Abstract
Objective
The persistence of myeloid-derived cells in the artery wall is a characteristic of advanced atherosclerotic plaques. However, the mechanisms by which these cells are retained are poorly understood. Semaphorins, a class of neuronal guidance molecules, play a critical role in vascular patterning and development, and recent studies suggest that they may also have immunomodulatory functions. The present study evaluates the expression of Semaphorin 3E (Sema3E) in settings relevant to atherosclerosis and its contribution to macrophage accumulation in plaques.
Approach and Results
Immunofluorescence staining of Sema3E, and its receptor PlexinD1, demonstrated their expression in macrophages of advanced atherosclerotic lesions of Apoe–/– mice. Notably, in two different mouse models of atherosclerosis regression, Sema3E mRNA was highly downregulated in plaque macrophages, coincident with a reduction in plaque macrophage content and an enrichment in markers of reparative M2 macrophages. In vitro, Sema3E mRNA was highly expressed in inflammatory “M1” macrophages, and in macrophages treated with physiological drivers of plaque progression and inflammation, such as oxidized LDL and hypoxia. To explore mechanistically how Sema3E affects macrophage behavior, we treated macrophages with recombinant protein in the presence/absence of chemokines, including CCL19, a chemokine implicated in the egress of macrophages from atherosclerotic plaques. Sema3E blocked actin polymerization and macrophage migration stimulated by the chemokines, suggesting that it may immobilize these cells in the plaque.
Conclusions
Sema3E is up-regulated in macrophages of advanced plaques, is dynamically regulated by multiple atherosclerosis-relevant factors, and acts as a negative regulator of macrophage migration, which may promote macrophage retention and chronic inflammation in vivo.
Keywords: Atherosclerosis, Semaphorin 3E, macrophage, migration, regression
INTRODUCTION
The persistence of myeloid-derived cells in the artery wall is characteristic of advanced atherosclerotic plaques, and these inflammatory cells contribute not only to atherosclerosis progression, but also plaque instability through the secretion of extracellular matrix-degrading proteases and cytotoxic factors. Not surprisingly, then, there has been a major effort to understand macrophage dynamics in the atherosclerotic plaque. Studies examining monocyte recruitment in atherosclerosis have revealed a number of different chemokines and receptors involved in monocyte homing to the arterial wall1. Perturbations in blood flow at areas of arterial branching, as well as both foreign and endogenous insults (including the retention of atherogenic lipoproteins) to the endothelium, are thought to be responsible for the upregulation of adhesion molecules and chemokines leading to monocyte recruitment and influx into the subendothelial layer of the aortic wall. As atherosclerosis is a chronic, progressive disease, it is thought that the accumulation of monocyte-derived macrophages compounds over time and is a major contributor to the persistence of the disease state2.
As part of the resolution phase of acute inflammation, activated macrophages normally emigrate from the site of localized inflammation to the draining lymphatics3. However, unlike other acute inflammatory states, atherosclerotic inflammation does not readily resolve and cholesterol-laden macrophages (foam cells) persist in the arterial wall. Macrophages in atherosclerotic plaques appear to be impaired in their ability to emigrate to draining lymph nodes4, 5. Trapped in an inflammatory milieu, some macrophages die locally, either by apoptosis or secondary necrosis, and the retention of activated macrophages further aggravates plaque inflammation, which can lead to instability and rupture1. Yet, the mechanisms by which macrophages are retained in the plaque are poorly understood. It has been shown that emigration of macrophages from atherosclerotic lesions can occur under conditions of plaque regression induced either by reducing plasma cholesterol4, 5 or by increasing levels of high-density lipoprotein (HDL)6, 7. Notably, macrophage emigration was largely dependent in a transplant model of atherosclerosis regression on the expression of the chemokine receptor CCR75, 8 implicating the CCR7-specific ligands CCL19 and CCL21 in promoting the egress of cells from the artery wall in this model.
In addition to chemokine pathways serving to regulate the emigration of macrophages from plaques, there are also factors that are likely to actively inhibit macrophage chemotaxis. A source of potential candidates has come from the realization that the vascular and nervous systems of vertebrates share common features both in their anatomy and in the molecular factors that regulate their development 9. Indeed, the role of neuronal guidance molecules in the vasculature has been a recent focus of investigation, and a growing body of evidence demonstrates the participation of classical neuronal guidance molecules in the development of the vascular system9. In addition, emerging evidence is revealing roles for neuronal guidance molecules in the immune system where they appear to exert diverse effects on leukocyte migration, adhesion and inflammatory responses10–12. Notably, we recently reported the involvement of netrin-1, a neuronal guidance molecule, in promoting chronic inflammation in atherosclerosis by retaining macrophages in plaque13.
As noted above, the development of a mouse model of atherosclerosis regression involving the transplant of an atherosclerotic aortic arch into a normolipidemic donor has permitted the study of the egress of monocyte-derived cells, primarily macrophages, from aortic arch plaques 4, 6, 14, 15. In addition, the study of gene expression changes specifically in macrophages from atherosclerotic plaques has become possible with the advent of laser capture microdissection16. Using these tools, we recently profiled the transcriptome changes in progressing and regressing atherosclerotic plaques15. Of the genes significantly changed during regression of atherosclerotic plaques, we were intrigued to see that Semaphorin 3E (Sema3e) was among the most highly downregulated (– 6 fold) compared to that in macrophages in progressing plaque. The Semaphorins are a large family of neuronal guidance cues that have been described as having roles in vascular development and neuroimmune signaling17–19. In particular, the class 3 Semaphorins, of which Sema3E is a member, are highly conserved secreted and matrix-associated proteins that can signal through various transmembrane receptors in the Plexin or Neuropilin family, mediating both repulsive and attractive signaling that appears to be cell type and context specific 20. Given our recent finding that netrin-1 expression in atheroma macrophages promotes chronic inflammation, we wanted to further investigate the expression and function of Sema3E in atherosclerosis to determine whether it had similar effects.
As will be presented, the data clearly show that Sema3E is expressed in macrophages of advanced atherosclerotic plaques in mice, and in vitro potently inhibits migration of macrophages to chemokines implicated in the recruitment of inflammatory macrophages to the draining lymph nodes (e.g., CCL2 and CCL19). Sema3E expression in macrophages in vitro is up-regulated by physiological drivers of plaque inflammation, such as oxidized low density lipoprotein (oxLDL) and hypoxia, and reduced under conditions that promote cholesterol efflux. Furthermore, Sema3E is highly expressed in inflammatory M1, but not anti-inflammatory M2 macrophages, and consistent with this is the reduction in expression of Sema3E in regressing atherosclerotic plaques, in which there is also a shift in macrophage phenotype from a predominantly M1 to M2 phenotype. Together, these data suggest a role for Sema3E in the retention of macrophages in atherosclerosis and highlight the expanding functions of neuroimmune guidance cues in regulating the persistence of inflammation in atherosclerotic plaques.
