Abstract
Interleukin-15 (IL-15) is an important IL-2 related cytokine, whose role in Th17 cell biology has not been fully elucidated. Here we show that exogenous IL-15 decreased IL-17A production in Th17 cultures. Neutralizing IL-15 using an antibody led to increases in IL-17A production in Th17 cultures. Both Il15−/− and Il15r−/− T cell cultures displayed higher frequency of IL-17A producers and higher amounts of IL-17A in the supernatants, compared to WT cells in vitro. IL-15 downmodulated IL-17A production, independently of ROR-γt, Foxp3 and IFN-γ expression. Both Th17 and APC cells produced IL-15, which induced binding of STAT5, an apparent repressor to the Il17 locus in CD4 T cells. Also, in a model of myelin oligodendrocyte glycoprotein (MOG) induced EAE, Il15−/− mice displayed exacerbated inflammation, correlating with increased IL-17A production by their CD4+ T cells, compared to WT controls. Exogenous IL-15 administration and IL-17A neutralization reduced the severity of EAE in Il15−/− mice. Taken together, these data indicate that IL-15 has a negative regulatory role in fine-tuning IL-17A production and Th17 mediated inflammation.
T helper (Th) 17 cells are a subset of CD4+ T lymphocytes that are implicated in host defense against extracellular bacteria and fungi (1–4). Although Th17 responses are important for host defense, they also contribute to immunopathology in various disease settings. Th17 responses have been documented in chronic inflammation and autoimmune diseases such as inflammatory bowel disease (IBD), multiple sclerosis (MS) and rheumatoid arthritis (RA) (5, 6). Because both protective and harmful effects of Th17 cells during infection and chronic inflammation have been described, it is critical to study how Th17 responses are regulated in vivo. At the molecular level, transcription factors such as RAR-related orphan receptor (ROR) -α and -γt and Signal-Transducer and Activator of Transcription (STAT) 3 and cytokines such as IL-6, IL-21 and IL-23 promote Th17 differentiation (7, 8). On the other hand, STAT5, IL-2 and IL-27 negatively regulate Th17 responses (9–12). However, the function of IL-15, a key cytokine for the development and proliferation of CD8 T cells and natural killer (NK) T cells, in regulating Th17 responses has not been fully elucidated.
IL-15 is one of the common gamma chain (γc) cytokines similar and structurally homologous to IL-2 and has pleiotropic roles in immune system development and lymphocyte homeostasis (13, 14). The IL-15 receptor (R) consists of IL-15Rα (CD215), IL-2Rβ (CD122), and the common γ (CD132) chains. In addition to sharing the β and γc receptor subunits, IL-2 and IL-15 also share biological actions namely, anti-apoptotic effects on T cells, stimulation of natural killer (NK) cell proliferation, and cytolytic activity (15–18). However, striking differences are observed between the phenotypes of IL-2 and IL-15 knockout (−/−) mice (17). This may be partly due to differences in the expression patterns of these two cytokines (19). Whereas IL-2 is expressed primarily by activated CD4 T cells, IL-15 is expressed mainly in non-lymphoid cells including the parenchymal cells of many organs, keratinocytes, skeletal muscle cells, and antigen presenting cells (APC) such as monocytes and dendritic cells (DC) (20–24). In the nervous system, IL-15 is expressed by neurons, astrocytes, and microglia (25, 26). Although initially it was believed that IL-15 is expressed in lymphocytes, recent studies have suggested that activated lymphocytes indeed express IL-15 (21–24). The major cells responding to IL-15 are lymphocytes, especially NK and memory T cells. Macrophage- and dendritic cell (DC)-derived IL-15Rα binds to N-glycosylated IL-15 within the endoplasmic reticulum and brings IL-15 to the cell surface to deliver membrane-bound agonistic signals. This cell-contact dependent mechanism, called trans-presentation, is an important step in transmitting physiological IL-15Rα-IL-15 signals to most cells (27). Following receptor engagement, IL-15 signaling activates both STAT3 and STAT5 transcription factors, to exert its regulatory functions in immune cells (28).
IL-15 has been implicated in a variety of inflammatory conditions. Increased circulating IL-15 levels have been found in Crohn’s and ulcerative colitis patients, implying IL-15 promotes the disease (29, 30). However, one study has shown that IL-15 protects against colitis in mice (31). Whether IL-15 controls IBD directly by regulating Th17 cells is unknown. In patients with RA, IL-15 is considered pro-inflammatory, and blocking IL-15 using anti-IL-15 monoclonal antibody (HuMax-IL-15), ameliorated symptoms (32). In the mouse collagen-induced arthritis (CIA) model, although WT and Il15−/− mice showed equal susceptibility to the disease, transgenic over-expression of IL-15 increased the disease severity by enhancing the proliferation of CD4 T cells and their production of IFN-γ and IL-17A (33). Again, whether IL-15 exerts a direct effect on IL-17A expression in CD4 cells and its role in induction of arthritis was unclear. Elevated IL-15 is also observed in peripheral blood mononuclear cells, serum and cerebrospinal fluid of MS patients (34–36). IL-15 produced by astrocytes in MS lesions may promote CD8 T cell functions in the experimental autoimmune encephalomyelitis (EAE) model in mice (37). On the other hand, two independent studies have shown that Il15−/− mice exhibit heightened EAE, implying that IL-15 suppresses rather than promotes inflammation (38, 39). However, the mechanism by which IL-15 suppresses EAE and its role in Th17 responses has not been investigated.
It has recently been shown that IL-2 and IL-21, members of the same γc cytokine family, perform opposite functions in restraining and promoting Th17 cells respectively (8, 40). IL-15, known to activate both STAT3 and STAT5 (28), may be inferred to either inhibit or promote Th17 cells. Hence, elucidating the exact mechanism of IL-15 action on Th17 cells is important and may shed new light on the mechanisms of autoimmune and inflammatory conditions.
In this study, we identify a novel mechanism by which IL-15 mediates inhibition of Th17 differentiation in vitro and immunopathology in the EAE model. We find that both Th17 cells and APC produce IL-15, which serves to control IL-17A production by activating STAT5 and inducing its binding to Il17a promoter in CD4+ T cells. Thus, our data show the mechanistic details of inhibitory action of IL-15 on IL-17A production in CD4+ T cells, both in vitro and during EAE inflammation in vivo.
Materials and Methods
Mice
C57BL/6 WT or Rag2−/−, Il15r−/− mice and the appropriate control mice were purchased from Jackson Laboratories. C57BL/6, Il15−/− mice, CD45.1 mice in Rag1−/− background were purchased from Taconic farms (Germantown, NY). All animal experiments were done at NIAID under an approved protocol and in compliance with the NIAID Institutional Animal Care and Use Committee’s guidelines.
Reagents and antibodies
Purified α-CD3 (145-2C11), α-CD28, α-CD25 (3C7), CD4, CD25, IL-2, IL-4 and IFN-γ antibodies were all purchased from BD Biosciences (San Diego, CA). Purified IL-17F, IL-17A, TNF-α, Foxp3, CD45.1, CD45.2, antibodies were purchased from eBiosciences (San Diego, CA). PE-conjugated IL-22 antibody was purchased from R&D Systems (Minneapolis, MN). Mouse CD4 isolation kit II, and anti-Biotin micro-beads were purchased from Miltenyi Biotec (Auburn, CA). IL-2 and IL-17A Quantikine ELISA kits, recombinant mouse IL-6, IL-2 and human TGF-β were purchased from R & D systems. IL-15 protein levels were determined by ELISA using antibodies from R&D systems. Mouse cells were cultured in complete RPMI-1640 (Bio-Whittaker) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM glutamine and 50 µM β-mercaptoethanol.
Th17 differentiation
CD4+ CD44low CD62Lhigh CD25− naïve responder T cells (5 ×104) were co-cultured in U-bottom 96 well plates in the presence of soluble 1 µg/ml α-CD3 and 3 µg/ml α-CD28 antibodies under Th0 or Th17 conditions for 3–8 days. Th0 cells were stimulated only with α-CD3 and α-CD28 antibodies with no added cytokines and Th17 cells were polarized using IL-6 (20 ng/ml), TGF-β (2 ng/ml), α-IFN-γ (6 µg/ml) and α-IL-4 (6 µg/ml). Where indicated, APCs were added at a T cell:APC ratio of 1:1 and T cells were carboxyfluorescein succinimidyl ester (CFSE) labeled to assess their proliferation. When indicated, cytokines, α-IL-15 (20 µg/ml) and α-IL-2 (10 µg/ml) were added at the beginning of stimulation of co-cultures.
Quantitative PCR (q-PCR) analyses
For q-PCR analyses of ROR-α, ROR-γt, Foxp3 IL-17A mRNA, naïve CD4 T cells were stimulated in Th17 cultures as above and RNA was recovered using an RNeasy Micro Kit (Qiagen). In some experiments, CD4 cells were first separated from APC using CD90 magnetic beads (Miltenyi Biotech, Auburn CA). DNase was added to the columns during RNA purification. cDNA was synthesized from total RNA using Superscript III Reverse Transcriptase plus random primers (Invitrogen), and purified on a MinElute spin column (Qiagen) following treatment with RNase A plus RNase H and extraction with phenol/chloroform. Five nano grams of cDNA was used in one reaction using a QuantiFast SYBR Green PCR Kit (Qiagen) in a real time PCR machine ABI7900HT (Applied Biosystems). All primers (Invitrogen) for PCR were designed to amplify a coding region within a single exon. The relative amount of cDNA of interest was estimated from its Ct value plotted on a standard curve acquired from the Ct values of a diluted series of genomic DNA. These quantified amounts were normalized to the amount of ribosomal 18S RNA, assigning values of “1” or “100” to unstimulated control samples. The following primer sequences were used: 18S: 5’-AAACGGCTACCACATCCAAG-3’ 5’-ATTCCAATTACAGGGCCTCG-3’, β-actin: 5’-GGAATGGGTCAGAAGGACT 3’, 5’ CGTCCCAGTTGGTAACAATG-3’, R O R-α: 5 ’-TTGGTCGGATGTCCAAGAAG 3’, 5’-TGGCTGAGATGTTGTAGGTG-3’, ROR-γt: 5’-CAGTCTACATGCAGAAGTGC-3’, 5’-ATGTAAGTGTGTCTGCTCCG-3’, FoxP3 : 5 ’-CAAGGGCTCAGAACTTCTAG-3’, 5’-GGTTCAAGGAAGAAGAGGTG-3’, IL-17A: 5’-TCCAGAAGGCCCTCAGACTA-3’, 5’-AGCATCTTCTCGACCCTGAA-3’, IL-1 5 : 5’-AATCCACCTTGACACATGGC-3’, 5’-AGGCTGGTTATCTGCTGACA-3’.
