Abstract
The immunological synapse (IS) formed between immune cells and antigen-presenting cells (APCs) provides a platform for signaling. Protein kinase C-θ (PKC-θ) localizes in the T cell IS within the central supramolecular activation cluster (cSMAC), where it associates with CD28 and mediates TCR/CD28 signals leading to effector T (Teff) cell activation. In regulatory T (Treg) cells, PKC-θ is sequestered away from the IS, and inhibits suppressive function. Other PKCs localizing in the IS mediate additional functions in various immune cells. Further work is needed to identify mechanisms underlying PKC recruitment or exclusion at the IS, potential redundancy among IS-localized PKCs, and the relevance of PKC localization for IS dynamics and lymphocyte activation.
The immunological synapse
Immune cells require extensive contact-dependent communications for the relay of signals and execution of effector functions. In the case of T lymphocytes, triggering of the antigen-specific T cell receptor (TCR) and costimulatory receptors such as CD28 by antigen-presenting cells (APCs) results in the activation of signal transduction pathways that induce differentiation into effector T (Teff) cells via defined gene expression programs. Thus, the effector function of T and other immune cells depends on their interaction with partner cells, just as neurons communicate with neighboring cells to send signals that evoke particular responses. Hence, the term “synapse”, originally coined to describe the structure that permits a neuron to pass an electrical or chemical signal to another cell, was transposed to the field of immunology, initially referring to the intimate contact between an antigen-specific T helper (Th) cell and an APC, which triggers directional cytokine secretion by the Th cell [1], and later expanded to various cytotoxic lymphocytes and even B cells. This contact area is now known as the immunological synapse (IS) [2] or the supramolecular activation cluster (SMAC) [3]. The IS has been reviewed extensively [4–6], and our understanding of its organization and function has evolved substantially since its discovery (Box 1). Over the years, several members of the protein kinase C (PKC) family, particularly PKCθ, were found to localize at the IS. With the expanding number of IS-residing PKCs and the growing list of functions they play in lymphocyte activation and effector functions, the time seems ripe to review the current knowledge accumulated in this area.
Box 1. The immunological synapse - then and now.
Then
Originally analyzed by confocal imaging and deconvolution microscopy in fixed T cells
Represents the contact region between Th cells and APCs that forms upon TCR stimulation with peptide-major histocompatibility complex (pMHC) ligands, as defined originally [2, 3]
Receptors, cytoskeletal proteins, and intracellular enzymes and adaptor proteins are concentrated in the IS contact area in a highly compartmentalized manner to initiate and sustain signal transduction pathways leading to immune cell activation and differentiation [4–6]
In addition to naive and effector Th cells, also found in other immune system cells, e.g., CTLs, NK cells, NKT cells, and B cells [81, 86–89]
A concentric “bull’s eye” structure consisting of three subregions: a central core enriched in TCR and pMHC (cSMAC), an intermediate ring enriched in adhesion molecules (pSMAC), and an outer ring containing receptors with large extracellular tails, e.g., CD45 and CD43 [90, 91]
Now
Improved, higher resolution real time analysis via the use of supported lipid bilayers and TIRFM in vitro, and two-photon microscopy in vivo to reveal the spatiotemporal dynamics T-APC interactions.
TCR microclusters (MCs) containing additional signaling molecules defined as the minimal active signaling unit in IS
MCs form in the periphery (dSMAC) of the T-APC junction in an actin-dependent manner and move centripetally to the cSMAC [6, 21, 92, 93]
Existence of kinapses, short-lived asymmetric synapses, in motile T cells [55]
The cSMAC is a site of signal termination through endocytosis and ubiquitin-mediated degradation of signaling complexes [19–21]
Segregation of the cSMAC into two distinct subregions - a central, CD3high region (signal termination) and an outer CD3low annular ring enriched in CD28 and PKCθ, a site of sustained signaling [13, 23]
PKCs in the IS of Th cells
PKCθ IS localization and T cell activation
The contact area between Th cells and APCs has long been recognized as a site where TCRs and costimulatory receptors are localized and engage their counter-receptors on APCs for efficient activation and polarized cytokine secretion driven by the microtubule-organizing complex (MTOC) [1, 7]. However, whether intracellular signaling molecules also localize at this site remained unknown for some time. Using digital immunofluorescence microscopy, protein kinase C-θ (PKCθ) (Box 2) was the first such molecule found to localize at the IS following APC stimulation; this localization was highly selective since other T cell-expressed PKCs (α, β1, δ, η and ζ) were not initially found in the IS [8]. However, with higher resolution imaging techniques, three of these PKCs, (δ, η and ζ) as well as another one (PKCε), were later also found to partition into the IS (see below). PKCθ translocation to the IS occurred at a high stoichiometry and was persistent, and it was associated with kinase activation. Weaker stimulation conditions that failed to induce PKCθ or MTOC IS translocation also did not cause T cell proliferation, implicating this selective PKCθ localization as important for productive T cell activation [8]. This discovery was soon followed by the identification and description of the SMAC [3] or the IS [2] as three-dimensional assemblies where the TCR and signaling molecules segregate into distinct subdomains in a highly compartmentalized fashion - the TCR and PKCθ as a central cluster (cSMAC), and adhesion molecules (LFA-1, talin) as an outer concentric ring (pSMAC).
Box 2. PKCθ (reviewed in [83, 84]).
