Abstract
Oncotype DX is a commercial assay frequently used for making chemotherapy decisions in estrogen receptor (ER)-positive breast cancers. The result is reported as a recurrence score ranging from 0 to 100, divided into low-risk (<18), intermediate-risk (18–30), and high-risk (≥31) categories. Our pilot study showed that recurrence score can be predicted by an equation incorporating standard morphoimmunohistologic variables (referred to as original Magee equation). Using a data set of 817 cases, we formulated three additional equations (referred to as new Magee equations 1, 2, and 3) to predict the recurrence score category for an independent set of 255 cases. The concordance between the risk category of Oncotype DX and our equations was 54.3%, 55.8%, 59.4%, and 54.4% for original Magee equation, new Magee equations 1, 2, and 3, respectively. When the intermediate category was eliminated, the concordance increased to 96.9%, 100%, 98.6%, and 98.7% for original Magee equation, new Magee equations 1, 2, and 3, respectively. Even when the estimated recurrence score fell in the intermediate category with any of the equations, the actual recurrence score was either intermediate or low in more than 80% of the cases. Any of the four equations can be used to estimate the recurrence score depending on available data. If the estimated recurrence score is clearly high or low, the oncologists should not expect a dramatically different result from Oncotype DX, and the Oncotype DX test may not be needed. Conversely, an Oncotype DX result that is dramatically different from what is expected based on standard morphoimmunohistologic variables should be thoroughly investigated.
Keywords: breast cancer, ER/PR/HER2/Ki-67, immunohistochemistry, morphology, Oncotype DX recurrence score prediction
A surgical pathology report on breast cancer contains various aspects of the tumor biology that provide prognostic and predictive information. These include, but are not limited to, tumor type, size, tumor grade, and lymph node status.1, 2 Tumor proliferation is reported as the mitotic count component of the Nottingham grade.3 Tumor proliferation can also be quantified with Ki-67 immunohistochemistry.4 Over the past 15 years, it has also become standard to report the tumor steroid hormone receptor status and ERBB2 (HER2) gene/protein status.
Tumor receptor status is critical for medical management of breast cancer. Tumors that are estrogen receptor (ER) negative behave more aggressively than ER-positive tumors and most derive substantial benefit from chemotherapy. Patients with ER-positive tumors are almost always offered hormonal therapy. A select subset of these patients also benefit from chemotherapy. Recently, several multigene assays have been developed in an attempt to identify the patients with ER-positive breast cancers that will benefit from chemotherapy.
Oncotype DX (Genomic Health, Redwood City, CA, USA), also known as the 21 gene assay, is one such assay. It is a quantitative reverse transcription-polymerase chain reaction-based assay, used to estimate the risk of distant recurrence for patients with ER-positive, lymph node-negative breast cancers. It is reported as a numerical score (recurrence score) ranging from 0 to 100 and divided into low-risk (<18), intermediate-risk (18–30), and high-risk (≥31) categories. It was validated using tissue blocks from the tamoxifen-treated arm of the National Surgical Adjuvant Breast and Bowel Project clinical trial B-14.5 B-14 compared the disease-free and overall survival of patients with ER-positive, axillary lymph node-negative breast cancer following curative resection, randomized to adjuvant tamoxifen versus placebo. The predictive value of Oncotype DX assay was shown in National Surgical Adjuvant Breast and Bowel Project clinical trial B-20.6 Tissue blocks from B-20 were used to perform the Oncotype DX assay and calculate recurrence scores for each patient. Patients with a high recurrence score were shown to benefit from chemotherapy with low- and intermediate-risk categories deriving minimal or no benefit.6
Oncotype DX quantifies the expression of 16 genes, grouped into the estrogen group (which includes ER and PR), the proliferation group, the HER2 group, the invasion group, and others.5 The remaining five genes are used to check RNA quality and normalize the expression levels. A formula is used to calculate the recurrence score from the polymerase chain reaction results, and gives the highest weight to the proliferation, HER2 and ER groups. Four of the 16 genes in the Oncotype DX assay are routinely measured at the protein level by immunohistochemistry, viz. ER, PR, HER2, and Ki-67. For HER2 immunohistochemical equivocal cases, the HER2 gene status is assessed via fluorescence in situ hybridization (FISH).