Chromatin Immunoprecipitation (ChIP)-qPCR
ChIP assays were performed using Th17 cells stimulated for 4 or 5 days in cultures. In brief, after being fixed in 1% formaldehyde, T cells were lysed for 10 minutes on ice. Chromatin was sheared by sonication in Branson 2510 digital sonifier. Lysates equivalent to 107 cells were used for each immunoprecipitation. After being precleared with protein-A agarose beads (Upstate), cell lysates were immunoprecipitated overnight at 4 °C with 2 µl of STAT5A-STAT5B (PA-ST5A and PA-ST5B; R&D Systems). After washing and elution, crosslinks were reversed for 4 h at 65 °C. Eluted DNA was purified and samples were analyzed by quantitative PCR with Taqman qPCR Mastermix (ABI, Roche N08430) and custom-designed primers and probes. Enrichment of chromatin was analyzed using the 7500 Fast Real-Time PCR instrument (Applied Biosystems). Data were normalized to input values and expressed as “% input”. Primer-probe sets used to detect the enrichment of STAT5 binding sites in Il17a locus were: P2: Forward: 5’-CACCTCACACGAGGCACAAG-3’, Reverse: 5’-ATGTTTGCGCGTCCTGATC-3’, Probe 2: 5’-CACCCAGCACCAGC-3’, P3: Forward: 5’-GGATTAAGGGCACACGTGTTG-3’, Reverse: 5’-TTTCCCCACTCTGTCTTTCCA-3’, Probe 3: 5’-TGGCCCAATGTTTATT-3’, P4: Forward: 5’-TCATCGGCTCCCACACAGA-3’, Reverse: 5’-GGCAGTACCGAAGCTGTTTCA-3’, Probe 4: 5’-CATGCCGCACTCAA-3’. Primer-probe sets used to detect the enrichment of STAT5 non binding sites in Il17a locus were NB1: Forward: 5’-CACCAGCCAACCCAATTAAAA-3’, Reverse: 5’-CCTTCCTCATGTGATATGGCAAA-3’, Probe: 5’-TAGTCACTTTACTAATGGAGACCA-3’, NB2: Forward: 5’-CCAGCCCTTGGGAAGCA-3’, Reverse: 5’ CCAGGGTGGCTTCAAACTCA-3’, Probe: 5’-AGGCAGACAAGTTC-3’
Retrovirus transduction
Constitutively active STAT5-IRES-GFP pMIGR and control GFP retroviral supernatants (gift from Dr. William E. Paul, NIAID) were used for transduction. 2 × 106 naive CD4+ T cells were plated in one well of a 24 well plate and were stimulated using soluble 1 µg/ml of anti-CD3, and 2 µg/ml of anti-CD28 under Th0 conditions for 48 hr. 0.5ml of retrovirus supernatants were added to the cells, replacing 0.5 ml of medium from the cultures, and incubated for 20 minutes in the incubator. The cells were spun at 2500 rpm for 1.5 hr at room temperature with 8 µg/ml of polybrene (Sigma). After 24 hr, infected cells were differentiated under Th17 cell conditions for 48 hr and stained for intracellular cytokines.
Tissue histology
For immunocytochemical staining, colon tissues were washed with PBS, fixed with 10% formalin overnight and suspended in 70% ethanol to prevent over fixation. Paraffin sectioning and Hematoxylin & Eosin (H&E) staining were performed by Histoserv, Inc, MD.
Intracellular staining of cytokines and STAT proteins and Flow cytometry
For single-cell staining, cells were cultured in Th17 conditions, washed in PBS, fixed with CytoFix/Cytoperm kit (BD BioSciences). Before fixation, co-cultures were re-stimulated with phorbol myristate acetate (PMA) (50 ng/ml) and Ionomycin (500 ng/ml) for 4 hours, with brefeldin-A (10 µg/ml) added in the last 2 hours. For pSTAT3 and pSTAT5 staining, the cells were washed, fixed and were stained with Phosflow staining kit from BD Biosciences using the manufacturer’s protocol. Data were acquired using BD FACSCalibur cytometers and were analyzed using FlowJo 9.1 software.
EAE in mice
EAE experiments were performed using the MOG/complete freund’s adjuvant (CFA) and pertussis toxin kit (# EK0115) from Hooke’s laboratories. EAE was induced in age and sex matched C57BL/6 or Il15−/− mice, at least 5 per group, according to the Hooke’s laboratory protocol. EAE was scored as follows: 0, healthy; 1, flaccid tail; 1.5, flaccid tail and impaired righting reflex; 2, impaired righting reflex and hind limb weakness; 2.5, one hind leg paralyzed; 3, both hind legs paralyzed with residual mobility in both legs; 3.5, both hind legs completely paralyzed; 4, both hind legs completely paralyzed and beginning front limb paralysis; 5, moribidity or death of the animal after preceding clinical disease.
IBD induction by Th17 cell transfer in vivo
CD45.1 Rag1−/− mice received 4 × 105 WT or Il15r−/− CD45.2 Th17 cells by intraperitoneal injection (in 100 µl PBS). CD45.2 CD25− CD44lowCD62LhighCD4+ naïve cells were cultured under Th17 polarizing conditions and were used as the source of Th17 cells for the injections. Histology scores were assigned as follows: 1, Low level of leukocyte infiltration with infiltration seen in <10% of the section. No structural changes; 2, Moderate leukocyte infiltration with infiltration seen in 10% to 25% of the section, crypt elongation and bowel wall thickening that does not extend beyond the mucosal layer; 3, high level of leukocyte infiltration seen in 25% to 50%, crypt elongation, infiltration beyond the mucosal layer, thickening of the bowel wall and mild loss of goblet cells; 4, marked degree of leukocyte infiltration seen in >50% of the section, elongated crypts, prominent loss of goblet cells and bowel-wall thickening; and 5, leukocyte infiltration seen in >70% and elongated and distorted crypts, bowel-wall thickening, significant or complete loss of goblet cells and extensive ulcerations.
Statistical analyses
P values were calculated by Students t-test in Microsoft Excel software using unpaired, two tailed distribution and two-sample equal variance parameters or unpaired t-test and Mann-Whitney test in Prism 4.0 (GraphPad Software, Inc.).