RESULTS
Sema3E is Expressed in Macrophages of Advanced Atherosclerotic Mouse Plaques and its Expression Decreases in Models of Plaque Regression
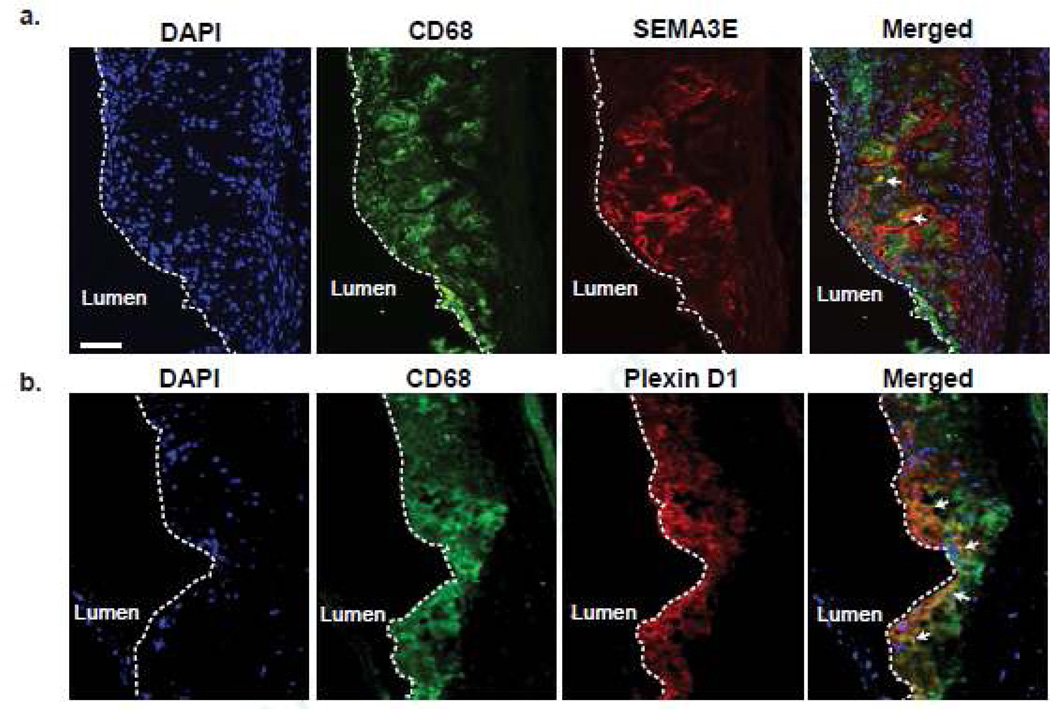
To investigate the expression of Sema3E at the protein level in atherosclerosis, we performed immunohistochemical staining on aortic root plaques of Apoe–/– mice fed a western diet for 12 weeks. In these progressing atherosclerotic plaques, double-staining for Sema3E and the macrophage marker CD68 showed Sema3E protein present in lesional macrophages (Fig. 1a, arrows). In addition, there appeared to be extracellular Sema3E staining in macrophage-rich regions of the plaque, consistent with Sema3E being a secreted protein that can bind to extracellular matrix. Furthermore, staining for the Sema3E receptor PlexinD1 also co-localized with CD68-positive macrophages in these advanced atherosclerotic plaques (Fig. 1b, arrows), suggesting that these cells may be both the source and target of Sema3E secreted in the plaque.
Figure 1. Sema3E and its Receptor PlexinD1 are Expressed by Macrophages in Advanced Atherosclerotic Plaques.
Immunofluorescent staining of CD68 (green), DNA (DAPI, blue) and (a) Sema3E (red) or (b)4, 5, PlexinD1 (red) in aortic root atherosclerotic plaques of Apoe−/− mice fed a Western diet for 12 weeks. Areas of co-localization are shown in yellow in the merged image (arrows). Scale bar, 50 µm. Images are representative of n ≥ 3 mice.
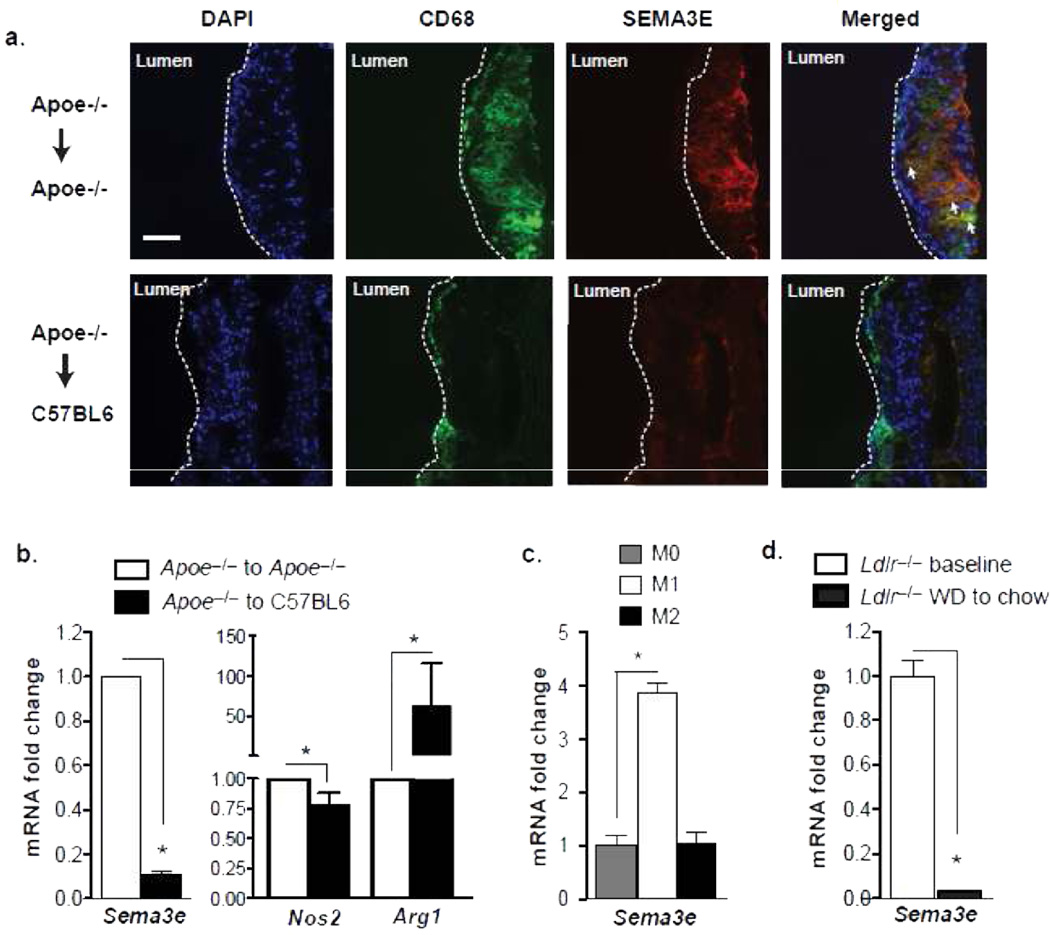
To understand the dynamics of Sema3E expression in atherosclerosis, we used an established model of atherosclerosis regression in which the aortic arch from Apoe–/– mice fed a western diet (WD) for 16 weeks is transplanted into either a hyperlipidemic Apoe–/– (progressive environment) or normolipidemic wild type C57BL6 (regressive environment) recipient mouse for 3 days14. Similar to its expression in aortic root plaques, Sema3E was abundantly expressed in aortic arch plaques in the progressive environment (Apoe–/– → Apoe–/–) in regions that stained positive for the macrophage marker CD68 (Fig. 2a). By contrast, staining for Sema3E was markedly reduced in plaques of aortic arch segments transplanted into the regressive environment (Apoe–/– → C57BL6), which as in previous studies (21), also show a decrease in plaque size and macrophage content. To confirm that macrophages are a source of Sema3E expression in the plaque, we isolated mRNA from lesional CD68+ macrophages by laser capture microdissection and measured gene expression by quantitative RT-PCR (qPCR). Consistent with our immunohistochemical analyses and the microarray studies15, macrophages isolated from plaques in the progressive environment expressed abundant Sema3e mRNA (Fig. 2b), which was reduced by 90% in macrophages from plaques transplanted into a regressive environment for only 3 days. This decrease in macrophage Sema3e mRNA also correlated with a reduction in the inflammatory M1 macrophage marker Nos2 and an increase in the anti-inflammatory M2 macrophage marker Arg1 (Fig. 2b), which we have previously shown to characterize plaques undergoing regression6, 7, 22. To investigate further whether Sema3e expression is associated with the M1 or M2 macrophage phenotype, we measured its expression in bone marrow derived macrophages (BMDM) polarized in vitro. Sema3e mRNA is highly increased in M1 macrophages polarized with LPS and IFNγ, but not M2 macrophages polarized with IL-4, compared to untreated macrophages (Fig. 2c), indicating that its expression is correlated with inflammatory macrophages.
Figure 2. Sema3E Expression by Lesional Macrophages is Downregulated during Atherosclerosis Regression.