Given the weight assigned to the ER, HER2, and proliferation groups in the Oncotype DX recurrence score, we examined the concept if standard histologic variables, in combination with semiquantitative ER, PR, HER2, and Ki-67 results, can provide information similar to that found in the Oncotype DX recurrence score. In a pilot study from Magee-Womens Hospital, we used tissue from 42 breast cancers that had been evaluated by Oncotype DX to show that standard histopathologic factors and immunohistochemical markers can be used to predict the recurrence score.7 In this study, we have analyzed a much larger data set of over 800 cases to create new equations that predict recurrence score and also validated them on a separate set of more than 200 cases.
Materials and methods
A study data set of 817 cases with available Oncotype DX test results (cases from 2004 to 2009, from Magee-Womens Hospital of the University of Pittsburgh Medical Center) was used to build the prediction models. The study was conducted independent of genomic health involvement. All cases included in the study were sent for Oncotype DX testing owing to clinical requests received by the Department of Pathology. Tumor grading information (including the Nottingham grade, score, and the individual components of grading), tumor size, semiquantitative immunohistochemical results for ER, PR, HER2, and Ki-67 were available from pathology reports. At our institution, ER and PR results are reported using a semiquantitative immunohistochemical score (commonly known as ‘H-score'), which details the percentage of positive cells showing none, weak, moderate, or strong staining.7, 8 The score is given as the sum of the percent staining multiplied by an ordinal value corresponding to the intensity level (0: none; 1+: weak; 2: moderate; 3+: strong). The resulting score ranges from 0 (no staining in the tumor) to 300 (diffuse intense staining). HER2 semiquantitated immunohistochemical results were reported according to the College of American Pathologist/American Society of Clinical Oncology (CAP/ASCO) guidelines.9 Immunohistochemical scores of 0 and 1+ were considered a negative result and a score of 3+ was considered a positive result. The final HER2 status on HER2 2+ immunohistochemical cases was determined based on the FISH result and classified as negative (negative for amplification, ie HER2:CEP17 ratio of <1.8), positive (positive for unequivocal amplification, HER2:CEP17 ratio of >2.2), or equivocal (HER2:CEP17 ratio of 1.8–2.2).
Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA). A significance level was set at 0.05 and all P-values reported were two-sided. Multiple linear regression analysis was performed to model the prediction of the Oncotype DX recurrence score by Nottingham score (range 3–9), Ki-67 labeling index (0–100), tumor size (in cm), H-scores (range: 0–300) for ER and PR, and HER2 status (negative, equivocal, or positive). Three models were built based on different hypotheses and data availability. The first regression model included all available parameters (including Ki-67 index) for prediction of Oncotype DX recurrence score. The second regression model was similar to the first but did not include Ki-67. The third regression model included only semiquantitative immunohistochemical expression level for ER, PR, HER2, and Ki-67. Ki-67 was available in less than half of the study data set cases, as it has only been routinely used at our institution since 2007. Using the estimated coefficients derived by the three regression models, three equations were created and were labeled as new Magee equations. The new Magee equations were tested on a validation set of 255 cases (cases from our institution, sent for Oncotype Dx testing from 2010 until early 2011). In addition, our previously published equation (original Magee equation)7 was also applied to the validation set cases. The original Magee equation is represented as follows: recurrence score=13.424+5.420*(nuclear grade)+5.538*(mitotic count)−0.045*(ER H-score)−0.030*(PR H-score)+9.486*(0 for negative/equivocal and 1 for HER2 positive).
Once the recurrence score was estimated using the four equations in Microsoft Excel worksheets (see Supplementary Data), the cases were categorized as low risk, intermediate risk, and high risk, using the same cutoffs as the actual recurrence score. Concordance statistics were performed to compare the actual Oncotype DX recurrence score category to the estimated/predicted recurrence scores category derived from each equation. Pearson's correlation coefficients were also calculated.
Results
Based on data availability, the linear regression analyses on study cases resulted in three new Magee equations and are represented below:
New Magee equation 1: Recurrence score=15.31385+Nottingham score*1.4055+ERIHC*(−0.01924)+PRIHC*(−0.02925)+(0 for HER2 negative, 0.77681 for equivocal, 11.58134 for HER2 positive)+tumor size*0.78677+Ki-67 index*0.13269.
New Magee equation 2: Recurrence score=18.8042+Nottingham score*2.34123+ERIHC*(−0.03749)+PRIHC*(−0.03065)+(0 for HER2 negative, 1.82921 for equivocal, 11.51378 for HER2 positive)+tumor size*0.04267.