Results
IL-15 downregulates IL-17A in Th17 cells in vitro
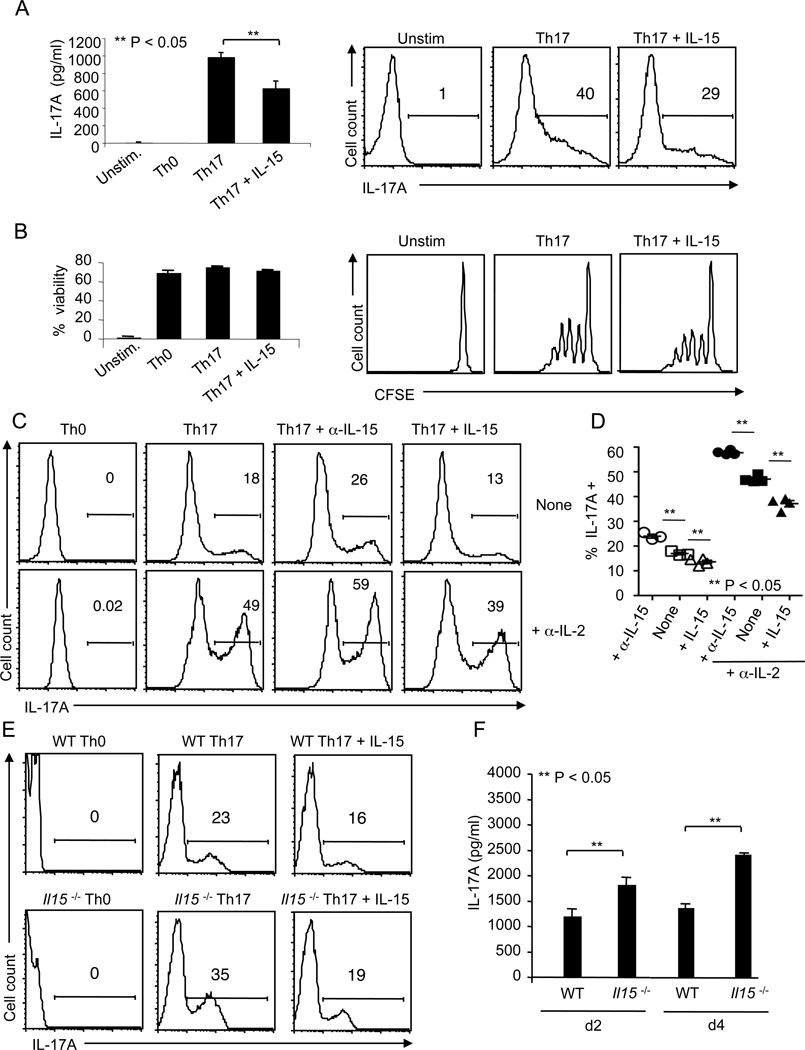
IL-15 is an activator of both STAT3 and STAT5 transcription factors, which are shown to perform opposing roles in regulating Th17 cells (28, 40). IL-15 may induce STAT3 activation to promote Th17 differentiation. Alternatively, by inducing STAT5, IL-15 may restrict Th17 cells. To test these possibilities, we stimulated CD4+ CD44low CD62Lhigh CD25− naïve T cells under Th17 polarizing conditions (8), consisting of 1 µg/ml soluble α-CD3 antibody and antigen presenting cells (APCs) with 20 ng/ml IL-6, 2 ng/ml TGF-β, 5 µg/ml α-IFN-γ, and 5 µg/ml α-IL-4, in the presence of IL-15. As controls, we also tested other individual γc cytokines including IL-2, IL-21 and IL-7. While IL-2 suppressed and IL-21 enhanced IL-17A production as shown by others (8, 40, 41), IL-7 moderately restrained IL-17A induction (data not shown). When IL-15 was added at the beginning of stimulation under Th17 polarizing conditions, IL-17A produced by CD4 cells was reduced in a dose dependent manner, as assessed by enzyme linked immunosorbent assay (ELISA) and intracellular staining on day 4 (d4) after stimulation (Fig.1A, S1). The unstimulated cells showed no detectable IL-17A (Fig.1A). However, the survival and proliferation, as assessed by propidium iodide staining and CFSE dilution, were unaffected by IL-15 (Fig.1B). Because the effect of IL-15 was only moderate, we speculated that the inhibitory effect of endogenous IL-2 in the cultures could mask the effects of IL-15 (10, 40). Therefore, we stimulated the cells under Th17 polarizing conditions with soluble α-CD3 and α-CD28 for 5 days in the presence or absence of α-IL-2 blocking antibody. In some experiments, α-IL-15 blocking antibody was also used. As expected, blocking IL-2 dramatically increased the frequency of IL-17A+ cells (Fig. 1C, D). In addition, blocking IL-15 further increased the frequency of IL-17A producers. Also, when we added exogenous IL-15, it strikingly decreased the frequency of IL-17A expressing cells (Fig. 1 C, D). Considering the possibility that endogenous IL-15 produced in cultures could also contribute to IL-17A suppression, we used Il15−/− T cells in our Th17 conditions. On d4 after stimulation, Il15−/− cells displayed increased frequency of IL-17A producers compared to WT T cells, and addition of IL-15 caused the frequency to drop to nearly the same level as WT cells treated with IL-15 (Fig.1E). ELISA data showing that IL-17A in the supernatants from Il15−/− cell cultures was increased compared to WT controls on d2 and d4, validated the flow cytometry data (Fig.1F). No detectable levels of IL-17A were found in WT control and Il15−/− Th0 cells (data not shown). Taken together, these results reveal that IL-15 plays an important role in limiting IL-17A production in differentiating Th17 cells.
Figure 1. IL-15 decreases the frequency of IL-17A+ cells and IL-17A production in Th17 cultures.
A) Naïve T cells were not stimulated (Unstim.) or cultured under Th0 or Th17 conditions along with or without 20 ng/ml of recombinant mouse IL-15 for 4 days. IL-17A levels were assessed in culture supernatants by ELISA (left). Intracellular cytokine staining was performed in PMA/ionomycin restimulated cells. Data from flow cytometric analyses show the percentage of IL-17A+ cells (right). (B) Cells were stimulated as in (A). The percentage of viable cells (propidium iodide negative cells, left) and proliferation (CFSE dilution, right) were assessed by flow cytometry. (C) Naïve CD4 cells were stimulated under Th17 skewing conditions using anti-CD3 and anti-CD28, with or without α-IL-2 blocking antibody (10 µg/ml) for 5 days. α-IL-15 antibody and IL-15 were added at 50 µg/ml and 20 ng/ml, respectively. Gates were drawn based on “Unstained” and “Th0” controls (not shown). (D) Cells were stimulated as in (C). The frequency of IL-17A+ cells on d4 from 3 independent experiments is depicted. Data points +/− SEM are plotted. (E) WT naïve CD4 cells were stimulated with WT APC and Il15−/− naïve cells were stimulated with Il15−/− APC. They were stained for IL-17A after 3 days of stimulation (gated on CD4 cells). (F) ELISA quantification of IL-17A in the supernatants of cultures stimulated as in (E) under Th17 conditions. The results are representative of at least 3 independent experiments.
Activated Th17 cells and APC produce IL-15 in Th17 cultures
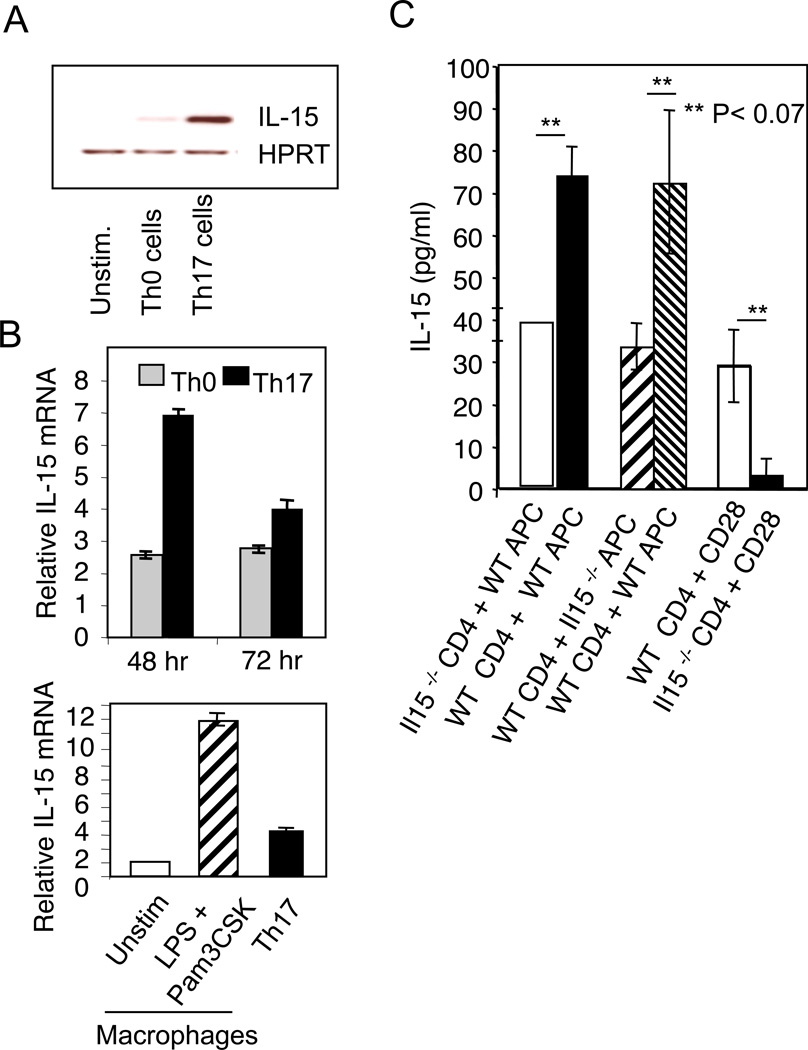
Next we sought to clearly delineate the source(s) of IL-15 in Th17 cultures. Although APCs are likely sources of IL-15, we speculated that like IL-2, endogenous IL-15 produced by Th17 cells themselves could be an autocrine negative feedback regulator restricting IL-17 production. This tenet was based on our finding that Th17 cells exposed to anti-IL-15 blocking antibody, had increased IL-17A production in the absence of APC (Fig. 1C, D). Although it has been reported previously that activated CD4 cells express IL-15, they are not considered to be an immunologically important source of IL-15 (21, 22, 24). Therefore we separated CD4 cells from Th0 or Th17 cultures stimulated with soluble α-CD3 and APC and measured steady state IL-15 mRNA. As previously reported, on d3 after stimulation, we detected IL-15 mRNA in activated Th0 cells (22). However, we observed higher levels of steady state IL-15 mRNA in Th17 cells (Fig. 2A). Furthermore, quantitative real time PCR (q-RT PCR) confirmed higher levels of IL-15 mRNA in flow cytometrically sorted naïve cells that were differentiated under Th17 conditions (Fig. 2B). As a positive control, we measured IL-15 mRNA in macrophages that were either left unstimulated or activated with LPS and Pam3CSK4 and found that the activated macrophages expressed significantly higher levels of IL-15 compared to T cells and unstimulated macrophages (24, 42). We validated the results by performing an ELISA to detect IL-15 protein levels in Th17-APC co-cultures. We quantified IL-15 protein levels in co-cultures where WT or Il15−/− Th17 cells were stimulated with WT APC. We also stimulated WT Th17 cells with either WT or Il15−/− APC. We observed obvious reduction of IL-15 expression in the co-cultures where either the Th17 cells or APCs were derived from IL-15 deficient mice (Fig. 2C). Thus, both T cells and APC contribute to IL-15 in Th17 cultures stimulated with α-CD3 and APC. To test whether Th17 cells produce IL-15 independently of APC, we stimulated WT or Il15−/− CD4 T cells alone with α-CD3 and α-CD28 in the absence of APC. We found that WT, but not Il15−/− Th17 cells produced IL-15, verifying that Th17 cells can express IL-15 independently of APC (Fig. 2C). Taken together, these results reveal that Th17 cells and APC produce IL-15, downmodulating IL-17A production in differentiating Th17 cells.