(a) Immunofluorescent staining of CD68 (green) and Sema3E (red) in aortic arch plaques transplanted into a progressive (Apoe−/−- recipient) or regressive (C57BL6 recipient) environment. Areas of co-localization (yellow) are shown in the merged image. Images are representative of n ≥ 3 mice. (b) qPCR analysis of mRNA from laser captured CD68+ cells in aortic arch plaques from mice in (a) (n=4 mice/group). (c) qPCR analysis of Sema3e mRNA in BMDMs polarized towards M1, M2 or unpolarized (M0). (d) qPCR analysis of Sema3e mRNA from laser captured CD68+ cells in aortic root plaques from Ldlr−/- mice fed a western diet for 14 weeks (baseline) and then switched to a chow diet for 4 weeks (regression conditions) (n=5 mice/group). Data in b-d are the mean of triplicate samples ± SEM. Statistical analyses were performed by Student’s t-test (b,d) or ANOVA (c). *P0003C;0.05
As the atherosclerosis regression observed in the aortic arch transplant model is quite rapid, we next sought to determine whether Sema3e was also regulated in plaques under conditions simulating less aggressive lipid-lowering management. To do this, Ldlr–/– mice were fed a WD for 14 weeks and either sacrificed for baseline plaque measurements or switched to a chow diet for an additional 4 weeks. This switch to chow is associated with reductions in total plasma cholesterol and plaque lipid content7. Consistent with our findings in the transplant model of regression, macrophages isolated from aortic root plaques of Ldlr–/– mice switched to chow diet showed a marked decrease in Sema3e mRNA compared to macrophages from baseline progressing atherosclerotic plaques (Fig. 2d). Collectively, these data indicate that Sema3e expression by lesional macrophages differentially responds to progressive and regressive atherosclerotic environments.
Macrophage Sema3E Expression is Up-Regulated by Physiological Drivers of Plaque Inflammation
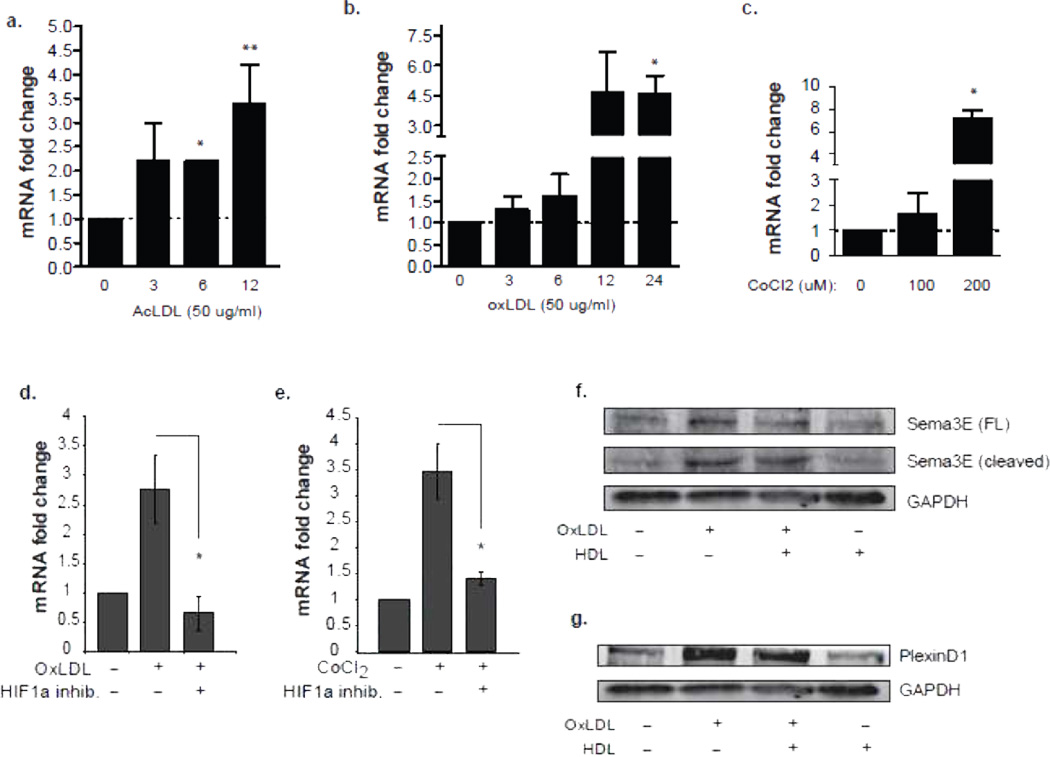
To further understand the molecular mechanisms of Sema3E expression in plaque macrophages and to extend the above observations in M1 and M2 polarized macrophages, we investigated whether Sema3E is induced by pro-inflammatory/atherogenic stimuli in vitro. We treated macrophages with acetylated or oxidized LDL (modifications that promote cholesterol loading of macrophages) and measured expression of Sema3e mRNA by qPCR. AcLDL or oxLDL induced a 3-4-fold increase in Sema3e mRNA (Fig. 3a-b), whereas native LDL did not alter expression of these genes (data not shown). As oxLDL promotes plaque oxidative stress and induces the hypoxia-inducible factor 1α (HIF1α) transcription factor, we also treated BMDM with a chemical mimic of hypoxia, CoCl2, which stabilizes HIF1α Similar to oxLDL, CoCl2 increased macrophage Sema3e mRNA 5-fold (Fig. 3c). Notably, the increase in Sema3e mRNA induced by oxLDL and CoCl2 was blocked by pretreating macrophages with an inhibitor of HIF-1α (Fig. 3d-e), implicating this transcription factor in the upregulation of Sema3e. Immunoblot analysis confirmed an increase in full-length Sema3E (89 kDa) in the supernatant of oxLDL-treated macrophages, which was paralleled by an increase in the cleaved form (69 kDa) of Sema3E (Fig. 3f). Notably, treatment with HDL, which promotes cholesterol efflux from the cell and blocks the inflammatory actions of oxLDL23, reduced levels of Sema3E secreted by oxLDL-stimulated cells, whereas treatment of macrophages with HDL alone did not alter levels of full-length or cleaved Sema3E (Fig. 3f). Similarly, macrophages treated with oxLDL, but not HDL, show increased abundance of PlexinD1 protein (250 kDa), and the effects of oxLDL on PlexinD1 are partially reversed by co-incubation with HDL (Fig. 3g). Collectively, these data demonstrate that Sema3E and PlexinD1 expression in macrophages is dynamically regulated by pro- and anti-atherosclerotic stimuli.