Structure and expression
A Ca2+-independent novel PKC predominantly expressed in T cells and muscle
Contains a novel N-terminal C2-like pTyr-binding domain (also found in PKCδ)
Proline-rich motif in the V3 (hinge) domain mediates interaction with CD28 (see below)
Ectopic expression and a marker in gastrointestinal stromal tumors
IS organization and signaling
Translocates to lipid rafts and found in the IS (cSMAC) in antigen-stimulated T cells
Required for T cell activation and proliferation in vitro
Major targets in mature T cells are NF-κB and AP-1 transcription factors and, to a lesser extent, NFAT
Provides a CD28-dependent signal that prevents T cell anergy
Forms an inducible complex with CD28 in the outer ring of the cSMAC, a site of sustained T cell signaling; CD28 association mediated by the PKCθ V3 domain required for transcriptional activation and T cell proliferation
Mediates a T cell survival signal
Effector functions
Minor role in T cell development (cooperation with PKCη)
Required for Th2 and Th17 differentiation and function, GvH disease, and allograft rejection, but largely dispensable for pathogen-specific protective responses and graft-versus-leukemia response
Mediates MTOC polarization in stimulated CD4+ T cells (in cooperation with PKCε and -η)
Prkcq SNPs associated with human type 1 diabetes, rheumatoid arthritis, and celiac disease
Sequestered from the IS of Treg cells, and inhibits their suppressive function
The molecular basis and biological significance of the selective cSMAC localization of PKCθ remained unknown for a long time. Conventional (cPKCs: α, β, γ) and novel (nPKCs: δ, ε, θ, η) possess two tandem cysteine-rich zinc finger C1 domains, which bind the plasma membrane (PM)-localized PKC-activating lipid second messenger, diacylglycerol (DAG), a step required for kinase activation. However, C1 domains alone cannot account for the selective recruitment of PKCθ to the IS, and particularly to the cSMAC, for a number of reasons. First, other PKCs containing very similar tandem C1 domains do not localize at the IS [8], despite the presence of high local DAG concentration at this site [9, 10]. Second, some C1-containing proteins, e.g., Ras-GRP1, are found in internal cellular membranes [11]. And, third, in contrast to the stable IS localization of full-length PKCθ, the tandem PKCθ C1 domains localize at the IS in an unstable and transient manner [11]. Therefore, another mechanism, perhaps involving a protein-protein interaction, seems responsible for the unique cSMAC localization of PKCθ following antigen stimulation.
A recent study unveiled the structural basis for PKCθ recruitment to the cSMAC and demonstrated that it is essential for PKCθ-dependent downstream signaling functions [12]. Antigen stimulation was found to induce a physical association, previously observed in phorbol ester-stimulated T cells [13], between PKCθ and CD28. This association accounted for the cSMAC localization of PKCθ because PKCθ mutants that were unable to associate with CD28 also failed to translocate to the cSMAC and, instead, were present in a diffuse manner throughout the IS. Furthermore, such mutants failed to activate PKCθ-dependent downstream signaling. The association between PKCθ and CD28 may occur early in TCR microclusters (MCs) formed at the IS periphery as CD28 was initially recruited coordinately with the TCR into peripheral MCs [13]. The coalescence of MCs at the cSMAC was accompanied by the segregation of CD28 from the TCR, resulting in the translocation of both CD28 and PKCθ to a spatially unique annular subregion of the cSMAC distinct from a more central CD3high region [13]. These findings explain earlier reports documenting the importance of CD28 costimulation in recruiting PKCθ to the cSMAC [13, 14] and activating the transcription factor NF-κB, a known PKCθ target [15–18]. However, it remains unclear whether the formation of CD3low annular ring depends on signaling initiated by TCR MCs, or how the CD28-PKCθ complex segregates into the CD3low annular ring.
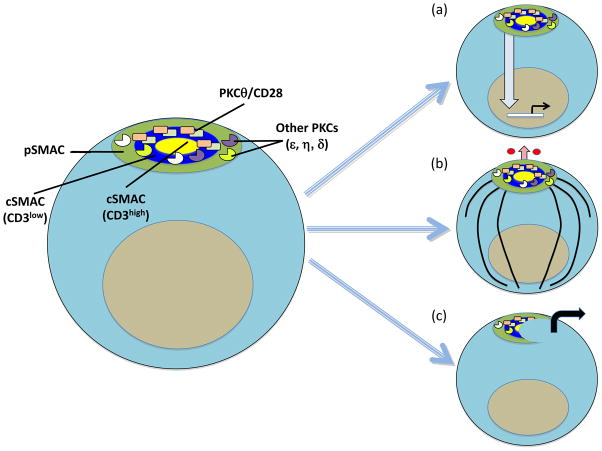
These recent studies [12, 13] resolve a glaring dilemma related to the function of the cSMAC. namely, whether it serves as a site for signal termination and degradation [19–21] and/or a site for sustained activation, as proposed initially [2, 3] and implied by the prolonged residence of PKCθ in the IS [8] and the findings that TCR-mediated tyrosine phosphorylation occurred primarily at the IS periphery and was largely terminated before a mature IS had formed [22]. Thus, we can now view the “traditional” cSMAC as containing two subregions with distinct biological functions: 1) a peripheral CD3low ring where a CD28-PKCθ complex sustains prolonged signaling important for productive T cell activation; and 2) a central CD3high core where signaling is terminated through endocytosis and degradation of the TCR and associated signaling molecules [6, 23] (Figure 1a)
Figure 1.
Functions of PKCθ in the IS of CD4+ T cells. The left image shows the pSMAC (green); the CD3low outer ring of the cSMAC (blue), where an inducible CD28-PKCθ complex (which also includes Lck) sustains signaling; and the CD3high core of the cSMAC (yellow), which is the site of signal termination. PKCθ has at least three distinct functions in the IS: (a) Intracellular signaling involving activation of NF-κB, AP-1 (and, to a lesser extent, NFAT) leading to productive T cells activation; (b) polarization of the MTOC (black circle) and microtubules (black lines) to the IS for directional cytokine (small red circles) secretion, indicated by the upward pointing arrow; PKCθ function in this respect requires the redundant action of PKCε and PKCη, which are localized diffusely over the entire IS; and (c) breaking of the stable IS to promote the formation of kinapses, short-term asymmetric synapses that characterize motile T cells; motility is indicated by the right pointing arrow. It remains to be determined whether association with CD28 is required for the PKCθ functions depicted in (b) and (c).
The CD28-PKCθ association is indirect and mediated by the Lck tyrosine kinase, although other modes of interaction cannot be entirely excluded. Lck associates with a phosphotyrosine (pTyr)-containing C-terminal proline (Pro)-rich motif (Pro-pTyr188-Ala-Pro) in the cytoplasmic tail of mature CD28 via either its SH2 or SH3 domains [24, 25], although the SH2 domain has a much higher affinity for this motif [24]. This would potentially leave the Lck SH3 domain free to interact with other proteins. A more recent study showed that the V3 (hinge) region of PKCθ is necessary for its cSMAC recruitment and ability to induce downstream signaling, including CD69 and CD25 upregulation, proliferation, and IL-2 production. A Pro-rich motif was mapped within the V3 domain that binds the SH3 domain of Lck and is critical for PKCθ cSMAC/IS translocation and its functionality [12]. This motif is evolutionally highly conserved in PKCθ orthologues, but is not present in other PKCs, providing a molecular basis for the selective cSMAC localization of the enzyme. Hence, the preferred binding model supported by our study [12] implicates the Lck SH2 and SH3 domains in binding the phosphorylated Tyr188 of CD28 and the Pro-rich motif of the PKCθ V3 domain, respectively, thereby mediating formation of a competent PKCθ-containing trimolecular signaling complex at the IS. The importance of the PKCθ V3 domain was further established by demonstrating that ectopic V3 expression functioned in a dominant negative manner to disrupt the stimulation-induced endogenous CD28-PKCθ complex. Under those conditions, endogenous PKCθ could not promote T cell activation [12], including the differentiation and function of Th2 and Th17 cells that is PKCθ-dependent [26, 27], leaving Th1 function, which is largely PKCθ-independent [26, 28], intact [12].