Figure 2. IL-15 is produced by both Th17 cells and APCs in Th17 cultures.
(A) Th0 or Th17 were stimulated in the presence of APC as in Fig.1E. 48 hrs after stimulation, RNA was isolated from CD4 cells that were magnetically separated to measure the IL-15 RNA levels by reverse transcription and semi-quantitative PCR. Unstimulated naïve CD4 cells were used as a control. As a housekeeping control gene we assessed the levels of HPRT RNA. (B) Naive CD4 cells were flow cytometrically sorted and stimulated under Th0 or Th17 conditions using anti-CD3 and anti-CD28 in the absence of APC. 48 or 72 hrs after stimulation, we isolated RNA and assessed the IL-15 RNA levels by quantitative PCR (upper panel). Macrophages derived from splenic CD14+ monocytes were left unstimulated or stimulated with LPS and Pam3CSK4 (each 10 µg/ml) for 24 hours and analyzed similarly (lower panel). Relative values are calculated normalizing to unstimulated naïve CD4 cell controls. (C) ELISA quantification of IL-15 in cultures stimulated as in (A) under indicated conditions for 3 days. Results represent the mean +/− SD. At least three independent experiments showed similar results.
IL-15 limits IL-17A and IL-17F production independently of IFN-γ, Foxp3 and RORγt
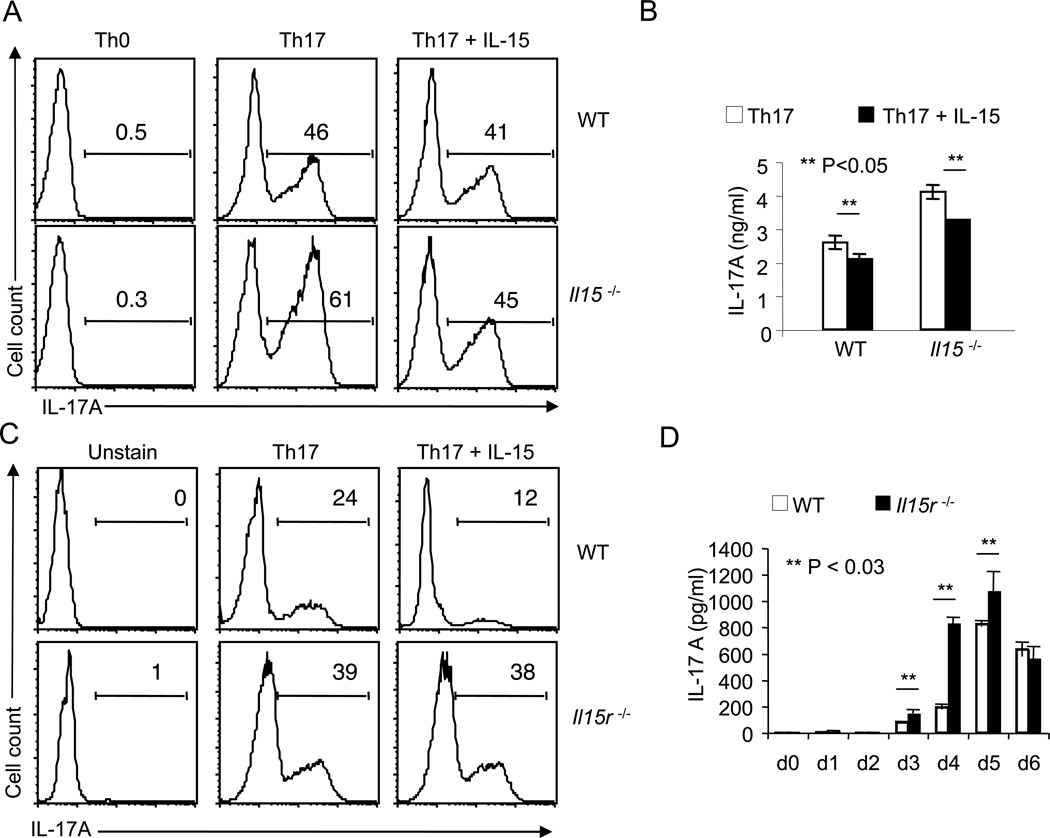
Because both APC and Th17 CD4 cells produce IL-17A, we examined the function of autocrine IL-15 in Th17 cells excluding APC effects. Therefore, we stimulated WT or Il15−/− naïve CD4 cells with α-CD3 and α-CD28 in the absence of APC under Th17 conditions for 4 days. As a negative control, we also stimulated naïve CD4 T cells under neutral conditions (Th0). Intracellular staining of IL-17A revealed that the frequency of IL-17A producers was increased in Il15−/− T cells compared to WT T cells (Fig.3A). Th0 cells showed no IL-17A production whether or not Il15 gene was intact. Addition of exogenous IL-15 independently caused the frequency of IL-17A producers to drop, especially in Il15−/− Th17 cells (Fig. 3A). Also, ELISA data showed that supernatants from Il15−/− cultures contained increased levels of IL-17A as compared to WT cells. Moreover, exogenous IL-15 significantly decreased IL-17A (Fig. 3B). We confirmed these findings by comparing WT cells with IL-15 receptor-alpha (Il15r)−/− cells, showing that the latter had a higher frequency of IL-17A producers under Th17 inducing conditions (Fig. 3C, D). As expected, because of the lack of receptor expression in Il15r−/− cells, 20 ng/ml of exogenous IL-15 did not affect the increased production of IL-17A, indicating that the observed suppression was specific (Fig. 3C). Both WT and Il15r−/− cultures polarized towards Th17 lineage and did not deviate to iTreg or Th1 lineage, because we found very low frequencies of Foxp3 and IFN-γ expressing cells, which was comparable in WT and Il15r−/− cells (Fig. S2). Although an increase in IL-17F producers was also observed in Il15r−/− CD4 cells, the frequency of IL-22 and TNF-α producers was measurably less compared to WT cells, indicating that certain hallmark cytokines of Th17 cells were not down-regulated by IL-15. The activation status was apparently the same in both the cell types, since they were comparable in terms of CD25 expression (Fig. S2). GM-CSF was recently found to be produced by Th17 cells, when stimulated with IL-23 in vitro and in vivo (43). Therefore, we tested for GM-CSF production in our WT and Il15r−/− Th17 cultures. Surprisingly, we were unable to detect GM-CSF in either WT and Il15r−/− cell cultures (Fig. S3A). It is likely that our Th17 cells don’t produce GMCSF because they are stimulated in the absence of IL-23. Because GMCSF is considered to be one of the pathogenic factors of Th17 cells for causing autoimmunity, and our Th17 cells did not produce GMCSF in cultures, we sought to test if these Th17 cells can still cause autoimmune disease. We transferred both WT and Il15r−/− Th17 cells in Rag1−/− mice and found that both the cell types induced IBD in the recipients. Most importantly, mice in to which Il15r−/− Th17 cells were transferred showed more severe disease scores, correlating with their increased production of IL-17A in vivo, compared to those transferred with WT Th17 cells (Fig S3B, data not shown). These data also confirms that Th17 cells with impaired IL-15 signaling produce higher levels of IL-17A in vivo. Taken together, these data suggest that IL-15 can function in an autocrine negative feed back manner, regulating IL-17 expression during Th17 differentiation.
Figure 3. Genetic deficiency of IL-15 or IL-15 receptor increases IL-17A production in Th17 cultures.
(A) Flow cytometric histograms of IL-17A expression of naive WT (upper panel) or Il15−/− CD4 cells (lower panel) stimulated using anti-CD3 and anti-CD28 under non-polarizing conditions (Th0) or Th17 conditions for 96 hours with or without 20 ng/ml of IL-15. (B) ELISA quantification of IL-17A in supernatants from cells that were stimulated as in (A) under Th17 conditions (white bars) in the absence or presence of IL-15 (black bars). (C) Flow cytometric histograms of IL-17A expression of naive WT (upper panels) or Il15r−/− CD4 cells (lower panels) stimulated under Th0 or Th17 conditions as in (A). (D) ELISA quantification of IL-17A in supernatants from WT (white bars) or Il15r−/− (black bars) Th17 cells that were stimulated as in (C) at different days after stimulation.