Figure 3. Sema3E Expression is Up-regulated by Physiological Drivers of Plaque Inflammation.
qPCR analysis of Sema3e mRNA in BMDMs treated with (a) 50 µg/ml of AcLDL or (b) oxLDL for the indicated times, or (c) CoCl2 for 24 h. (d-e) qPCR analysis of Sema3e mRNA expression in peritoneal macrophages treated with (d) oxLDL (50 µg/ml) or (e) CoCl2 (200 µM) with or without HIF-1α inhibitor (100 µmol/L). (f) Western blot of full-length and cleaved Sema 3E in supernatants from BMDMs stimulated with 50 µg/ml oxLDL, 10 µg/ml HDL, or both for 24 h. (g) Western blot of PlexinD1 in cell lysates of BMDMs stimulated with 50 µg/ml oxLDL, 10 µg/ml HDL, or both for 24 h. Data in a-c are the mean of triplicate samples ± SEM. Statistical analysis was performed by ANOVA; (a-c) * P<0.05 compared to untreated, (d-e) * P<0.05 compared to OxLDL or CoCl2 alone.
Sema3E Inhibits the Migration of Macrophages
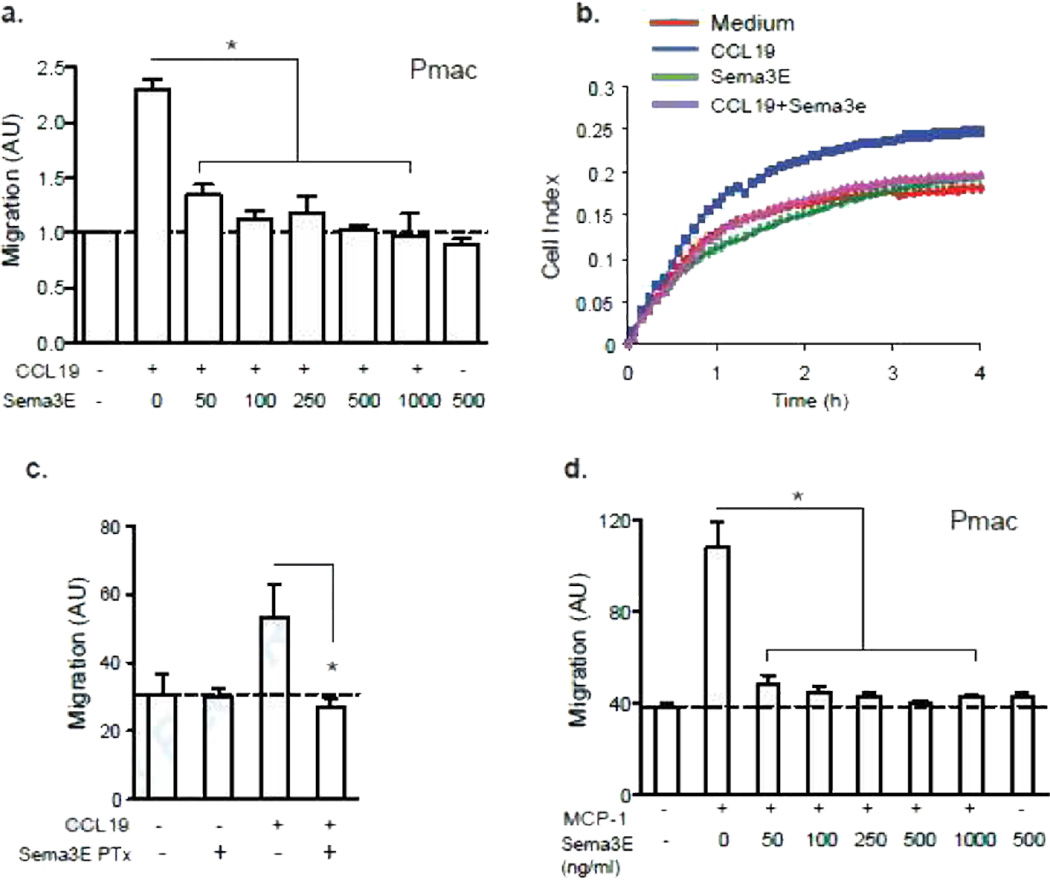
Sema3E has previously been reported to act as a negative guidance cue for thymocytes, and inhibits the migration of these cells to CCL25 and CCL2124. As recent studies have identified the importance of chemokine-mediated macrophage emigration from the plaque during atherosclerosis regression4–6, 8, 13, 15, 21, 22, we next examined the effect of Sema3E on macrophage chemotaxis using both transwell Boyden chambers and a real-time detection method (xCelligence). Whereas Sema3E had little effect on macrophage migration in the absence of chemokine, it potently inhibited peritoneal macrophage migration towards the CCR7 ligand, CCL19, a chemokine receptor-ligand pair implicated in macrophage egress from atherosclerotic plaques5 (Fig 4a). Analogous results were obtained using real time detection of macrophage migration, where peritoneal macrophages showed an increase in migration to CCL19 in the lower chamber within 30 min, whereas for up to 4 h, macrophages treated with 250 ng/ml Sema3E and CCL19 showed no increase migration above that in cells treated with media alone (Fig. 4b). Peritoneal macrophages pre-treated with Sema3E for 45 min, and then washed before exposure to chemokines, also showed impaired migration to CCL19 (Fig. 4c), indicating that Sema3E does not require direct interaction with the chemokine to mediate its inhibitory effect. This effect was not chemokine-specific, as Sema3E also blocked migration of peritoneal macrophages to CCL2 (Fig. 4d), a second chemokine implicated in the emigration of inflammatory macrophages to the lymph nodes25, 26.