New Magee equation 3: Recurrence score=24.30812+ERIHC*(−0.02177)+PRIHC*(−0.02884)+(0 for HER2 negative, 1.46495 for equivocal, 12.75525 for HER2 positive)+Ki-67*0.18649.
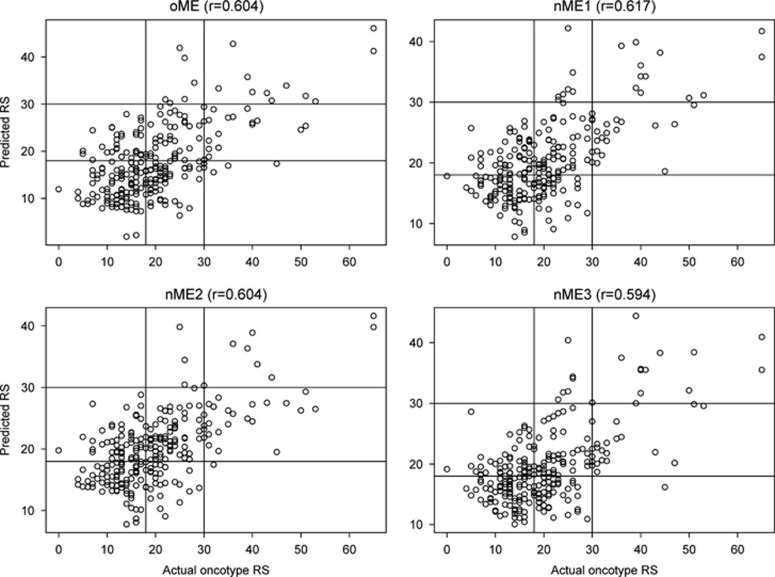
The original and new Magee equations were then applied to each of the 255 validation set cases to estimate the recurrence score. Using this data set, the mean (median) recurrence score for actual Oncotype DX was 20 (19) compared with 17.8 (16.3) for original Magee equation, 20 (19.1) for new Magee equation 1, 19.9 (19.6) for new Magee equation 2, and 19.5 (18.5) for new Magee equation 3 (Table 1). The concordance between actual Oncotype DX recurrence score and the estimated recurrence score calculated from Magee equations with respect to categorization ranged from 54.3 to 59.4% (Tables 2, 3, 4, 5). The Pearson's correlation coefficient (Figure 1) between estimated and actual recurrence score was similar for each of the equations (0.60404, 0.61661, 0.60386, and 0.59407 for original Magee equation, new Magee equation 1, new Magee equation 2, and new Magee equation 3, respectively). With the exclusion of the intermediate-risk categories for both the actual recurrence score and estimated recurrence score, the concordance for each equation increased to more than 95%, reflecting the very low two-step discordance (concordance 96.9% (95/98), 100% (76/76), 98.6% (75/76), and 98.7% (79/80) for original Magee equation, new Magee equation 1, new Magee equation 2, and new Magee equation 3, respectively).
Table 1. Comparison of score averages on 255 validation set cases obtained by different equations to actual Oncotype DX recurrence score (RS).
| Mean | Median | S.d. | Range | |
|---|---|---|---|---|
| Actual Oncotype DX RS | 20 | 19 | 10 | 0–65 |
| Original Magee equation | 17.8 | 16.3 | 7.5 | 1.9–46.0 |
| New Magee equation 1 | 20 | 19.1 | 6.2 | 7.8–42.2 |
| New Magee equation 2 | 19.9 | 19.6 | 5.7 | 7.8–41.6 |
| New Magee equation 3 | 19.5 | 18.5 | 6.2 | 10.0–44.4 |
Table 2. Comparison of recurrence score (RS) categories on validation set cases between original Magee equation and Oncotype DX (ODX).
| ODX RS high | ODX RS intermediate | ODX RS low | Total | |
|---|---|---|---|---|
| Estimated RS high | 9 | 4 | 0 | 13 |
| Estimated RS intermediate | 18 | 43 | 32 | 93 |
| Estated RS low | 3 | 59 | 86 | 148 |
| Total | 30 | 106 | 118 | 254 |
Results of 254 of the total 255 cases were available. Nuclear grade was not available in one case. Concordance: 138/254 (54.3%); one-step discordance: 113/254 (44.5%); two-step discordance: 3/254 (1.2%); Pearson's correlation coefficient: 0.60404.