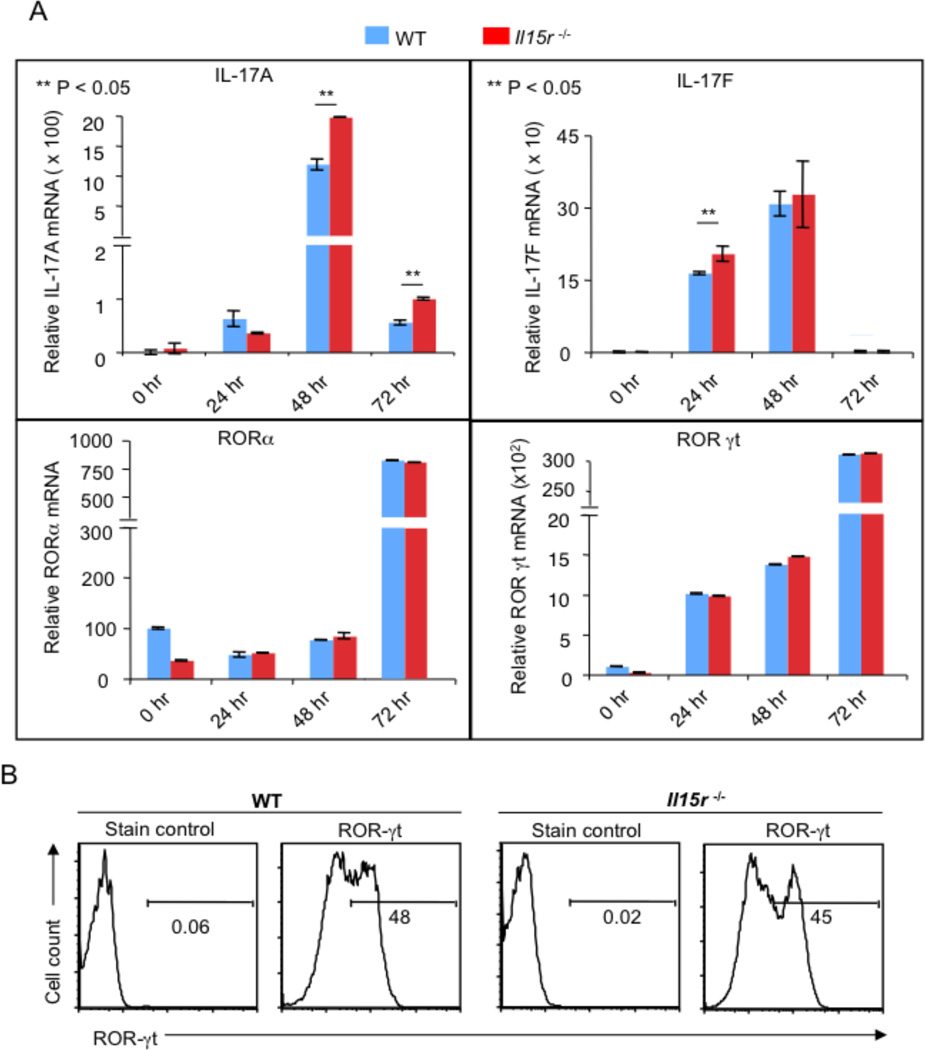
To test whether IL-15 also regulates steady state mRNA levels of IL-17, we quantified IL-17 mRNA by q-RT PCR. We found that IL-17A mRNA was increased in Il15r−/− cells compared to WT cells at 48 and 72 hours after stimulation (Fig. 4A, top panel, left). However, IL-17F was only slightly higher in IL15r−/− cells than WT cells (Fig. 4A, top panel, right). Next, we determined the steady state mRNA levels of the master regulators of Th17 differentiation, ROR-γt and ROR-α. To our surprise, ROR-γt and ROR-α mRNA levels were the same in both the cell types (Fig. 4A, bottom panel). Also, ROR-γt protein levels were equivalent in both the cell types and IL-15 did not regulate ROR-γt in WT cells (Fig. 4B, data not shown). Taken together, our data reveal that IL-15 signaling inhibits IL-17A at the mRNA and protein levels, independently of lineage commitment, activation profile, or regulating ROR-α and ROR-γt expression in differentiating Th17 cells.
Figure 4. IL-15 limits IL-17A expression independently of ROR-γt expression.
(A) WT or Il15r−/− cells were stimulated as in Fig. 3C. RNA was isolated at indicated times of stimulation to assess the il-17a, il-17f, ror-α or ror-γt RNA levels (indicated by Y axes). These data are representation of 2 independent experiments with triplicate samples. (B) WT or Il15r−/− cells stimulated for 48 hours as in (A) were stained with isotype control antibody (Stain control) or anti Ror-γt antibody (Ror-γt). Shown is the fraction of cells in the indicated gate. Gates were drawn based on “Unstained” controls (not shown). These data represent 3 independent experiments.
IL-15 limits IL-17A production by activating STAT5
Th17 cells require TGF-β-mediated SMAD signaling and IL-6-mediated STAT3 activation for differentiation (7). We first examined the levels of the activated or phosphorylated SMAD2 (pSMAD2) and pSMAD3 as a measure of TGF-β signaling (11). Upon TGF-β stimulation, Il15r−/− and WT cells displayed equivalent levels of pSMAD2 and pSMAD3 (data not shown). Similarly, phosphorylated STAT3 (pSTAT3) levels were also the same in WT and Il15r−/− cells at different time points after stimulation in the presence of 100 ng/ml of IL-15 or IL-6 (Fig. 5A, upper panel, data not shown). The basal levels of pSTAT3 were high in both of the cell types, possibly due to constitutive IL-6 and IL-21 signaling in these cultures. These data suggest that IL-15 does not affect TGF-β and IL-6 signals during Th17 differentiation.
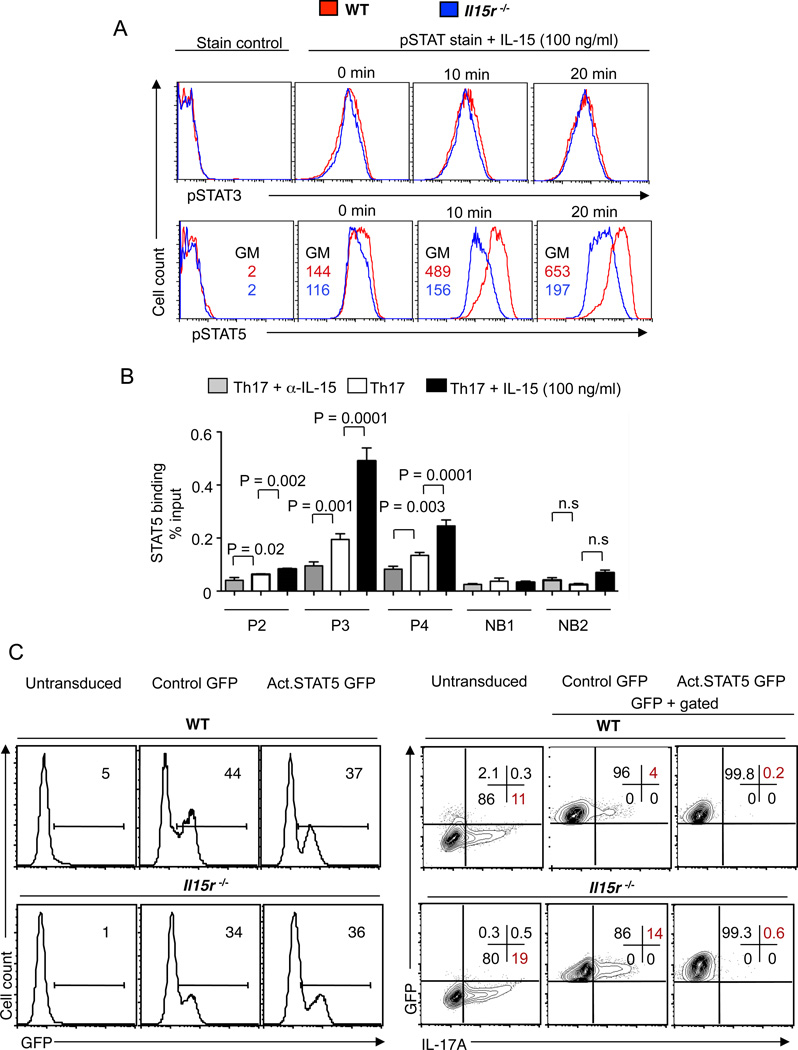
Figure 5. IL-15 restricts IL-17A production by activating STAT5.
(A) WT (red) or Il15r−/− (blue) cells were stimulated as in Fig. 3C. On day 4, the cells were washed, restimulated with IL-15 for indicated times and stained for pSTAT3 (upper panel) or pSTAT5 (lower panel). The geometric mean fluorescence intensity (56) above and below refer to WT and Il15r−/− cells respectively. (B) CD4 T cells were stimulated under Th17 conditions with α-IL-2 antibody (10 µg/ml, all cultures), without (white bars) or with α-1L-15 (50 µg/ml) (grey bars) or with exogenous IL-15 (black bars). On day 5, the cells were restimulated with IL-15 for 20 minutes, fixed and used for ChIP followed by Taqman qPCR. P2, P3, P4 are the primer probe sets for STAT5 binding sites and NB1 and NB2 are the primer probe sets for STAT5 non-binding sites in the il-17 locus (C) WT (upper panel) or Il15r−/− (lower panel) CD4 T cells were used for transduction with control-GFP virus (control GFP) or constitutively-active STAT5 retrovirus (Act.STAT5 GFP) followed up by polarization under Th17 conditions. Untransduced cells were used as control. Flow cytometric plots showing GFP expression (left panels) and the frequency of IL-17A producers (right panels), (gated on GFP+ cells). Three independent experiments showed similar results.