Interestingly, Lck may play a dual role in this scheme because, in addition to the adaptor function of its SH2 and SH3 domains, it also phosphorylates a regulatory tyrosine residue (Tyr-90) in the N-terminal C2-like domain of PKCθ [25], an event that increases its affinity for PM-localized DAG, and promote its IS translocation and signaling [25, 29–31]. This phosphorylation, in conjunction with TCR-dependent activating phosphorylation of Thr-538 by GLK3 [32], may induce a conformational change, relaxing the binding of the kinase domain to its pseudosubstrate site at the N-terminus, which keeps it in a resting state. The exposed kinase domain would then autophosphorylate at Thr-219 located between the two tandem C1 domains, which is required for the IS recruitment of PKCθ and for its ability to activate reporter genes [33]. The same conformational change may also condition the V3 domain for Lck-dependent binding to CD28, which would then serve as a second step to concentrate PKCθ at the cSMAC subsequent to its DAG-dependent enrichment at the entire IS. Another report, demonstrating the importance of an active kinase domain for the IS recruitment of PKCθ [34], may reflect the need for PKCθ to autophosphorylate Thr-219. Hence, the activation, IS localization, and downstream signaling functions of PKCθ appear to be regulated in a complex manner by auto- and transphosphorylation events [35] (and, by extension, by the opposing action of phosphatases), and additional work is needed to elucidate the precise nature of, and crosstalk among, these post-translational modifications.
The PKCθ-cytoskeleton connection in relation to the IS
The actin and microtubule systems constitute dynamic cellular networks that are required to induce integrin-mediated adhesion, IS formation, cellular polarization and migration, and ultimately control T-cell activation [36, 37]. In the stable IS, the outer, concentric pSMAC is enriched with adhesion molecules and polymerized actin [38]. The antigen-induced translocation of PKCθ to the IS and to colocalized lipid rafts [29] depends on actin cytoskeleton reorganization and, in turn, on a signaling axis consisting of the guanine nucleotide exchange factor Vav1 and Rac1, its small GTPase target [39, 40]. In fact, antigen stimulation causes ~50% of PKCθ to translocate to a cytoskeleal compartment [39].
The molecular basis and the functional relevance of the association of PKCθ with the actin cytoskeleton is not entirely clear, but several clues have emerged. First, PKCθ may play an indirect role in reorganization of the cytoskeleton that is tightly linked to IS formation, as it phosphorylates the Wiskott-Aldrich Syndrome protein (WASp)-interacting protein (WIP) in CD4+ T cells [41]. When bound to WASp, WIP inhibits the activity of the latter. Phosphorylation of WIP dissociates it from WASp and relieves this inhibition, allowing WASp to promote actin polymerization and F-actin stabilization in hematopoietic cells [41].
Second, following T cell activation the actin-binding scaffolding protein, filamin A (FLNa), was recruited to the IS, where it colocalized and physically associated with PKCθ [42] and with CD28 [43]. RNAi-mediated FLNa depletion did not affect TCR-induced early tyrosine phosphorylation or actin polymerization per se but, nevertheless, resulted in impaired IL-2 expression and activation of key transcription factors, as well as in reduced PM (and, presumably, IS) translocation of PKCθ [42]. FLNa depletion also inhibited CD28-mediated raft accumulation at the IS and T-cell costimulation [43]. Interestingly, the same C-terminal Pro-pTyr188-Ala-Pro motif in the CD28 cytoplasmic tail, which binds Lck kinase and is critical for the IS recruitment of PKCθ and its association with CD28 [12], was also required for FLNa translocation to the IS [43]. More studies are needed to establish the precise relationship and order of interaction among these signaling proteins and with CD28. Hence, FLNa likely represents a functional and potentially physical link between PKCθ and the actin cytoskeleton on one hand, and CD28 on the other hand.
PKCs and T cell polarity
Directional secretion of soluble mediators by Teff cells provides a means for a highly specific and efficient communication with APCs and other target cells and, in the case of lytic granules, it is also critical to prevent damage to bystander cells and to the cytotoxic T lymphocytes (CTLs) themselves [44–46]. In antigen-stimulated Th cells, DAG produced upon PLCγ1 activation is focused at the IS [10, 47], where it drives MTOC polarization toward the IS [47]. Using single-cell photoactivation of the TCR coupled with TIRFM, Quann et al. reported that PKCθ was required for MTOC reorientation. TCR stimulation caused PKCθ to rapidly localize at a central region (presumably the cSMAC) of the IS. However, PKCθ recruitment was preceded by two other nPKCs, PKCε and PKCη, which also accumulated at the IS but, unlike PKCθ, were distributed in a broad pattern over the entire IS [9]. Moreover, these two PKCs were required in a redundant manner for the subsequent cSMAC recruitment of PKCθ. The diffuse presence of PKCη in the T cell IS was also reported by others [48, 49]. This study establishes an additional, previously unrecognized role for PKCθ in the IS and, furthermore, demonstrates that a cascade of distinct IS-localized PKCs regulates CD4+ T cell polarity (Figure 1b). However, the molecular mechanism of how IS recruitment of PKCε and PKCη leads to the subsequent recruitment of PKCθ remains to be defined. The DAG- and Ca2+-independent PKCζ may play a similar role because it was found to be rapidly activated at the IS of human Th cells interacting with cognate dendritic cells (DCs) and, moreover, an active PKCζ was required for polarization of the Th cell secretory machinery toward DCs [50]. Thus, substantial redundancy and cooperation among PKC family members likely exists at the level of T cell polarization and MTOC organization, at least in CD4+ T cells.
