conclusions
High exposure to PM may be associated with plaque neovascularization, and thereby potentially indicates worsening atherogenic profile of “Ground Zero” workers. The results of this study also indicate that more future work is warranted to examine the long-term cardiovascular effects of particulate matter exposure in WTC exposure patients as well as in other groups who might have excessive particulate exposure from occupational or environmental sources.
Acknowledgments
Partial support was provided by: NIH/NHLBI R01 HL071021 (ZAF).
Abbreviations
- AICC
Akaike information criteria corrected model
- AUC
Area under the signal intensity versus time curve
- CVD
Cardiovascular disease
- DCE-MRI
Dynamic contrast enhanced MRI
- DIR
Double inversion recovery
- IMT
Intima-media thickness
- LDL
Low-density lipoprotein
- MRI
Magnetic resonance imaging
- NO
Nitric oxide
- PCB
Polychlorinated biphenyls
- PDW
Proton density weighted
- PM
Particulate matter
- T1W
T1-weighted
- T2W
T2-weighted
- TSE
Turbo spin echo
Footnotes
Conflict of interest None.
References
- 1.Simkhovich BZ, Kleinman MT, Kloner RA. Particulate air pollution and coronary heart disease. Curr Opin Cardiol. 2009;24:604–609. doi: 10.1097/HCO.0b013e32833161e5. [DOI] [PubMed] [Google Scholar]
- 2.Gill EA, Curl CL, Adar SD, Allen RW, Auchincloss AH, O'Neill MS, Park SK, Van Hee VC, Diez Roux AV, Kaufman JD. Air pollution and cardiovascular disease in the multi-ethnic study of atherosclerosis. Prog Cardiovasc Dis. 2011;53:353–360. doi: 10.1016/j.pcad.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mateen FJ, Brook RD. Air pollution as an emerging global risk factor for stroke. JAMA. 2011;305:1240–1241. doi: 10.1001/jama.2011.352. [DOI] [PubMed] [Google Scholar]
- 4.Fang SC, Cassidy A, Christiani DC. A systematic review of occupational exposure to particulate matter and cardiovascular disease. Int J Environ Res Public Health. 2010;7:1773–1806. doi: 10.3390/ijerph7041773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pope CA, III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 6.Grahame TJ, Schlesinger RB. Cardiovascular health and particulate vehicular emissions: a critical evaluation of the evidence. Air Qual Atmos Health. 2010;3:3–27. doi: 10.1007/s11869-009-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: a statement for health-care professionals from the expert panel on population and prevention science of the American heart association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 8.Nemmar A, Hoylaerts MF, Hoet PH, Dinsdale D, Smith T, Xu H, Vermylen J, Nemery B. Ultrafine particles affect experimental thrombosis in an in vivo hamster model. Am J Respir Crit Care Med. 2002;166:998–1004. doi: 10.1164/rccm.200110-026OC. [DOI] [PubMed] [Google Scholar]
- 9.Calcagno C, Mani V, Ramachandran S, Fayad ZA. Dynamic contrast enhanced (DCE) magnetic resonance imaging (MRI) of atherosclerotic plaque angiogenesis. Angiogenesis. 2010;13:87–99. doi: 10.1007/s10456-010-9172-2. [DOI] [PubMed] [Google Scholar]
- 10.Calcagno C, Vucic E, Mani V, Goldschlager G, Fayad ZA. Reproducibility of black blood dynamic contrast-enhanced magnetic resonance imaging in aortic plaques of atherosclerotic rabbits. J Magn Reson Imaging. 2010;32:191–198. doi: 10.1002/jmri.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Li F, Zhao X, Yuan C, Rutt B, Kerwin WS. Extended graphical model for analysis of dynamic contrast-enhanced MRI. Magn Reson Med. 2011;66:868–878. doi: 10.1002/mrm.22819. [DOI] [PubMed] [Google Scholar]
- 12.Kerwin WS, Cai J, Yuan C. Noise and motion correction in dynamic contrast-enhanced MRI for analysis of atherosclerotic lesions. Magn Reson Med. 2002;47:1211–1217. doi: 10.1002/mrm.10161. [DOI] [PubMed] [Google Scholar]
- 13.Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif JC, Rudd JH, Farkouh ME, Tawakol A. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-plaque): a randomised clinical trial. Lancet. 2011;378:1547–1559. doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGee JK, Chen LC, Cohen MD, Chee GR, Prophete CM, Haykal-Coates N, Wasson SJ, Conner TL, Costa DL, Gavett SH. Chemical analysis of world trade center fine particulate matter for use in toxicologic assessment. Environ Health Perspect. 2003;111:972–980. doi: 10.1289/ehp.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson DA, Norris GA, Landis MS, Vette AF. Chemical characterization of ambient particulate matter near the world trade center: elemental carbon, organic carbon, and mass reconstruction. Environ Sci Technol. 2004;38:4465–4473. doi: 10.1021/es030689i. [DOI] [PubMed] [Google Scholar]