pSTAT3 binds to multiple DNA recognition sites at the IL-17A locus and promotes IL-17 transcription (10). However, its effects are counter-regulated by the fact that IL-2 limits IL-17A production through a STAT5 dependent mechanism (10). Activated STAT5 (phosphorylated form of STAT5 (pSTAT5)) competes with pSTAT3 for the same cognate binding sites and inhibits Il17a transcription and to a lesser extent Il17f transcription. Interestingly, we found that the pSTAT5 expression was markedly reduced in Il15r−/− cells compared to the WT cells, indicating that IL-15 promoted inhibitory STAT5 phosphorylation in Th17 cells (Fig. 5A, lower panel). To further examine the effects of IL-15 on Il17 transcription, we stimulated WT or Il15r−/− CD4 cells under Th17-inducing conditions and determined the binding of STAT5 in Il17a–Il17f locus by chromatin immunoprecipitation (ChIP) and qRT-PCR. Previous studies using ChIP followed by massive parallel sequencing (ChIP-seq) have mapped STAT5 binding sites to the Il-17a–Il-17f locus (10). We chose to examine the enrichment of three strong STAT5 binding sites (P2, P3 and P4) in the Il17 locus, as described previously (10). We also designed the primers for two non-binding sites, NB1 and NB2. We found that the STAT5 binding in the Il17a–Il17f locus was higher in WT cells than in Il15r−/− cells on d5 after stimulation (Fig. S4). To further validate the effects of IL-15 in WT cells from the same mice, we cultured them alone, with α-IL-15 blocking antibody or with exogenous IL-15 in Th17 inducing conditions and performed the ChIPq-PCR on d5. We found that blocking of IL-15 decreased the binding of STAT5 and exogenous IL-15 dramatically increased the binding at all 3 sites in the il-17a locus (Fig. 5B). STAT5 non-binding sites (NB1, NB2) were detected at very low and equivalent levels. Thus, IL-15 promoted direct binding of STAT5 in the il-17a–il-17f locus and may have inhibited il-17 transcription by displacing STAT3 proteins. To support the hypothesis that IL-15-mediated STAT5 signaling indeed limited IL-17A production, we transduced WT and Il15r−/− CD4 cells with a retrovirus constitutively expressing active STAT5 and GFP. Il15r−/− cells in both untransduced and control GFP virus cultures showed increased frequencies of IL-17A+ cells compared to their WT counterparts (Fig. 5C). By contrast, transduction with constitutively active STAT5 reduced IL-17A production both in WT and Il15r−/− cells (Fig. 5C). Because, STAT5 upregulates Bcl-2 expression in T cells, we tested for Bcl-2 expression. We found that cells overexpressing STAT5 indeed up-regulated Bcl-2 expression, confirming the action of STAT5 (data not shown). These results show that STAT5 signaling inhibits IL-17A production in differentiating Th17 cells.
IL-15 regulates the severity of EAE and limits IL-17A production in CD4 cells in mice
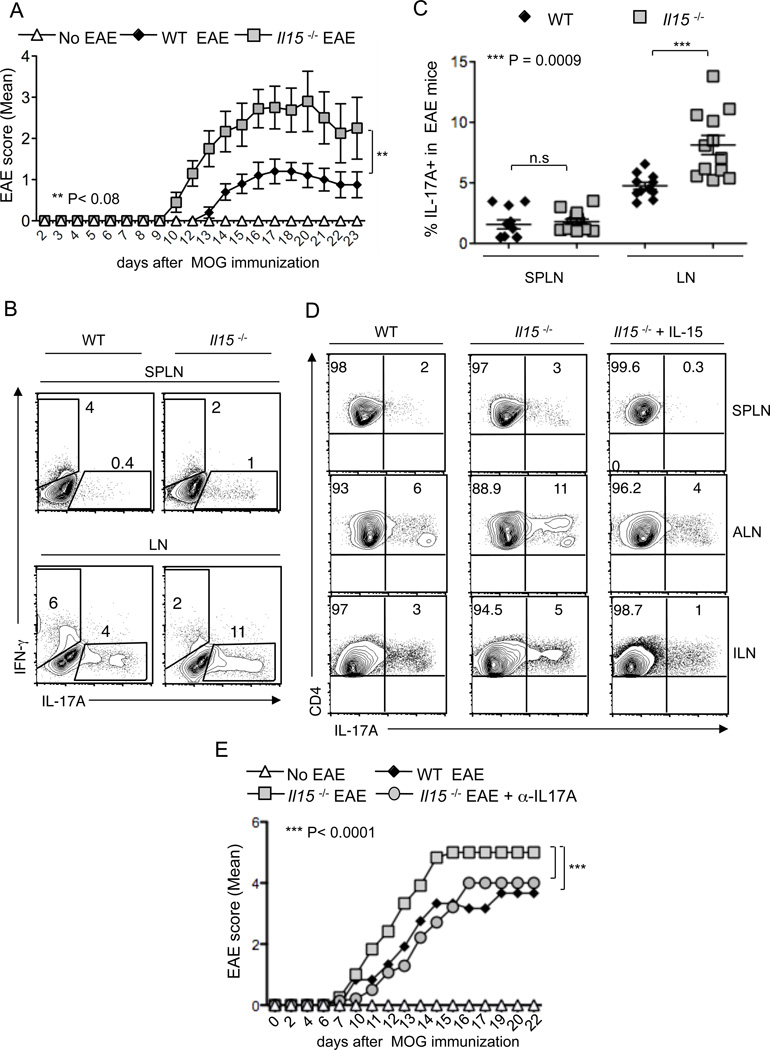
IL-17A plays an important role in the development of EAE in mice (44). Therefore, we used the EAE model to examine the effect of IL-15 on Th17 cells in a disease setting and to validate the regulatory role of IL-15 (38). We induced EAE in WT and Il15−/− mice using MOG (35–55) peptide and pertussis toxin. In line with the previous studies, we found that Il15−/− mice exhibited earlier onset of EAE with higher disease scores compared to WT mice (38, 39) (Fig.6A). This correlated with increased serum IL-17A in Il15−/− mice (data not shown). However, we found that serum GM-CSF levels were comparable in WT and Il15−/− mice induced with EAE, showing that GM-CSF did not exacerbate EAE in Il15−/− mice (data not shown). On day 9 after EAE induction, we isolated CD4 single cell suspensions from the spleen (SPLN) as well as axillary and inguinal lymph nodes to assess cytokine production. Flow cytometry analyses revealed that CD4+ T cells in lymph nodes of Il15−/− mice contained a small but clearly increased population of IL-17A-producing T cells compared to WT mice (Fig. 6B, X axis, and 6C). A slightly higher frequency of IL-17A+ cells was seen in splenic CD4 cells in Il15−/− mice compared to WT mice, but the differences were not significant, because of low cell numbers (Fig.6C). Interestingly, the increased frequency of IL-17A+ cells in Il15−/− mice correlated with a decreased frequency of IFN-γ+ CD4 cells (Fig.6B, Y axis). Thus the removal of IL-15 augmented the Th17 response in vivo, and that was associated with a higher susceptibility to EAE. Although the direct effect of IL-15 on Th17 cells was not investigated, it has been previously shown that injection of IL-15 can suppress the inflammation in EAE induced mice (39). Therefore we examined the IL-17A production in CD4 cells upon administration of IL-15 in Il15−/− mice, after the induction of EAE. Similar to previous findings, we also found that the administration of two doses of 200 ng of IL-15 in Il15−/− mice suppressed EAE inflammation (data not shown) (39). More importantly, we found that the suppression of EAE by IL-15 correlated with lower frequencies of IL-17A producing CD4+ T cells in the EAE-induced mice (Fig.6D).
Figure 6. Loss of IL-15 exacerbates Th17 inflammation and EAE disease in vivo.
(A) 8 week old WT (n = 10) or Il15−/− (n = 10) mice were immunized with MOG in CFA to induce EAE. Control mice were left untreated (No EAE). EAE scores were recorded in blinded fashion. Mean scores +/− SEM are plotted. The data are pooled from 2 independent experiments. (B) On day 9 after EAE induction, CD4+ cells from spleens (SPLN) or pooled axillary/inguinal lymph nodes (LN) were harvested for intracellular staining of IL-17A (X axis) and or IFN-γ (Y axis). (C) The frequencies of IL-17A expressing cells in LN or SPLN are plotted (gated on CD4+ cells). Each data point represents data from a single mouse. (D) IL-15 suppresses the IL-17A production in EAE induced mice. 10–11 week old WT (n = 4) or Il15−/− (n = 6) mice induced with MOG-mediated EAE. Three mice were intraperitoneally injected with recombinant IL-15 (200 ng) on day 3 and day 9 after EAE induction. On day 16, CD4+ cells from spleens (SPLN) or pooled axillary (ALN) or inguinal lymph nodes were harvested and restimulated with MOG peptide (10 µg/ml) for 48 hours and PMA/ionomycin for 4 hours before intracellular staining of IL-17A (gated on CD4+ cells). These data are pooled from 3 independent experiments. (E) Blocking IL-17A reduces the severity of EAE in Il15−/− mice. EAE was induced in 12 week old WT (n = 4) or Il15−/− (n = 7) mice using MOG kit from Hooke’s laboratories. Four Il15−/− mice were intraperitoneally injected with anti-IL-17A antibody (Ebiosciences) (150 µg) on days 1, 3, 6 and 9 after EAE induction. Il15−/− control mice did not receive MOG injection (No EAE). EAE scores were assigned in a blinded fashion. Data are plotted using values from 2 out of 3 independent experiments showing similar results.
Finally, to confirm that increased IL-17A contributed to more severe EAE in Il15−/− mice, we blocked IL-17A in vivo by administering α-IL-17A blocking antibody, on days 2, 4, 7, 9 days after EAE induction. We observed that the anti-IL17 treated Il15−/− mice presented with significantly reduced EAE inflammation scores, comparable to the WT levels (Fig.6E). This finding confirms that the increased IL-17A indeed contributed to more severe inflammation scores in Il15−/− mice. Taken together, these results show that IL-15 controls EAE by restraining IL-17A production in CD4+ T cells.
Discussion
Here we have shown that IL-15 induces the activation of STAT5 and restricts Th17 differentiation in vitro and in vivo. Our results from in vitro experiments demonstrate that IL-15, by itself modulated IL-17A production consistently, but only moderately, in normal Th17 cultures. This was due to the presence of IL-2 produced by Th17 cells, wherein IL-2 could independently control IL-17A production. When we removed the effect of IL-2 by using α-IL-2 antibody, the ability of IL-15 to suppress IL-17A production was much more evident in vitro. On the other hand, blocking IL-15, or genetic deficiency of either IL-15 or IL-15 receptor, all increased the frequency of IL-17A+ cells in Th17 differentiation conditions. We found only moderate increases in the IL-17A+ population in cells deficient in IL-15 signaling compared to WT cells. Nevertheless, these data together with the observations of increased levels of IL-17 mRNA, and supernatant IL-17A protein, from the cultured Il15−/− or Il15r−/− cells, support our conclusion that IL-15 plays an important role in controlling Th17 differentiation.