Asymmetric T cell division
Asymmetric cell division is an evolutionary conserved metazoan process that allows a dividing cell to produce two daughter cells that give rise to distinct cell fates. This process is likely important during lymphocyte activation and differentiation given the fact that T and B cells can differentiate into distinct functional subsets [51]. Antigen-activated CD4+ and CD8+ T cells preparing to undergo their first cell division were found to display unequal partitioning of proteins that mediate signaling, cell fate specification, and asymmetric cell division [52]. One of these proteins was PKCζ, known to be an important component of the ancestral polarity-determining machinery. In pre-mitotic cells, upon interaction with a cognate peptide-MHC complex, PKCζ was localized to the distal pole complex (DPC), which is the subcellular region opposite CD3 and the MTOC [53]. Upon cell division, PKCζ segregated into the distal daughter cell, i.e., the cell away from the interacting APC [52, 54]. This asymmetric segregation required prolonged T cell-APC interaction before division, and the proximal and distal daughter T cells displayed phenotypic and functional markers characteristic of effector and memory T cell lineages, respectively [52]. Hence, formation of a stable IS may play an important role in dictating asymmetric T cell division by segregating signaling proteins, including some PKC family members, in (PKCθ) or out (PKCζ) of the IS.
PKCθ and the T cell kinapse
The long held view of the T cell IS as a stable, symmetric “bull’s eye” structure was mostly based on the analysis of antigen-primed static T cells that have been restimulated in vitro. With the development of higher resolution imaging techniques and spatial-temporal imaging of dynamic T-APC interactions, it became clear that these T cells can form short-term, asymmetric contacts with APCs and, moreover, that these short contacts are sufficient to trigger signaling responses (e.g., T cell anergy) that differ qualitatively and quantitatively from the signals generated in the stable IS [55]. These transient T-APC interactions can be observed both in vivo and in vitro, and have been termed kinapses [38]. The kinapse is considered to represent a platform that integrates activation signals during short, transient contacts that T cells establish with APCs when scanning the surface of APCs for foreign antigens. The duration and stability of contact between T cells and APCs and, consequently, the type of IS that forms, is determined by the balance between stop signals provided by adhesive interactions and chemokine gradients that induce T cell migration, and a T cell can undergo a reversible transition between a stable IS and a kinapse when serially interacting with APCs.
PKCθ and WASp regulate the conversion between synapses and kinapses, respectively, in an opposite manner [56]. PKCθ promotes breaking of the stable IS at the pSMAC and drives T cell migration (Figure 1c). Conversely, reassembly of a stable IS is driven by WASp, and Was−/− T cells form a concentric, stable IS after migration only if PKCθ is inhibited pharmacologically [56]. This opposite regulation of IS stability may account for findings that Prkcq deletion (i.e., a more stable IS) favors Th1 differentiation and function [26, 27], while Was−/− T cells (with tendency to form kinapses) display impaired Th1, but not Th2, differentiation [57]. Indeed, Th2 cells respond more poorly to low-affinity peptide stimulation [58], a condition that favors kinapse formation. The negative regulation of IS stability by PKCθ also has important potential implications for the function of regulatory T (Treg) cells as discussed later. Based on the transient, unstable nature of the kinapse, it can be predicted that kinapses would not support NF-κB activation or asymmetric cell division, which require a prolonged contact (i.e., a stable IS) between T cells and APCs [52, 59]. In summary, it is clear that, in addition to forming stable, persistent synapses, T cells can also form transient kinapses, which result in distinct functional outcomes for the responding T cell.
PKCs at the IS of cytotoxic lymphocytes
Cytotoxic lymphocytes, including CD8+ CTLs, NK cells, and NKT cells, polarize their secretory machinery toward their target cells via formation of an IS in a process driven by the MTOC [44–46]. The CTL IS is generally similar to the one observed in CD4+ T cells, with a peripheral ring (pSMAC) containing adhesion molecules and a central region equivalent to the cSMAC, where PKCθ and Lck are enriched [60, 61]. However, there are some differences, including the presence of some PKCθ in the CTL pSMAC and an asymmetric secretory cleft found on one side of the cSMAC [61]. Similar to CD4+ T cells, the CTL IS delivers TCR signals but, in addition, it directs the delivery of lytic granule contents to the target cells [44, 61, 62]. Both naive and acutely activated CD8+ T cells form stable conjugates with target cells, but only effector cells developed a cSMAC defined by focusing of PKCθ, which was phosphorylated (and, thus, likely active). In contrast, naïve CD8+ cells displayed a uniform distribution of PKCθ over the entire IS, but could still polarize their MTOC, produce IL-2, proliferate, and differentiate into effector CTLs [63]. In fact, CTL lines derived from Prkcq−/ − mice were found to degranulate normally, suggesting that other PKCs may compensate for the loss of PKCθ [64]. For example, PKCδ, the nearest relative of PKCθ, localizes to secretory lysosomes (lytic granules) of CD8+ CTLs, where it directly mediates TCR signals leading to granule exocytosis [65]. Additionally, PKCα has also been shown to translocate to the CTL IS and, in synergy with PKCθ, to mediate lytic granule exocytosis by augmenting Ca2+ signaling [66]. Therefore, similar to CD4+ Th cells [9], CTLs may recruit several distinct PKCs that function in a redundant manner to promote degranulation at the IS.
PKCθ was reported to destabilize the IS in CD4+ CTLs since a selective PKCθ inhibitor increased IS stability and sensitivity of specific target cell lysis [67]. Conversely, disruption of the pSMAC by treatment with anti-LFA-1 antibody destabilized the CD8+ CTL IS and decreased target cell sensitivity to lysis. This study also demonstrated that CD4+ CTLs form a less stable IS with target cells than their CD8+ counterparts, which correlates with relatively reduced lytic efficiency. These results suggest that formation of a stable pSMAC, which is inhibited by PKCθ, functions to confine the released lytic molecules at the synaptic interface and to enhance the effectiveness of target cell lysis. However, evidence also exists indicating that PKCθ promotes IS stability. Thus, PKCθ was reported to activate the β2 integrin LFA-1 (i.e., increase its avidity for its ligand ICAM-1) downstream of the TCR by phosphorylating the guanine nucleotide exchange factor Rap-GEF2, an activator of the small GTPase Rap1 [68]. Additional studies will be required to settle this apparent discrepancy.
In NK cells, the balance between stimulatory and inhibitory receptors regulates IS formation and signaling. The NK cell IS also displays a pSMAC containing F-actin and adhesion molecules, and a cSMAC where perforin is found [69]. PKCθ is expressed in NK cells, but its role in NK activation and effector function in the context of the IS is not entirely clear [69]. The most direct evidence for PKCθ localization and function in the NK cell IS comes from a very recent study showing that PKCθ accumulated in MCs, and that these MCs coalesced within the NK cell IS [70]. This clustering was dependent on the N-terminal C2 domain of PKCθ, which we recently found to constitute a novel pTyr-binding domain [71]. In contrast to wild-type PKCθ, PKCθ carrying a C2 point mutation that abolishes pTyr binding [71] was incapable of forming MCs and rescuing the defect in cytokine production by Prkcq−/− NK cells [70]. These results suggest that the pTyr-binding function of PKCθ is important for MC formation and clustering, and raise the interesting possibility that a PKCθ C2-bound pTyr-containing protein participates in MC formation and is necessary for NK cell effector function.