Figure 4. Sema3E Inhibits the Migration of Macrophages.
(a) Transwell migration of peritoneal macrophages to CCL19 (500 ng/ml) with or without recombinant Sema3E at the concentrations indicated. (b) Real-time migration (xCelligence) of peritoneal macrophages to CCL19 with or without 250 ng/ml Sema3E. (c) Transwell migration of peritoneal macrophages pre-treated with 250 ng/ml Sema3E for 45 minutes, washed and then exposed to 500 ng/ml CCL19. (b) Transwell migration of peritoneal macrophages to 100 ng/ml CCL2 with or without recombinant Sema3E at the concentrations indicated. Data are the mean ± s.d. of triplicate samples in a single experiment and are representative of an experimental n=3. Statistical analysis was performed by ANOVA followed by Tukey test; (a,c,d) * P<0.01.
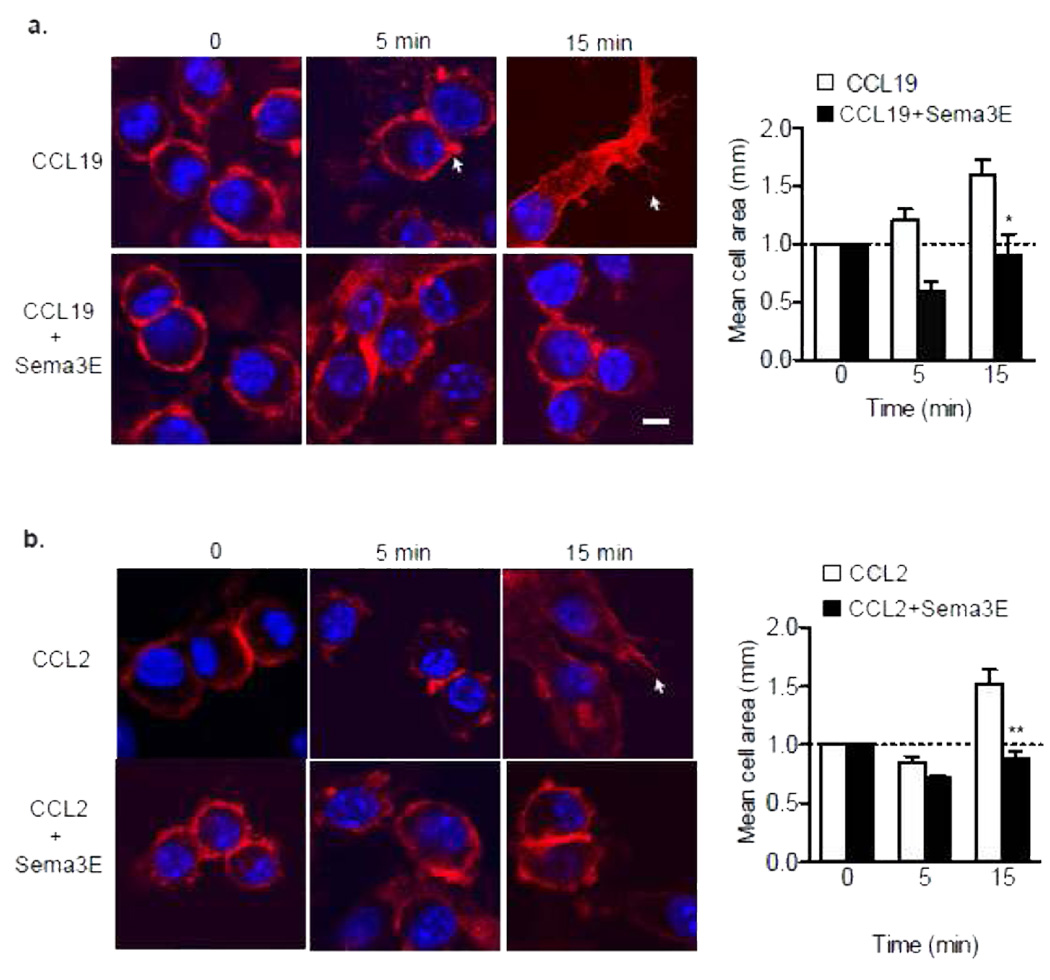
To gain insight into the mechanisms by which Sema3E inhibits directed chemotaxis of macrophages to multiple chemokines, we measured its effect on the organization of the actin cytoskeleton. Stimulation of pMø with CCL19 or CCL2 induced a marked reorganization of actin, characterized by the appearance of membrane ruffles, lamellipodia and filapodia (Fig. 5a-b). Pre-treatment with Sema3E prior to stimulation with CCL19 or CCL2 inhibited these effects, and cells maintained a rounded morphology consistent with their non-motile phenotype. Accordingly, whereas pMø exhibit rapid cell spreading in response to chemokine stimulation, cell pre-treated with Sema3E showed no increase in mean cell area (Fig. 5a-b).
Figure 5. Sema3E Affects the Organization of the Actin Cytoskeleton.
Peritoneal macrophages stained with Phalloidin to detect polymerized actin after treatment with (a) 500 ng/ml CCL19 or (b) 100 ng/ml CCL2 in the presence or absence of recombinant Sema3E (250 ng/mL). Arrows indicate membrane ruffles, scale bar 10 µm. Mean cell surface area of macrophages is graphed at right. Statistical analysis was performed by ANOVA; * P<0.05 compared to CCL19 (a) or CCL2 (b) alone.