The data in this report indicates that IL-15 may function in conjunction with IL-2 to limit Th17 cells. We previously found that IL-2 restrains IL-17A only during the early induction period and may play a less prominent role at later time-points of differentiation, or in the maintenance of Th17 cells (45). Hence, IL-15 may play a more prominent role in restricting Th17 cells late in the immune response or when CD4 cells produce non-optimal levels of IL-2. The evidence suggest that IL-2 is a stronger negative regulator of Th17 cells, whereas IL-15 may be involved in fine-tuning Th17 inflammatory responses at late phase of the immune response. This tenet is based on the fact that, whereas Il2−/− and Stat5−/− mice exhibit widespread autoimmunity that associates with increased Th17 differentiation, Il15−/− mice don’t show spontaneous autoimmune symptoms. It would be interesting to analyze Il2−/− and Il15−/− double knockout mice. It is noteworthy that, in vivo, the major source of IL-2 is CD4 cells, whereas IL-15 is produced by a variety of cell types including activated CD4 T cells, monocytes, skeletal muscle cells and keratinocytes (21–24). Thus IL-2 provides only an autocrine regulatory loop, whereas IL-15 may facilitate broader paracrine regulation by a host of non-lymphoid cells to limit Th17 differentiation in certain anatomical niches. Moreover, Th17 cells themselves also produce higher levels of IL-15 when compared to cells that are activated under Th0 conditions. Although there are a few reports showing activated CD4 cells produce IL-15 (7, 24), the general belief is that APCs and not T cells are the predominant source of IL-15 in an adaptive immune response. Here we show that CD4 cells, when activated under Th17 conditions, produced biologically significant levels of IL-15, which limits IL-17A production. IL-15 produced by APC also limits IL-17A production in paracrine manner in cultures (data not shown). Thus IL-15 takes on a new role in directly limiting Th17 functions.
At the molecular level, IL-15 inhibits Il17 expression independently of ROR-γt. Although IL-15 can activate STAT3, we found that STAT3 phosphorylation was comparable in WT and Il15r−/− Th17 cells (28). However, IL-15 induced STAT5 phosphorylation in WT cells, and this is impaired in Il15r−/− cells (28). Interestingly we found that excess of IL-15 (100 ng/ml) can cause some STAT5 phosphorylation also in Il15r−/− cells (Fig. 5A, lower panel). We speculate that this could be due to the effect of IL-15 bypassing the alpha-receptor at excess concentrations (27). Importantly, IL-15 clearly induced STAT5 phosphorylation which is required for the inhibitory effect on Th17 differentiation. This is illustrated by the results from retroviral transduction of active STAT5 in Th17 cells. Our ChIP data also indicates that IL-15 has a direct effect on il-17a expression, by enhancing the binding of inhibitory STAT5 at different sites in the Il-17a locus. Thus, IL-15 downregulated il-17a expression by inducing STAT5 binding and likely displacing STAT3 proteins at the il-17a locus (10). However, to gain insights in to the mechanistic details of STAT5 mediated il-17a repression, further investigation is needed.
Although IL-15 promotes inflammation in patients with Rheumatoid Arthritis (RA), the direct effect of IL-15 in inducing Th17 cells and in promoting inflammation in vivo is unclear (13, 32). In RA, IL-15 promotes synovial fluid T cell activation by upregulating the expression of CD69 and CD154 and promoting the survival and activation of APC, synovial neutrophils, NK cells, fibroblasts and vascular endothelial cells (46–48). Although Th17 cells are present in the synovial compartments in RA, IFN-γ+ Th1 rather than Th17 cells predominate in the synovial joints of RA patients (49, 50). Thus, IL-15 could worsen the disease probably by promoting the proliferation of memory T cells (Th1 cells) or by a completely different mechanism, such as promoting RANTES in myeloid cells independently of its effects on T cells in synovial fluid (51). Recent studies clearly show that IL-15 signals differently in myeloid immune cells and lymphocytes, which may also account for different functional outcomes. The details of these different signaling mechanisms will provide insights into biology and therapeutic applications of IL-15 (51, 52). Anti-IL-15 antibody (HuMax-IL-15) ameliorates disease activity in RA patients, but it was associated with decreased release of IFN-γ (Th1) and CD69 expression by synovial mononuclear cells and was likely independent of Th17 cells (32, 33). However, Halvorsen et al showed that adding exogenous IL-15 resulted in increased IL-17A, as well as IFN-γ, IL-6, IL-10, IL-22 and TNF-α in CD45RO+ memory synovial T cells culture supernatants (50). This study showing the effect of IL-15 in promoting Th17 cells is in apparent contradiction with our study. However they have not clarified whether IL-15 increased the survival and proliferation of differentiated memory T cells, which might have contributed to the overall increases in cytokines in the supernatants, or if it directly induced IL-17A in naïve CD4 cells (15). We support the hypothesis that similar to IL-2, IL-15 likely increases the proliferation and cytokine production of differentiated T cells, in general. Also, the differences between human and mice may also be responsible for the differences (53). Based on our current findings, it seems unlikely that the effect is due to direct induction of IL-17A in naïve cells, but we have not examined synovial T cells to directly address this question. Another study showed that mast cells and necessarily not the T cells are the main source of IL-17 in the inflamed joint, in the rheumatoid arthritis (RA) condition (54). Therefore, distinguishing between the effects of IL-15 on Th17 cells and mast cells from the synovial tissue is important and needs further investigation. Whether IL-15 can also promote Th17 differentiation in certain contexts, depending on the cell types and their STAT3/STAT5 ratio and their binding to il-17a locus remains a possibility to be investigated.
Consistent with the hypothesis that IL-15 limits Th17 cells, Il15−/− mice showed heightened susceptibility to EAE, which is also in agreement with previous studies (38, 39). Whereas those studies did not address the mechanism of IL-15 mediated suppression of EAE, we now show that IL-15 limits Th17 cells in EAE induced mice. Also in our EAE experiments, we found that IL-15 mediated regulation of Th17 cells only in the draining lymph nodes of the hind and forelimbs but not in the spleen. The site-specific functions of IL-15 is interesting and needs to be investigated in further detail. Importantly, we found that IL-15 decreased EAE susceptibility not by reducing IFN-γ+ CD4 cells. Actually, IL-15 enhanced the frequency of IFN-γ+CD4 cells, while reducing IL-17A+CD4 cells. Therefore, altered IL-17A and not IFN-γ increased the susceptibility of Il15−/− mice to EAE. Our experiments blocking IL-17A in Il15−/− mice, which showed less severe EAE, further confirm the role of increased IL-17A in their exaggerated EAE. Taken together, our results strongly support the notion that IL-15 is not only involved in promoting the development of certain innate cells and adaptive memory cells; it also controls inflammation involving Th17 responses. These findings shed new light on the increase in the serum IL-15 in MS and IBD patients, which could be indicative of a compensatory mechanism to ameliorate the Th17 mediated disease. Clinical trials using anti-IL-15 antibody in inflammatory diseases other than RA are not encouraging, implying that IL-15 may not be responsible for the pro-inflammatory effects in those conditions (47, 55). Our data suggest a cautionary note that targeting and blocking IL-15 may actually exacerbate immunological and inflammatory diseases that involve Th17 cells (55). Also, our study opens new avenues to study potential therapeutic applications of IL-15 in suppressing Th17 responses and the associated autoimmune diseases.
Supplementary Material
Acknowledgements
We thank Carol Trageser and Austin Swafford for assessing histology data, and Dr. Yuka Kanno for valuable suggestions and help. We also thank Julie Edwards for FACS sorting.
PP was partially supported by a fellowship from the National Research Council (NRC), National Academy of Sciences. This work was supported by the intramural research program of National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH).
Footnotes
Author contributions. PP designed the study, performed experiments and analyzed data with the supervision of MJL. PP wrote and MJL edited the manuscript; LZ measured the weight of the mice, helped with manuscript preparation, recorded EAE scores in masked fashion and contributed to discussions; SI and SSS performed Real Time PCR experiments and analyzed the data. JJO and XPY helped PP in ChIP experiments.