Engagement of activating NK receptors leads to rapid PKCθ activation; however, Prkcq−/− NK cells were deficient in transcriptional activation and IFNγ production, but displayed intact release of soluble cytolytic mediators [72], potentially reflecting a compensatory role for other PKC family members [9, 64]. The same explanation may account for the intact activation of NF-κB in Prkcq−/− NK cells (which is in contrast to CD4+ T cells), as the unique requirement of PKCθ for NF-κB activation may be T cell-specific [15]. PKCθ may also indirectly regulate actin cytoskeleton reorganization, which contributes to IS formation, in NK cells as it phosphorylates WIP in these cells [73], similar to the effect observed in CD4+ T cells [41] reviewed earlier. WIP polarizes toward the NK cell IS, and its knockdown inhibited lytic granule polarization and release [74].
Similar to CTLs and NK cells, NKT cells also form an IS upon engagement by CD1+ APCs presenting lipid antigen, and they polarize their MTOC and lytic granules toward the IS [62]. Moreover, a secretory cleft similar to that observed in CTLs [61], also exists in the NKT (and NK) cell IS. Inasmuch as the degranulation pathway in NKT cells appears to be similar to the one studied in CTLs and NK cells [62], it would not be surprising if PKCθ and/or other PKCs [9, 65] were also to be found to localize in the NKT cell IS, but this has not yet been formally established.
PKCθ and the Treg IS
CD4+Foxp3+ regulatory T cells (Tregs) maintain immune homeostasis and self-tolerance during the process of self/nonself discrimination [75, 76], and formation of an IS between Tregs and APCs is a prominent feature of contact-dependent suppression [77]. PKCθ is required for the thymic development of nTregs [78, 79]. This requirement is not absolute, however, since Prkcq−/− mice still have ~20% of the nTregs found in wild-type mice. Moreover, ex vivo Tregs isolated from Prkcq−/− mice display intact suppressive activity [79] The requirement of PKCθ reflects its important role in activating the NF-κB signaling pathway because, similar to PKCθ deletion, deletion of IKKβ and Bcl10, two critical components in the canonical NF-κB pathway, reduces nTreg development [79]. The importance of NF-κB in Treg development is also evident from the finding that the transcription factor c-Rel initiates Foxp3 transcription in thymic Treg precursors [80]. The nTreg development defect in Prkcq−/− mice is not related to a missing survival signal since transgenic expression of the anti-apoptotic survival protein BclxL does not restore the Treg cell population in these mice [81]. In addition, CN-Aβ-deficient mice also have a decreased Treg cell population similar to that observed in Prkcq−/− mice, suggesting that NFAT also plays an important role in nTreg development [81].
Recent findings that PKCθ is sequestered from the IS of Tregs, and that it negatively regulates induced Treg (iTreg) suppressive function [82], represent a significant breakthrough. Thus, in contrast to Teff cells, PKCθ was concentrated in the DPC of human Tregs engaged by APCs, i.e., opposite the IS. Pharmacological inhibition of PKCθ enhanced Treg function, protected Treg from inhibition of their suppressive function by TNFα, rescued the impaired function of Tregs from rheumatoid arthritis patients, and enhanced protection of mice from inflammatory colitis [82]. The exclusion of PKCθ, which promotes IS breaking in Teff cells [56], from the Treg IS may account for the fact that Tregs form a more stable IS than conventional Teff cells [77]. This higher stability is likely also due to the higher expression of adhesion molecules such as LFA-1 and neuropilin in Tregs [75, 76]. PKCθ may also negatively regulate the differentiation of naïve T cells into FoxP3+ functional Tregs, since the in vitro TGFβ-induced iTreg differentiation was enhanced in Prkcq−/− T cells or in wild-type cells treated with a PKCθ inhibitor [81]. Thus, in Treg cells, PKCθ seems to play a role, which is the opposite of its positive regulatory role in the activation of conventional T cells. This intriguing finding highlights the possibility that inhibition of PKCθ function or its IS localization may represent an attractive therapeutic modality for autoimmune diseases and prevention of transplant rejection by simultaneously and synergistically inhibiting the undesired function of pathogenic T cells and enhancing the beneficial activity of Tregs in these scenarios [77, 83, 84].
Concluding remarks
Although PKCθ was originally reported to be the only PKC family member to localize in the IS of antigen-stimulated T cells [8], later studies made it clear that other PKCs can also be found in the IS in T, NK and NKT cells. Whether the recruitment of these other PKCs to the IS is solely accounted for by the accumulation of DAG in the IS remains to be determined. Nevertheless, until demonstrated otherwise, PKCθ still retains the “honor” of being the only PKC that is uniquely concentrated in the cSMAC and, more specifically, in a newly identified annular ring surrounding the core of the cSMAC [13], where it forms a physical complex with CD28 that is essential for CD4+ T cell activation [12]. However, we do not yet definitively know whether the PKCθ pool found in this location is catalytically active. In this regard, reagents that can be used in high-resolution imaging studies to specifically report the activation status of PKCθ are sorely missing.
In CD8+ T cells, although PKCθ can also be found in the IS, it may be required for some, but not all, aspects of cell activation, differentiation and effector function, suggesting substantial differences between CD4+ and CD8+ T cells in terms of the importance of PKCθ in general, and its IS/cSMAC localization in particular. These differences may reflect a redundancy between PKCθ and other PKC family members vis-à-vis certain effector functions, particularly MTOC and lytic granule polarization in CTLs and NK cells. The use of compound PKC knockout mice may be necessary to resolve the relative importance of distinct PKCs in this process, their localization vis-à-vis the IS, and their interdependency.