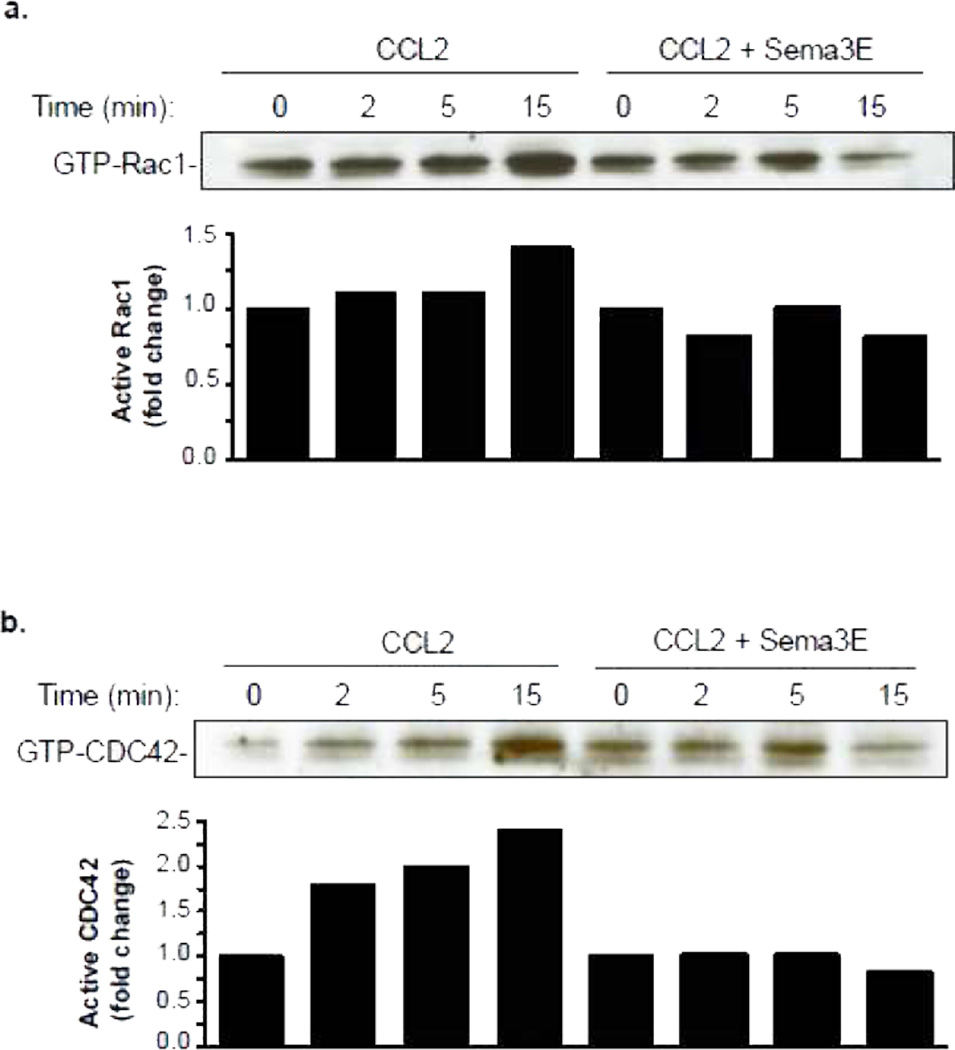
As members of the Rho family of GTPases play key roles in the reorganization of actin in macrophages, we next investigated whether Sema3E altered activation of Rac1 and CDC42 in response to chemotactic stimulation. Using GST beads conjugated to PAK1-PBD to immunoprecipitate the GTP-bound forms of Rac1/CDC42, we show that levels of activated Rac1 and CDC42 were rapidly increased in BMDM stimulated with CCL2 (Fig. 6a-b). By contrast, pretreatment of macrophages with Sema3E inhibited CCL2-induced Rac1 and CDC42 activation. Thus, Sema3E blocks activation of the effectors of lamellipodia (Rac1) and filopodia (CDC42) formation, which are essential components of the migratory machinery. Collectively, these data indicate that Sema3E inhibits the directional migration of macrophages by disrupting the Rho GTPase signaling cascade, re-organization of the actin cytoskeleton and cell polarization.
Figure 6. Sema3E impairs Rho GTPAse signaling.
Immunoblot of activated (a) Rac1 and (b) CDC42 in macrophages treated with 100 ng/ml CCL2 with and without 250 ng/ml Sema3E. Densitometry quantification of blots is shown graphically. Data shown is from one experiment, and is representative of an experimental n=3.
DISCUSSION
Atherosclerosis is a chronic and progressive inflammatory condition, driven by the accumulation of lipids and immune cells in plaques. Current concepts view the persistence of macrophages in plaques as a failure to resolve inflammation27. Recent studies in mouse models of atherosclerosis have revived the hope that plaque macrophage lipid homeostasis and emigration can be clinically restored with aggressive lipid management, leading to the achievement of atherosclerosis regression in humans28. These studies have indicated that macrophage egress from the plaque is likely to be actively inhibited during disease progression, such as with hypercholesterolemia, although the factors and their regulatory signals that impair egress remain largely unknown. In the present studies, we have marshaled support for Sema3E to be one such factor by showing that: 1) It is expressed in mouse atherosclerotic plaques, where there is significant co-localization with monocyte-derived cells; 2) Its expression in vivo is significantly lower in these cells in a plaque regression vs. progression environment; 3) Its expression in vitro is higher in inflammatory M1 macrophages than in tissue remodeling, M2 ones, which are enriched in regressing plaques; and 4) It retards the migration of macrophages in vitro to a number of chemokines, including CCL19, previously shown to be a mediator of macrophage egress from regressing plaques5, apparently by altering cytoskeletal reorganization. Thus, Sema3E is predicted to have a macrophage-retention, anti inflammation-resolving effect in plaques similar to what we recently established for another neuronal guidance molecule, netrin-113.
Neuronal guidance cues are increasingly recognized as important players in immune function and chronic inflammatory diseases, and recent studies have implicated members of the Semaphorin family in the pathogenesis of rheumatoid arthritis (Sema3E, Sema3C)29, 30 and atherosclerosis (Sema4D)31, 32. To our knowledge, this is the first time that Sema3E has been shown to be dynamically regulated in macrophages and identified in atherosclerotic plaques. Previous studies have shown the expression of Sema3E in the outer retina of embryonic chicks, in neurons throughout the ganglion cell layer of P4 mouse retinas, in thalamostriatal projection neurons and developing somites of mice, in mouse medullary thymocytes, and in mouse calvarial osteoblasts11, 19, 24, 33–37. Although there is a lack of understanding of the factors that mediate Sema3E expression in the majority of these cell types, in addition to the factors identified herein ( hyperlipidemia, oxLDL, hypoxia), 1,25-dihydroxyvitamin D3 has been reported to increase Sema3E expression in mouse osteoblasts33. PlexinD1, the canonical receptor for Sema3E, is highly expressed in many cell types, including macrophages38, as we also describe herein. In addition, we find that PlexinD1 is upregulated by oxLDL in vitro and is expressed by macrophages in atherosclerotic plaques.
Sema3E is unique from other Semaphorins in that it binds directly to PlexinD1, and does not require coreceptors to exert its repellant signal36. PlexinD1 is thought to exist at the plasma membrane in an inactive folded state, and upon binding with Sema3E undergoes a conformational change that activates its Guanosine triphosphate (GTP-ase)-Activating-Protein (GAP) domain, which may enable protein-protein interactions with other domains, such as its Rho GTPase-Binding Domain (RBD)39. The Sema3E activation of PlexinD1 can disrupt focal adhesions and integrin-mediated cell adhesion to the extracellular matrix, affect PI3K signaling and cause the RhoA dependant collapse of the cytoskeleton in neurons and endothelial cells10, 39, 40. Activation of PlexinD1 by Sema3E has also been shown to repress CCL25 signaling via its receptor CCR9 in thymocytes24, and to inhibit mouse osteoblast migration in wound healing assays33.