REFERENCES
- 1.Awasthi A, Kuchroo VK. Th17 cells: from precursors to players in inflammation and infection. Int Immunol. 2009;21:489–498. doi: 10.1093/intimm/dxp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 3.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O'Shea J, Holland SM, Paul WE, Douek DC. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Quinn DB, Palmer MT, Lee YK, Weaver CT. Emergence of the Th17 pathway and its role in host defense. Adv Immunol. 2008;99:115–163. doi: 10.1016/S0065-2776(08)00605-6. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Shen F, Crellin NK, Ouyang W. The IL-17 pathway as a major therapeutic target in autoimmune diseases. Ann N Y Acad Sci. 2011;1217:60–76. doi: 10.1111/j.1749-6632.2010.05825.x. [DOI] [PubMed] [Google Scholar]
- 6.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual review of immunology. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 7.Dong C. Genetic controls of Th17 cell differentiation and plasticity. Exp Mol Med. 2011;43:1–6. doi: 10.3858/emm.2011.43.1.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 9.O'Shea JJ, Lahesmaa R, Vahedi G, Laurence A, Kanno Y. Genomic views of STAT function in CD4+ T helper cell differentiation. Nature reviews. 2011;11:239–250. doi: 10.1038/nri2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, Kanno Y, O'Shea JJ, Laurence A. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nature immunology. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez GJ, Nurieva RI, Yang XO, Dong C. Regulation and function of proinflammatory TH17 cells. Ann N Y Acad Sci. 2008;1143:188–211. doi: 10.1196/annals.1443.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stumhofer JS, Silver J, Hunter CA. Negative regulation of Th17 responses. Seminars in immunology. 2007;19:394–399. doi: 10.1016/j.smim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Sabatino A, Calarota SA, Vidali F, Macdonald TT, Corazza GR. Role of IL-15 in immune-mediated and infectious diseases. Cytokine Growth Factor Rev. 2011;22:19–33. doi: 10.1016/j.cytogfr.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Lodolce J, Burkett P, Koka R, Boone D, Chien M, Chan F, Madonia M, Chai S, Ma A. Interleukin-15 and the regulation of lymphoid homeostasis. Mol Immunol. 2002;39:537–544. doi: 10.1016/s0161-5890(02)00211-0. [DOI] [PubMed] [Google Scholar]
- 15.Bulfone-Paus S, Ungureanu D, Pohl T, Lindner G, Paus R, Ruckert R, Krause H, Kunzendorf U. Interleukin-15 protects from lethal apoptosis in vivo. Nature medicine. 1997;3:1124–1128. doi: 10.1038/nm1097-1124. [DOI] [PubMed] [Google Scholar]
- 16.Giri JG, Anderson DM, Kumaki S, Park LS, Grabstein KH, Cosman D. IL-15, a novel T cell growth factor that shares activities and receptor components with IL-2. J Leukoc Biol. 1995;57:763–766. doi: 10.1002/jlb.57.5.763. [DOI] [PubMed] [Google Scholar]
- 17.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annual review of immunology. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 18.Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, Waldmann TA, Tagaya Y. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, DuBose R, Cosman D, Park LS, Anderson DM. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 2001;167:1179–1187. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 21.Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O'Mahony L, Palomares O, Rhyner C, Quaked N, Schaffartzik A, Van De Veen W, Zeller S, Zimmermann M, Akdis CA. Interleukins, from 1 to 37, and interferon-gamma: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127:701–721. e701–e770. doi: 10.1016/j.jaci.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 22.Bulfone-Paus S, Durkop H, Paus R, Krause H, Pohl T, Onu A. Differential regulation of human T lymphoblast functions by IL-2 and IL-15. Cytokine. 1997;9:507–513. doi: 10.1006/cyto.1996.0194. [DOI] [PubMed] [Google Scholar]
- 23.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science (New York, N.Y. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 24.Neely GG, Robbins SM, Amankwah EK, Epelman S, Wong H, Spurrell JC, Jandu KK, Zhu W, Fogg DK, Brown CB, Mody CH. Lipopolysaccharide-stimulated or granulocyte-macrophage colony-stimulating factor-stimulated monocytes rapidly express biologically active IL-15 on their cell surface independent of new protein synthesis. J Immunol. 2001;167:5011–5017. doi: 10.4049/jimmunol.167.9.5011. [DOI] [PubMed] [Google Scholar]
- 25.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Lee YB, Satoh J, Walker DG, Kim SU. Interleukin-15 gene expression in human astrocytes and microglia in culture. Neuroreport. 1996;7:1062–1066. doi: 10.1097/00001756-199604100-00022. [DOI] [PubMed] [Google Scholar]
- 27.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205:1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston JA, Bacon CM, Finbloom DS, Rees RC, Kaplan D, Shibuya K, Ortaldo JR, Gupta S, Chen YQ, Giri JD, et al. Tyrosine phosphorylation and activation of STAT5, STAT3, and Janus kinases by interleukins 2 and 15. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8705–8709. doi: 10.1073/pnas.92.19.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirman I, Nielsen OH. Increased numbers of interleukin-15-expressing cells in active ulcerative colitis. Am J Gastroenterol. 1996;91:1789–1794. [PubMed] [Google Scholar]
- 30.Liu Z, Geboes K, Colpaert S, D'Haens GR, Rutgeerts P, Ceuppens JL. IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J Immunol. 2000;164:3608–3615. doi: 10.4049/jimmunol.164.7.3608. [DOI] [PubMed] [Google Scholar]
- 31.Obermeier F, Hausmann M, Kellermeier S, Kiessling S, Strauch UG, Duitman E, Bulfone-Paus S, Herfarth H, Bock J, Dunger N, Stoeck M, Scholmerich J, Falk W, Rogler G. IL-15 protects intestinal epithelial cells. European journal of immunology. 2006;36:2691–2699. doi: 10.1002/eji.200535173. [DOI] [PubMed] [Google Scholar]
- 32.Baslund B, Tvede N, Danneskiold-Samsoe B, Larsson P, Panayi G, Petersen J, Petersen LJ, Beurskens FJ, Schuurman J, van de Winkel JG, Parren PW, Gracie JA, Jongbloed S, Liew FY, McInnes IB. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis and rheumatism. 2005;52:2686–2692. doi: 10.1002/art.21249. [DOI] [PubMed] [Google Scholar]
- 33.Yoshihara K, Yamada H, Hori A, Yajima T, Kubo C, Yoshikai Y. IL-15 exacerbates collagen-induced arthritis with an enhanced CD4+ T cell response to produce IL-17. European journal of immunology. 2007;37:2744–2752. doi: 10.1002/eji.200737229. [DOI] [PubMed] [Google Scholar]
- 34.Blanco-Jerez C, Plaza JF, Masjuan J, Orensanz LM, Alvarez-Cermeno JC. Increased levels of IL-15 mRNA in relapsing--remitting multiple sclerosis attacks. J Neuroimmunol. 2002;128:90–94. doi: 10.1016/s0165-5728(02)00146-7. [DOI] [PubMed] [Google Scholar]
- 35.Losy J, Niezgoda A, Zaremba J. IL-15 is elevated in sera of patients with relapsing-remitting multiple sclerosis. Folia Neuropathol. 2002;40:151–153. [PubMed] [Google Scholar]
- 36.Pashenkov M, Mustafa M, Kivisakk P, Link H. Levels of interleukin-15-expressing blood mononuclear cells are elevated in multiple sclerosis. Scand J Immunol. 1999;50:302–308. doi: 10.1046/j.1365-3083.1999.00598.x. [DOI] [PubMed] [Google Scholar]
- 37.Saikali P, Antel JP, Pittet CL, Newcombe J, Arbour N. Contribution of astrocyte-derived IL-15 to CD8 T cell effector functions in multiple sclerosis. J Immunol. 2010;185:5693–5703. doi: 10.4049/jimmunol.1002188. [DOI] [PubMed] [Google Scholar]
- 38.Gomez-Nicola D, Spagnolo A, Guaza C, Nieto-Sampedro M. Aggravated experimental autoimmune encephalomyelitis in IL-15 knockout mice. Exp Neurol. 2010;222:235–242. doi: 10.1016/j.expneurol.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 39.Wu X, Pan W, He Y, Hsuchou H, Kastin AJ. Cerebral interleukin-15 shows upregulation and beneficial effects in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;223:65–72. doi: 10.1016/j.jneuroim.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea J. J. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 42.Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–7120. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 44.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 45.Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernandez-Santos N, Edgerton M, Gaffen SL, Lenardo MJ. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34:422–434. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Monoclonal antibody and intravenous immunoglobulin therapy for rheumatic diseases: rationale and mechanisms of action. Nat Clin Pract Rheumatol. 2007;3:262–272. doi: 10.1038/ncprheum0481. [DOI] [PubMed] [Google Scholar]
- 47.Gabay C, McInnes IB. The biological and clinical importance of the 'new generation' cytokines in rheumatic diseases. Arthritis Res Ther. 2009;11:230. doi: 10.1186/ar2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mottonen M, Isomaki P, Luukkainen R, Toivanen P, Punnonen J, Lassila O. Interleukin-15 up-regulates the expression of CD154 on synovial fluid T cells. Immunology. 2000;100:238–244. doi: 10.1046/j.1365-2567.2000.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamada H, Nakashima Y, Okazaki K, Mawatari T, Fukushi JI, Kaibara N, Hori A, Iwamoto Y, Yoshikai Y. Th1 but not Th17 cells predominate in the joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:1299–1304. doi: 10.1136/ard.2007.080341. [DOI] [PubMed] [Google Scholar]
- 50.Halvorsen EH, Stronen E, Hammer HB, Goll GL, Sollid LM, Molberg O. Interleukin-15 induces interleukin-17 production by synovial T cell lines from patients with rheumatoid arthritis. Scand J Immunol. 2011;73:243–249. doi: 10.1111/j.1365-3083.2010.02498.x. [DOI] [PubMed] [Google Scholar]
- 51.Chenoweth MJ, Mian MF, Barra NG, Alain T, Sonenberg N, Bramson J, Lichty BD, Richards CD, Ma A, Ashkar AA. IL-15 Can Signal via IL-15Ralpha, JNK, and NF-kappaB To Drive RANTES Production by Myeloid Cells. J Immunol. 2012;188:4149–4157. doi: 10.4049/jimmunol.1101883. [DOI] [PubMed] [Google Scholar]
- 52.Harris KM. Monocytes differentiated with GM-CSF and IL-15 initiate Th17 and Th1 responses that are contact-dependent and mediated by IL-15. J Leukoc Biol. 2011;90:727–734. doi: 10.1189/jlb.0311132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, Melendez AJ, McInnes IB. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184:3336–3340. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- 55.Yokoyama S, Watanabe N, Sato N, Perera PY, Filkoski L, Tanaka T, Miyasaka M, Waldmann TA, Hiroi T, Perera LP. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes. Proc Natl Acad Sci U S A. 2009;106:15849–15854. doi: 10.1073/pnas.0908834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neufert C, Becker C, Wirtz S, Fantini MC, Weigmann B, Galle PR, Neurath MF. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. European journal of immunology. 2007;37:1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.