Lastly, the mechanism that sequesters PKCθ from the Treg IS remains to be elucidated. One possibility is that CTLA-4, which is expressed at high levels in Tregs, competes with CD28 for recruitment to the Treg IS/cSMAC [85], thereby displacing the PKCθ-CD28 complex [12]. Another possibility is that pharmacological inhibition of PKCθ prevents it from gaining access to the IS since its catalytic activity is important for IS localization [34]. These potential scenarios could explain why the Treg IS is devoid of PKCθ, but they do not account for a presumably active process that shuttles PKCθ from the IS to the DPC, nor do they explain how active, DPC-localized PKCθ inhibits Treg function. It would be interesting to determine whether in Tregs, PKCθ forms a complex with some other protein(s), which directs it to the DPC and/or mediate its negative effect on Treg function. Future studies will likely decipher the mechanisms underlying the recruitment to, or exclusion from, the IS of distinct PKCs, potential functional redundancies among IS-localized PKCs, and the biological relevance of this highly compartmentalized localization in relation to IS dynamics and lymphocyte activation.
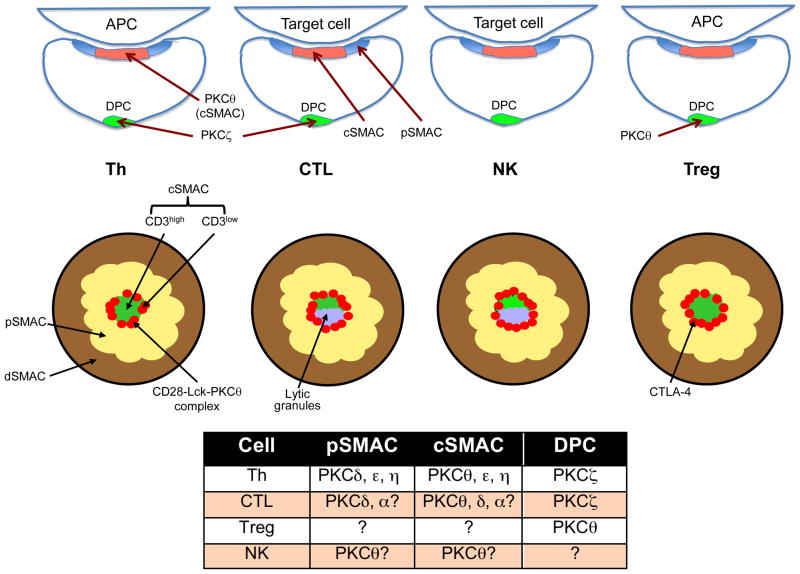
Figure 2.
PKCs in the IS of immune cells. The figure presents side view (top row), top (en face) view (middle row), and a summary table (bottom) displaying the localization and organization of IS subregions, the distal pole complex (DPC), and various PKC family members in Th, CTL, NK, and Treg cells. Question marks in the table refer to unresolved issues, i.e., the distribution of PKCθ and δ in the pSMAC vs. cSMAC of CTLs and NK cells, whether any PKC is found in the Treg cell IS, and formal demonstration of a DPC in NK cells.
Acknowledgments
This is publications number 1568 from the La Jolla Institute for Allergy and Immunology. Work from the author’s laboratory was supported by National Institutes of Health grant CA035299. I thank the many colleagues in my laboratory, who over a period of many years contributed to our work on PKCθ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 2.Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 3.Monks CR, et al. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 4.Bunnell SC. Multiple microclusters: diverse compartments within the immune synapse. Curr Top Microbiol Immunol. 2010;340:123–154. doi: 10.1007/978-3-642-03858-7_7. [DOI] [PubMed] [Google Scholar]
- 5.Dustin ML, et al. Understanding the structure and function of the immunological synapse. Cold Spring Harb Perspect Biol. 2010;2:a002311. doi: 10.1101/cshperspect.a002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokosuka T, Saito T. The immunological synapse, TCR microclusters, and T cell activation. Curr Top Microbiol Immunol. 2010;340:81–107. doi: 10.1007/978-3-642-03858-7_5. [DOI] [PubMed] [Google Scholar]
- 7.Podack ER, Kupfer A. T-cell effector functions: mechanisms for delivery of cytotoxicity and help. Annu Rev Cell Biol. 1991;7:479–504. doi: 10.1146/annurev.cb.07.110191.002403. [DOI] [PubMed] [Google Scholar]
- 8.Monks CR, et al. Selective modulation of protein kinase C-θ during T-cell activation. Nature. 1997;385:83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 9.Quann EJ, et al. A cascade of protein kinase C isozymes promotes cytoskeletal polarization in T cells. Nat Immunol. 2011;12:647–654. doi: 10.1038/ni.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spitaler M, et al. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity. 2006;24:535–546. doi: 10.1016/j.immuni.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Carrasco S, Merida I. Diacylglycerol-dependent binding recruits PKCθ and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Mol Biol Cell. 2004;15:2932–2942. doi: 10.1091/mbc.E03-11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong KF, et al. A motif in the V3 domain of the kinase PKC-θ determines its localization in the immunological synapse and functions in T cells via association with CD28. Nat Immunol. 2011;12:1105–1112. doi: 10.1038/ni.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokosuka T, et al. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C θ translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, et al. CD28 plays a critical role in the segregation of PKC θ within the immunologic synapse. Proc Natl Acad Sci USA. 2002;99:9369–9373. doi: 10.1073/pnas.142298399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coudronniere N, et al. NF-κB activation induced by T cell receptor/CD28 costimulation is mediated by PKCθ. Proc Natl Acad Sci USA. 2000;97:3394–3399. doi: 10.1073/pnas.060028097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin X, et al. Protein kinase C θ participates in NF-κB/Rel activation induced by CD3/CD28 costimulation through selective activation of IκB β (IKKβ) Mol Cell Biol. 2000;20:2933–2940. doi: 10.1128/mcb.20.8.2933-2940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfeifhofer C, et al. Protein kinase C θ affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J Exp Med. 2003;197:1525–1535. doi: 10.1084/jem.20020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Z, et al. PKC-θ is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 19.Lee KH, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 20.Vardhana S, et al. Essential role of ubiquitin and TSG101 protein in formation and function of the central supramolecular activation cluster. Immunity. 2010;32:531–540. doi: 10.1016/j.immuni.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varma R, et al. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KH, et al. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 23.Yokosuka T, Saito T. Dynamic regulation of T-cell costimulation through TCR-CD28 microclusters. Immunol Rev. 2009;229:27–40. doi: 10.1111/j.1600-065X.2009.00779.x. [DOI] [PubMed] [Google Scholar]
- 24.Hofinger E, Sticht H. Multiple modes of interaction between Lck and CD28. J Immunol. 2005;174:3839–3840. doi: 10.4049/jimmunol.174.7.3839-a. author reply 3840. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, et al. Regulation of protein kinase Cθ function during T cell activation by Lck-mediated tyrosine phosphorylation. J Biol Chem. 2000;275:3603–3609. doi: 10.1074/jbc.275.5.3603. [DOI] [PubMed] [Google Scholar]