Many of the properties of the Sema3E-PlexinD1 axis in other cell types, then, are consistent with what we observed in macrophages, namely the effects on the cytoskeleton and the inhibition of migration to a number of chemokines, including CCL19, previously implicated as an atherosclerosis regression factor5. Perhaps more striking, then, was the dynamic regulation of Sema3E expression in macrophages by factors related to atherosclerosis. That Sema3E expression at the protein or mRNA level was higher in progressing than in regressing plaques, and increased in vitro by oxLDL and a hypoxia mimic (CoCl2), strongly implies that it participates in the retention of macrophages and the failure to resolve inflammation under pro-atherogenic conditions. This is further supported in a model of in vitro macrophage polarization, in which we found that Sema3E is highly expressed in ‘classically activated’ inflammatory M1 macrophages, which are thought to be the predominant phenotype under conditions that are pro-atherogenic41, but less expressed in the reparative “M2” macrophages, which are considered mediators of inflammatory resolution42, and whose markers are enriched in regressing plaques6, 7, 15, 22, 43 coincident with decreased Sema3e mRNA expression.
Monocyte and macrophage death are well-recognized factors that determine the content of macrophages in mouse models of atherosclerosis27. An emerging picture (recently reviewed in 44) based on the present and prior studies4, 5, 13, 45 is that macrophage egress is also a determinant of plaque macrophage content and that it is regulated by factors that can be broadly classified as gas pedals [emigration signals (e.g. CCR7)], and brakes [inhibitory guidance cues (e.g. Sema3E and netrin-1), adhesion molecules (e.g. cadherins, vinculin), cellular motility factors (e.g. actin and myosin)]15. Based on the hundreds of differences in the transcriptomes of monocyte-derived cells in regressing vs. progressing plaques15, it is likely that the list of gas pedals and brakes, as well as their modifiers, will grow. This will not only expand our fundamental understanding of atherosclerosis and other chronic inflammatory conditions, but will also suggest strategies to reduce the considerable residual risk of heart disease observed with the currently available therapies.
Supplementary Material
SIGNIFICANCE.
This study identifies semaphorin 3E as a negative regulator of macrophage migration that is expressed in progressing atherosclerotic plaques and decreased in regressing plaques. Our data suggest a role for Sema3E in the retention of macrophages in atherosclerosis and highlight the expanding functions of neuroimmune guidance cues in regulating immune cell function.
ACKNOWLEDGEMENTS
a) Acknowledgments: none
b) Sources of Funding: Support for this work came from the National Institutes of Health (RC1HL100815 to K.J.M; R01 HL084312 to EAF; predoctoral fellowship AG-029748 to J.E.F.), American Heart Association (Grant-in-aid 0655840T to K.J.M; 09POST2080250 to J.M.vG), Canadian Institute of Health Research (CGS-MSFSS to T.S., URC #57093; IGO 94418 to EO’B) and German Research Foundation (DFG: HE 6092/1-1 to BH).
Abbreviations
- Sema3E
Semaphorin 3E
- oxLDL
oxidized LDL
- AcLDL
acetylated LDL
- BMDM
bone marrow derived macrophage
- HIF-1a
hypoxia inducible factor 1 alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: none
REFERENCES
- 1.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye D, Zhao Y, Hildebrand RB, Singaraja RR, Hayden MR, Van Berkel TJ, Van Eck M. The dynamics of macrophage infiltration into the arterial wall during atherosclerotic lesion development in low-density lipoprotein receptor knockout mice. Am J Pathol. 2011;178:413–422. doi: 10.1016/j.ajpath.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: Inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol. 1996;157:2577–2585. [PubMed] [Google Scholar]
- 4.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor ccr7 during atherosclerosis regression in apoe-deficient mice. Proc Natl Acad Sci U S A. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner KJ, Moore KJ, Garabedian MA, Fisher EA. Hdl promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ. Antagonism of mir-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feig JE, Pineda-Torra I, Sanson M, Bradley MN, Vengrenyuk Y, Bogunovic D, Gautier EL, Rubinstein D, Hong C, Liu J, Wu C, van Rooijen N, Bhardwaj N, Garabedian M, Tontonoz P, Fisher EA. Lxr promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J Clin Invest. 2010;120:4415–4424. doi: 10.1172/JCI38911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melani M, Weinstein BM. Common factors regulating patterning of the nervous and vascular systems. Annu Rev Cell Dev Biol. 2010;26:639–665. doi: 10.1146/annurev.cellbio.093008.093324. [DOI] [PubMed] [Google Scholar]
- 10.Perala N, Sariola H, Immonen T. More than nervous: The emerging roles of plexins. Differentiation. 2012;83:77–91. doi: 10.1016/j.diff.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Sakurai A, Gavard J, Annas-Linhares Y, Basile JR, Amornphimoltham P, Palmby TR, Yagi H, Zhang F, Randazzo PA, Li X, Weigert R, Gutkind JS. Semaphorin 3e initiates antiangiogenic signaling through plexin d1 by regulating arf6 and r-ras. Mol Cell Biol. 2010;30:3086–3098. doi: 10.1128/MCB.01652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirulli V, Yebra M. Netrins: Beyond the brain. Nat Rev Mol Cell Biol. 2007;8:296–306. doi: 10.1038/nrm2142. [DOI] [PubMed] [Google Scholar]
- 13.van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL, Alvarez-Leite JI, Rayner AJ, McDonald TO, O'Brien KD, Stuart LM, Fisher EA, Lacy-Hulbert A, Moore KJ. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–143. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chereshnev I, Trogan E, Omerhodzic S, Itskovich V, Aguinaldo JG, Fayad ZA, Fisher EA, Reis ED. Mouse model of heterotopic aortic arch transplantation. J Surg Res. 2003;111:171–176. doi: 10.1016/s0022-4804(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 15.Feig JE, Vengrenyuk Y, Reiser V, Wu C, Statnikov A, Aliferis CF, Garabedian MJ, Fisher EA, Puig O. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PLoS One. 2012;7:e39790. doi: 10.1371/journal.pone.0039790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trogan E, Choudhury RP, Dansky HM, Rong JX, Breslow JL, Fisher EA. Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein e-deficient mice. Proc Natl Acad Sci U S A. 2002;99:2234–2239. doi: 10.1073/pnas.042683999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura F, Kalb RG, Strittmatter SM. Molecular basis of semaphorin-mediated axon guidance. J Neurobiol. 2000;44:219–229. doi: 10.1002/1097-4695(200008)44:2<219::aid-neu11>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Raper JA. Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol. 2000;10:88–94. doi: 10.1016/s0959-4388(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 19.Steinbach K, Volkmer H, Schlosshauer B. Semaphorin 3e/collapsin-5 inhibits growing retinal axons. Exp Cell Res. 2002;279:52–61. doi: 10.1006/excr.2002.5595. [DOI] [PubMed] [Google Scholar]