- 16.Mani V, Itskovich VV, Aguiar SH, Mizsei G, Aguinaldo JG, Samber DD, Macaluso FM, Fayad ZA. Comparison of gated and non-gated fast multislice black-blood carotid imaging using rapid extended coverage and inflow/outflow saturation techniques. J Magn Reson Imaging. 2005;22:628–633. doi: 10.1002/jmri.20428. [DOI] [PubMed] [Google Scholar]
- 17.Mani V, Itskovich VV, Szimtenings M, Aguinaldo JG, Samber DD, Mizsei G, Fayad ZA. Rapid extended coverage simultaneous multisection black-blood vessel wall MR imaging. Radiology. 2004;232:281–288. doi: 10.1148/radiol.2321031022. [DOI] [PubMed] [Google Scholar]
- 18.El Aidi H, Mani V, Weinshelbaum KB, Aguiar SH, Taniguchi H, Postley JE, Samber DD, Cohen EI, Stern J, van der Geest RJ, Reiber JH, Woodward M, Fuster V, Gidding SS, Fayad ZA. Cross-sectional, prospective study of MRI reproducibility in the assessment of plaque burden of the carotid arteries and aorta. Nat Clin Pract Cardiovasc Med. 2009;6:219–228. doi: 10.1038/ncpcardio1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fayad ZA, Mani V, Woodward M, Kallend D, Bansilal S, Pozza J, Burgess T, Fuster V, Rudd JH, Tawakol A, Farkouh ME. Rationale and design of dal-plaque: a study assessing efficacy and safety of dalcetrapib on progression or regression of atherosclerosis using magnetic resonance imaging and 18f-fluorodeoxyglucose positron emission tomography/computed tomography. Am Heart J. 2011;162(214-221):e212. doi: 10.1016/j.ahj.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mani V, Muntner P, Gidding SS, Aguiar SH, El Aidi H, Weinshelbaum KB, Taniguchi H, van der Geest R, Reiber JH, Bansilal S, Farkouh M, Fuster V, Postley JE, Woodward M, Fayad ZA. Cardiovascular magnetic resonance parameters of atherosclerotic plaque burden improve discrimination of prior major adverse cardiovascular events. J Cardiovasc Magn Reson. 2009;11:10. doi: 10.1186/1532-429X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Geest RJ, de Roos A, van der Wall EE, Reiber JH. Quantitative analysis of cardiovascular MR images. Int J Card Imaging. 1997;13:247–258. doi: 10.1023/a:1005869509149. [DOI] [PubMed] [Google Scholar]
- 22.Itskovich VV, Samber DD, Mani V, Aguinaldo JG, Fallon JT, Tang CY, Fuster V, Fayad ZA. Quantification of human atherosclerotic plaques using spatially enhanced cluster analysis of multicontrast-weighted magnetic resonance images. Magn Reson Med. 2004;52:515–523. doi: 10.1002/mrm.20154. [DOI] [PubMed] [Google Scholar]
- 23.Calcagno C, Cornily JC, Hyafil F, Rudd JH, Briley-Saebo KC, Mani V, Goldschlager G, Machac J, Fuster V, Fayad ZA. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler Thromb Vasc Biol. 2008;28:1311–1317. doi: 10.1161/ATVBAHA.108.166173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnham KP, Anderson DR, Huyvaert KP. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol. 2011;65:23–35. [Google Scholar]
- 25.Jordan HT, Brackbill RM, Cone JE, Debchoudhury I, Farfel MR, Greene CM, Hadler JL, Kennedy J, Li J, Liff J, Stayner L, Stellman SD. Mortality among survivors of the sept 11, 2001, world trade center disaster: results from the world trade center health registry cohort. Lancet. 2011;378:879–887. doi: 10.1016/S0140-6736(11)60966-5. [DOI] [PubMed] [Google Scholar]
- 26.Deanfield JE, Shea M, Kensett M, Horlock P, Wilson RA, de Landsheere CM, Selwyn AP. Silent myocardial ischaemia due to mental stress. Lancet. 1984;2:1001–1005. doi: 10.1016/s0140-6736(84)91106-1. [DOI] [PubMed] [Google Scholar]
- 27.Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, O'Connor G, Betteridge J, Klein N, Steptoe A, Deanfield JE. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102:2473–2478. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- 28.Rudd JH, Myers KS, Sanz J, Fayad ZA. Multimodality imaging of atherosclerosis (magnetic resonance imaging/computed tomography/positron emission tomography-computed tomography) Top Magn Reson Imaging. 2007;18:379–388. doi: 10.1097/rmr.0b013e3181598db0. [DOI] [PubMed] [Google Scholar]
- 29.Silvera SS, Aidi HE, Rudd JH, Mani V, Yang L, Farkouh M, Fuster V, Fayad ZA. Multimodality imaging of athero-sclerotic plaque activity and composition using FDG-PET/CT and MRI in carotid and femoral arteries. Atherosclerosis. 2009;207:139–143. doi: 10.1016/j.atherosclerosis.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerwin WS, Oikawa M, Yuan C, Jarvik GP, Hatsukami TS. MR imaging of adventitial vasa vasorum in carotid atherosclerosis. Magn Reson Med. 2008;59:507–514. doi: 10.1002/mrm.21532. [DOI] [PubMed] [Google Scholar]