Table 3. Comparison of recurrence score (RS) categories on validation set cases between new Magee equation 1 and Oncotype DX (ODX).
| ODX RS high | ODX RS intermediate | ODX RS low | Total | |
|---|---|---|---|---|
| Estimated RS high | 11 | 5 | 0 | 16 |
| Estimated RS intermediate | 18 | 59 | 45 | 122 |
| Estimated RS low | 0 | 39 | 65 | 104 |
| Total | 29 | 103 | 110 | 242 |
Results available on 242 of total 255 cases; no tumor size available for six cases and one of these without Nottingham score either, no Ki-67 for seven cases. Concordance: 135/242 (55.8%); one-step discordance: 107/242 (44.2%); two-step discordance: 0/242 (0%); Pearson's correlation coefficient: 0.61661.
Table 4. Comparison of recurrence score (RS) categories on validation set cases between new Magee equation 2 and Oncotype DX (ODX).
| ODX RS high | ODX RS intermediate | ODX RS low | Total | |
|---|---|---|---|---|
| Estimated RS high | 7 | 2 | 0 | 9 |
| Estimated RS intermediate | 21 | 73 | 48 | 142 |
| Estimated RS low | 1 | 29 | 68 | 98 |
| Total | 29 | 104 | 116 | 249 |
Results available on 249 of total 255 cases; no tumor size available for six cases and one of these without Nottingham score either. Concordance: 148/249 (59.4%); one-step discordance: 100/249 (40.2%); two-step discordance: 1/249 (0.4%); Pearson's correlation coefficient: 0.60386.
Table 5. Comparison of recurrence score (RS) categories on validation set cases between new Magee equation 3 and Oncotype DX (ODX).
| ODX RS high | ODX RS intermediate | ODX RS low | Total | |
|---|---|---|---|---|
| Estimated RS high | 7 | 2 | 0 | 9 |
| Estimated RS intermediate | 21 | 73 | 48 | 142 |
| Estimated RS low | 1 | 29 | 68 | 98 |
| Total | 29 | 104 | 116 | 249 |
Results of 248 of the total 255 cases were available. Ki-67 was not available in seven cases. Concordance: 135/248 (54.4%); one-step discordance: 112/248 (45.2%); two-step discordance: 1/248 (0.4%); Pearson's correlation coefficient: 0.59407.
Figure 1.
Graphical representation of scores estimated/predicted by using Magee equations (Y axis) versus actual Oncotype DX recurrence scores (X axis). Intercepts are drawn at recurrence score 18 and 30. oME: Original Magee equation; nME1: new Magee equation 1; nME2: new Magee equation 2; nME3: new Magee equation 3.
When the estimated recurrence score fell in the intermediate category with any of the equations, the actual recurrence score was either intermediate or low in more than 80% of the cases (Tables 2, 3, 4, 5). With original Magee equation, 93 cases were estimated to be intermediate recurrence score (Table 2). Of these, 75 (81%) remained either low or intermediate with actual Oncotype DX recurrence score. The remaining 18 cases (19%) that were determined to be high on actual Oncotype DX recurrence score had a median score of 36 compared with the estimated median score of 26. With new Magee equation 1, 122 cases were estimated to be intermediate recurrence score (Table 3). Of these, 104 (85%) remained either low or intermediate with actual Oncotype DX recurrence score. The remaining 18 cases (15%) that were determined to be high on actual Oncotype DX recurrence score had a median score of 33 compared with the estimated median score of 25. With new Magee equation 2, 142 cases were estimated to be intermediate recurrence score (Table 4). Of these, 121 (85%) remained either low or intermediate with actual Oncotype DX recurrence score. The remaining 21 cases (15%) that were determined to be high on actual Oncotype DX recurrence score had a median score of 35 compared with the estimated median score of 26. With new Magee equation 3, 119 cases were estimated to be intermediate recurrence score (Table 5). Of these, 101 (85%) remained either low or intermediate with actual Oncotype DX recurrence score. The remaining 18 cases (15%) that were determined to be high on actual Oncotype DX recurrence score had a median score of 33 compared with the estimated median score of 23.