- 26.Marsland BJ, et al. Protein kinase C θ is critical for the development of in vivo T helper (Th)2 cell but not Th1 cell responses. J Exp Med. 2004;200:181–189. doi: 10.1084/jem.20032229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salek-Ardakani S, et al. Differential regulation of Th2 and Th1 lung inflammatory responses by PKCθ. J Immunol. 2004;173:6440–6447. doi: 10.4049/jimmunol.173.10.6440. [DOI] [PubMed] [Google Scholar]
- 28.Salek-Ardakani S, et al. Protein kinase Cθ controls Th1 cells in experimental autoimmune encephalomyelitis. J Immunol. 2005;175:7635–7641. doi: 10.4049/jimmunol.175.11.7635. [DOI] [PubMed] [Google Scholar]
- 29.Bi K, et al. Antigen-induced translocation of PKC-θ to membrane rafts is required for T cell activation. Nat Immunol. 2001;2:556–563. doi: 10.1038/88765. [DOI] [PubMed] [Google Scholar]
- 30.Melowic HR, et al. Mechanism of diacylglycerol-induced membrane targeting and activation of protein kinase Cθ. J Biol Chem. 2007;282:21467–21476. doi: 10.1074/jbc.M700119200. [DOI] [PubMed] [Google Scholar]
- 31.Diaz-Flores E, et al. Membrane translocation of protein kinase Cθ during T lymphocyte activation requires phospholipase C-γ generated diacylglycerol. J Biol Chem. 2003;278:29208–29215. doi: 10.1074/jbc.M303165200. [DOI] [PubMed] [Google Scholar]
- 32.Chuang HC, et al. The kinase GLK controls autoimmunity and NF-κB signaling by activating the kinase PKC-θ in T cells. Nat Immunol. 2011;12:1113–1118. doi: 10.1038/ni.2121. [DOI] [PubMed] [Google Scholar]
- 33.Thuille N, et al. Critical role of novel Thr-219 autophosphorylation for the cellular function of PKCθ in T lymphocytes. EMBO J. 2005;24:3869–3880. doi: 10.1038/sj.emboj.7600856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cartwright NG, et al. An active kinase domain is required for retention of PKCθ at the T cell immunological synapse. Mol Biol Cell. 2011;22:3491–3497. doi: 10.1091/mbc.E10-11-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, et al. Regulation of PKC-θ function by phosphorylation in T cell receptor signaling. Front Immunol. 2012;3:197. doi: 10.3389/fimmu.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat Immunol. 2000;1:23–29. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- 37.Gomez TS, Billadeau DD. T cell activation and the cytoskeleton: you can’t have one without the other. Adv Immunol. 2008;97:1–64. doi: 10.1016/S0065-2776(08)00001-1. [DOI] [PubMed] [Google Scholar]
- 38.Dustin ML. Cell adhesion molecules and actin cytoskeleton at immune synapses and kinapses. Curr Opin Cell Biol. 2007;19:529–533. doi: 10.1016/j.ceb.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villalba M, et al. Vav1/Rac-dependent actin cytoskeleton reorganization is required for lipid raft clustering in T cells. J Cell Biol. 2001;155:331–338. doi: 10.1083/jcb.200107080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villalba M, et al. A novel functional interaction between Vav and PKCθ is required for TCR-induced T cell activation. Immunity. 2000;12:151–160. doi: 10.1016/s1074-7613(00)80168-5. [DOI] [PubMed] [Google Scholar]
- 41.Sasahara Y, et al. Mechanism of recruitment of WASP to the immunological synapse and of its activation following TCR ligation. Mol Cell. 2002;10:1269–1281. doi: 10.1016/s1097-2765(02)00728-1. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi K, Altman A. Filamin A is required for T cell activation mediated by PKCθ. J Immunol. 2006;177:1721–1728. doi: 10.4049/jimmunol.177.3.1721. [DOI] [PubMed] [Google Scholar]
- 43.Tavano R, et al. CD28 interaction with filamin-A controls lipid raft accumulation at the T-cell immunological synapse. Nat Cell Biol. 2006;8:1270–1276. doi: 10.1038/ncb1492. [DOI] [PubMed] [Google Scholar]
- 44.Griffiths GM, et al. The immunological synapse: a focal point for endocytosis and exocytosis. J Cell Biol. 2010;189:399–406. doi: 10.1083/jcb.201002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huse M, et al. Shouts, whispers and the kiss of death: directional secretion in T cells. Nat Immunol. 2008;9:1105–1111. doi: 10.1038/ni.f.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stinchcombe JC, Griffiths GM. Secretory mechanisms in cell-mediated cytotoxicity. Annu Rev Cell Dev Biol. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- 47.Quann EJ, et al. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009;10:627–635. doi: 10.1038/ni.1734. [DOI] [PubMed] [Google Scholar]
- 48.Fu G, Gascoigne NR. The role of protein kinase Cη in T cell biology. Front Immunol. 2012;3:177. doi: 10.3389/fimmu.2012.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singleton KL, et al. Itk controls the spatiotemporal organization of T cell activation. Sci Signal. 2011;4:ra66. doi: 10.1126/scisignal.2001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bertrand F, et al. Activation of the ancestral polarity regulator protein kinase C ζ at the immunological synapse drives polarization of Th cell secretory machinery toward APCs. J Immunol. 2010;185:2887–2894. doi: 10.4049/jimmunol.1000739. [DOI] [PubMed] [Google Scholar]
- 51.Chang JT. Polarity and lymphocyte fate determination. Curr Opin Cell Biol. 2012;24:526–533. doi: 10.1016/j.ceb.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 53.Cullinan P, et al. The distal pole complex: a novel membrane domain distal to the immunological synapse. Immunol Rev. 2002;189:111–122. doi: 10.1034/j.1600-065x.2002.18910.x. [DOI] [PubMed] [Google Scholar]
- 54.Chang JT, et al. Asymmetric proteasome segregation as a mechanism for unequal partitioning of the transcription factor T-bet during T lymphocyte division. Immunity. 2011;34:492–504. doi: 10.1016/j.immuni.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dustin ML. T-cell activation through immunological synapses and kinapses. Immunol Rev. 2008;221:77–89. doi: 10.1111/j.1600-065X.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 56.Sims TN, et al. Opposing effects of PKCθ and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 57.Trifari S, et al. Defective Th1 cytokine gene transcription in CD4+ and CD8+ T cells from Wiskott-Aldrich syndrome patients. J Immunol. 2006;177:7451–7461. doi: 10.4049/jimmunol.177.10.7451. [DOI] [PubMed] [Google Scholar]
- 58.Balamuth F, et al. Distinct patterns of membrane microdomain partitioning in Th1 and Th2 cells. Immunity. 2001;15:729–738. doi: 10.1016/s1074-7613(01)00223-0. [DOI] [PubMed] [Google Scholar]