- 20.Puschel AW. Semaphorins: Repulsive guidance molecules show their attractive side. Nat Neurosci. 1999;2:777–778. doi: 10.1038/12140. [DOI] [PubMed] [Google Scholar]
- 21.Trogan E, Fayad ZA, Itskovich VV, Aguinaldo JG, Mani V, Fallon JT, Chereshnev I, Fisher EA. Serial studies of mouse atherosclerosis by in vivo magnetic resonance imaging detect lesion regression after correction of dyslipidemia. Arterioscler Thromb Vasc Biol. 2004;24:1714–1719. doi: 10.1161/01.ATV.0000139313.69015.1c. [DOI] [PubMed] [Google Scholar]
- 22.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–998. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yvan-Charvet L, Wang N, Tall AR. Role of hdl, abca1, and abcg1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi YI, Duke-Cohan JS, Ahmed WB, Handley MA, Mann F, Epstein JA, Clayton LK, Reinherz EL. Plexind1 glycoprotein controls migration of positively selected thymocytes into the medulla. Immunity. 2008;29:888–898. doi: 10.1016/j.immuni.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimenez F, Quinones MP, Martinez HG, Estrada CA, Clark K, Garavito E, Ibarra J, Melby PC, Ahuja SS. Ccr2 plays a critical role in dendritic cell maturation: Possible role of ccl2 and nf-kappa b. Journal of immunology. 2010;184:5571–5581. doi: 10.4049/jimmunol.0803494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato N, Ahuja SK, Quinones M, Kostecki V, Reddick RL, Melby PC, Kuziel WA, Ahuja SS. Cc chemokine receptor (ccr)2 is required for langerhans cell migration and localization of t helper cell type 1 (th1)-inducing dendritic cells. Absence of ccr2 shifts the leishmania major-resistant phenotype to a susceptible state dominated by th2 cytokines, b cell outgrowth, and sustained neutrophilic inflammation. The Journal of experimental medicine. 2000;192:205–218. doi: 10.1084/jem.192.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore KJ, Tabas I. Macrophage in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: Insights from the clinical and experimental literature. Nature clinical practice. Cardiovascular medicine. 2008;5:91–102. doi: 10.1038/ncpcardio1086. [DOI] [PubMed] [Google Scholar]
- 29.Mangasser-Stephan K, Dooley S, Welter C, Mutschler W, Hanselmann RG. Identification of human semaphorin e gene expression in rheumatoid synovial cells by mrna differential display. Biochem Biophys Res Commun. 1997;234:153–156. doi: 10.1006/bbrc.1997.6607. [DOI] [PubMed] [Google Scholar]
- 30.Miller LE, Weidler C, Falk W, Angele P, Schaumburger J, Scholmerich J, Straub RH. Increased prevalence of semaphorin 3c, a repellent of sympathetic nerve fibers, in the synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 2004;50:1156–1163. doi: 10.1002/art.20110. [DOI] [PubMed] [Google Scholar]
- 31.Yukawa K, Tanaka T, Kishino M, Yoshida K, Takeuchi N, Ito T, Takamatsu H, Kikutani H, Kumanogoh A. Deletion of sema4d gene reduces intimal neovascularization and plaque growth in apolipoprotein e-deficient mice. Int J Mol Med. 2010;26:39–44. doi: 10.3892/ijmm_00000432. [DOI] [PubMed] [Google Scholar]
- 32.Zhu L, Stalker TJ, Fong KP, Jiang H, Tran A, Crichton I, Lee EK, Neeves KB, Maloney SF, Kikutani H, Kumanogoh A, Pure E, Diamond SL, Brass LF. Disruption of sema4d ameliorates platelet hypersensitivity in dyslipidemia and confers protection against the development of atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1039–1045. doi: 10.1161/ATVBAHA.109.185405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes A, Kleine-Albers J, Helfrich MH, Ralston SH, Rogers MJ. A class iii semaphorin (sema3e) inhibits mouse osteoblast migration and decreases osteoclast formation in vitro. Calcif Tissue Int. 2012;90:151–162. doi: 10.1007/s00223-011-9560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S, Uemura A. Sema3e-plexind1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest. 2011;121:1974–1985. doi: 10.1172/JCI44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding JB, Oh WJ, Sabatini BL, Gu C. Semaphorin 3e-plexin-d1 signaling controls pathwayspecific synapse formation in the striatum. Nat Neurosci. 2012;15:215–223. doi: 10.1038/nn.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3e and plexin-d1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 37.Klagsbrun M, Shimizu A. Semaphorin 3e, an exception to the rule. J Clin Invest. 2010;120:2658–2660. doi: 10.1172/JCI44110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janabi M, Yamashita S, Hirano K, Sakai N, Hiraoka H, Matsumoto K, Zhang Z, Nozaki S, Matsuzawa Y. Oxidized ldl-induced nf-kappa b activation and subsequent expression of proinflammatory genes are defective in monocyte-derived macrophages from cd36-deficient patients. Arterioscler Thromb Vasc Biol. 2000;20:1953–1960. doi: 10.1161/01.atv.20.8.1953. [DOI] [PubMed] [Google Scholar]
- 39.Gay CM, Zygmunt T, Torres-Vazquez J. Diverse functions for the semaphorin receptor plexind1 in development and disease. Dev Biol. 2011;349:1–19. doi: 10.1016/j.ydbio.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, He H, Srivastava N, Vikarunnessa S, Chen YB, Jiang J, Cowan CW, Zhang X. Plexins are gtpase-activating proteins for rap and are activated by induced dimerization. Sci Signal. 2012;5:ra6. doi: 10.1126/scisignal.2002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A, Caligiuri G. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teupser D, Burkhardt R, Wilfert W, Haffner I, Nebendahl K, Thiery J. Identification of macrophage arginase i as a new candidate gene of atherosclerosis resistance. Arterioscler Thromb Vasc Biol. 2006;26:365–371. doi: 10.1161/01.ATV.0000195791.83380.4c. [DOI] [PubMed] [Google Scholar]
- 43.Parathath S, Grauer L, Huang LS, Sanson M, Distel E, Goldberg IJ, Fisher EA. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes. 2011;60:1759–1769. doi: 10.2337/db10-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerszten RE, Tager AM. The monocyte in atherosclerosis--should i stay or should i go now? The New England journal of medicine. 2012;366:1734–1736. doi: 10.1056/NEJMcibr1200164. [DOI] [PubMed] [Google Scholar]
- 45.Ly NP, Komatsuzaki K, Fraser IP, Tseng AA, Prodhan P, Moore KJ, Kinane TB. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci U S A. 2005;102:14729–14734. doi: 10.1073/pnas.0506233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.