- 59.Katzman SD, et al. Duration of antigen receptor signaling determines T-cell tolerance or activation. Proc Natl Acad Sci USA. 2010;107:18085–18090. doi: 10.1073/pnas.1010560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Potter TA, et al. Formation of supramolecular activation clusters on fresh ex vivo CD8+ T cells after engagement of the T cell antigen receptor and CD8 by antigen-presenting cells. Proc Natl Acad Sci USA. 2001;98:12624–12629. doi: 10.1073/pnas.221458898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stinchcombe JC, et al. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 62.Stinchcombe JC, et al. Centriole polarisation to the immunological synapse directs secretion from cytolytic cells of both the innate and adaptive immune systems. BMC Biol. 2011;9:45. doi: 10.1186/1741-7007-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Keefe JP, et al. Formation of a central supramolecular activation cluster is not required for activation of naive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:9351–9356. doi: 10.1073/pnas.0305965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puente LG, et al. A novel PKC regulates ERK activation and degranulation of cytotoxic T lymphocytes: Plasticity in PKC regulation of ERK. Eur J Immunol. 2006;36:1009–1018. doi: 10.1002/eji.200535277. [DOI] [PubMed] [Google Scholar]
- 65.Ma JS, et al. Protein kinase C δ localizes to secretory lysosomes in CD8+ CTL and directly mediates TCR signals leading to granule exocytosis-mediated cytotoxicity. J Immunol. 2008;181:4716–4722. doi: 10.4049/jimmunol.181.7.4716. [DOI] [PubMed] [Google Scholar]
- 66.Grybko MJ, et al. Protein kinase C activity is required for cytotoxic T cell lytic granule exocytosis, but the θ isoform does not play a preferential role. J Leukoc Biol. 2007;81:509–519. doi: 10.1189/jlb.0206109. [DOI] [PubMed] [Google Scholar]
- 67.Beal AM, et al. Protein kinase C θ regulates stability of the peripheral adhesion ring junction and contributes to the sensitivity of target cell lysis by CTL. J Immunol. 2008;181:4815–4824. doi: 10.4049/jimmunol.181.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Letschka T, et al. PKC-θ selectively controls the adhesion-stimulating molecule Rap1. Blood. 2008;112:4617–4627. doi: 10.1182/blood-2007-11-121111. [DOI] [PubMed] [Google Scholar]
- 69.Anel A, et al. Protein kinase C-θ (PKC-θ) in natural killer cell function and anti-tumor immunity. Front Immunol. 2012;3:187. doi: 10.3389/fimmu.2012.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Merino E, et al. Protein Kinase-θ clustering at immunological synapses amplifies effector responses in NK cells. J Immunol. 2012;189:4859–4869. doi: 10.4049/jimmunol.1200825. [DOI] [PubMed] [Google Scholar]
- 71.Stahelin RV, et al. Protein kinase Cθ C2 domain is a phosphotyrosine binding module that plays a key role in its activation. J Biol Chem. 2012;287:30518–30528. doi: 10.1074/jbc.M112.391557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tassi I, et al. NK cell-activating receptors require PKC-θ for sustained signaling, transcriptional activation, and IFN-γ secretion. Blood. 2008;112:4109–4116. doi: 10.1182/blood-2008-02-139527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krzewski K, et al. Formation of a WIP-, WASp-, actin-, and myosin IIA-containing multiprotein complex in activated NK cells and its alteration by KIR inhibitory signaling. J Cell Biol. 2006;173:121–132. doi: 10.1083/jcb.200509076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krzewski K, et al. WIP is essential for lytic granule polarization and NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2008;105:2568–2573. doi: 10.1073/pnas.0711593105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Josefowicz SZ, et al. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sakaguchi S, et al. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 77.Zanin-Zhorov A, et al. PKC-θ function at the immunological synapse: prospects for therapeutic targeting. Trends Immunol. 2011;32:358–363. doi: 10.1016/j.it.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gupta S, et al. Differential requirement of PKC-θ in the development and function of natural regulatory T cells. Mol Immunol. 2008;46:213–224. doi: 10.1016/j.molimm.2008.08.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmidt-Supprian M, et al. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-κB activation. Proc Natl Acad Sci USA. 2004;101:4566–4571. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hori S. c-Rel: a pioneer in directing regulatory T-cell lineage commitment? Eur J Immunol. 2010;40:664–667. doi: 10.1002/eji.201040372. [DOI] [PubMed] [Google Scholar]
- 81.Ma J, et al. Protein kinase C-θ inhibits inducible regulatory T cell differentiation via an AKT-Foxo1/3a-dependent pathway. J Immunol. 2012;188:5337–5347. doi: 10.4049/jimmunol.1102979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zanin-Zhorov A, et al. Protein kinase C-θ mediates negative feedback on regulatory T cell function. Science. 2010;328:372–376. doi: 10.1126/science.1186068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Altman A, Kong KF. PKCθ: a new target for selective immunosuppression. Expert Rev Clin Immunol. 2012;8:205–208. doi: 10.1586/eci.12.8. [DOI] [PubMed] [Google Scholar]
- 84.Zhang EY, et al. The Yin and Yang of protein kinase C-θ (PKCθ): A novel drug target for selective immunosuppression. Adv Pharmacol. 2013:66. doi: 10.1016/B978-0-12-404717-4.00006-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yokosuka T, et al. Spatiotemporal basis of CTLA-4 costimulatory molecule-mediated negative regulation of T cell activation. Immunity. 2010;33:326–339. doi: 10.1016/j.immuni.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 86.McCarthy C, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204:1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stinchcombe JC, Griffiths GM. The role of the secretory immunological synapse in killing by CD8+ CTL. Semin Immunol. 2003;15:301–305. doi: 10.1016/j.smim.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 89.Batista FD, et al. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–494. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 90.Freiberg BA, et al. Staging and resetting T cell activation in SMACs. Nat Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 91.Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol. 2003;3:973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- 92.Campi G, et al. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yokosuka T, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]