Discussion
Oncotype DX test is currently widely used by clinicians in the United States. Despite the known pitfalls of Oncotype DX assay in evaluation of single gene status,10 the overall assay has been reported to have prognostic and predictive value. This is somewhat expected as many of the genes analyzed in the assay have individually been shown to have prognostic and predictive value. Although the seminal paper was reported to show benefit of 21 gene/Oncotype DX assay over individual standard histopathologic parameters,5 there are dearth of studies that have compared the combined prognostic/predictive power of standard histology and immunohistochemical markers with Oncotype DX assay. Recently, the study by Cuzick et al11 provide evidence that standard clinical and pathologic parameters can be even better than Oncotype DX assay for prognostication.
This study was undertaken to estimate the Oncotype DX recurrence score using standard histologic and immunohistologic findings that are routinely reported. The concordance between the estimated recurrence score category and the Oncotype DX recurrence score category ranged from 55 to 60%. The two-step discordance (meaning an estimated recurrence score that is high, when the Oncotype DX recurrence score is low, and vice versa) was either not present (for new Magee equation 1) or negligible (for the other equations). One can therefore conclude that if the estimated recurrence score is clearly in the high or low categories, it is predictive of the Oncotype DX recurrence score category with >95% certainty. Even in cases where the estimated recurrence score category predicted an intermediate category, the results were clinically useful. An estimated recurrence score (using new Magee equations) in the intermediate category means that the actual Oncotype DX recurrence score category will be either intermediate or low approximately 85% of the time. The remainder 15% cases in the validation set that were determined to be high risk by actual Oncotype DX recurrence score had median score close to the 31 point cutoff. These results are important as the National Surgical Adjuvant Breast and Bowel Project-B20 trial showed that cases with intermediate recurrence score derive minimal or no benefit with adjuvant chemotherapy.6 Therefore, majority of the patients with estimated recurrence score in the intermediate category are unlikely to derive large benefit from chemotherapy. The cases that have estimated recurrence score at the higher end of intermediate category may be considered for chemotherapy based on individual benefit and risk. These equations provide clinically useful information, using data from standard pathologic reporting of breast cancer. Of our four equations, new Magee equation 2 had the highest concordance for categorization (60%), and includes Nottingham score, ER H-score, PR H-score, HER2 status, and tumor size (and does not include Ki-67). This may indicate some confounding results from the equations that use Ki-67 (new Magee equations 1 and 3). Given that all of the equations utilize either the Nottingham score or the mitotic count component of the Nottingham score, the actual mitotic activity is either similar or better predictor of tumor proliferation than Ki-67 immunohistochemistry. The previous studies have shown the importance of Ki-67 in determining the Oncotype DX recurrence score,12, 13 but Ki-67 alone appears to be inferior to combined histologic and immunophenotypic data.
Although the overall correlation between estimated and actual recurrence score was good, it was not perfect. There are several possibilities. First, the estimated recurrence score measures the morphologic and immunophenotypic data and actual recurrence score measures gene expression levels and some variability is expected if two different parameters are measured. Secondly, interobserver variability for grading and semiquantifying immunohistochemical results among pathologists can lead to under- or overestimation of recurrence score. Last, but not the least, there may be characteristics intrinsic to the Oncotype DX assay that can alter the recurrence score. One major issue is the possible effect of various benign breast components on recurrence score. Despite gross macrodissection or even microdissection of tumor tissue for polymerase chain reaction analysis, many non-invasive tumor tissue components (such as stromal fibroblasts, adipose tissue, lymphocytes, macrophages, normal ducts and lobules, in situ carcinoma, epithelial proliferation, etc) can be admixed during mRNA extraction and may alter the recurrence score by at least a few points. Recently, Acs et al14 demonstrated the impact of mitotically active cellular stroma on Oncotype DX recurrence score. Their results suggested that increased stromal cellularity and/or associated inflammatory cells in low-grade invasive breast cancer may contribute to apparent increase in recurrence score. Apart from tumor macrodissection/microdissection, reproducibility of the assay between the same tumor block and in between different tumor blocks of the same tumor has not been extensively studied. Cronin et al15 reported the analytical validation of the Oncotype DX assay, but did not address the reproducibility of recurrence score on re-extracted mRNA from the same block or difference in recurrence score values between different blocks of the same tumor. It is also possible that variation in tissue handling and fixation can alter recurrence score, but there is no published data on this subject. Delay to formalin fixation can not only alter immunohistochemical results and mitotic counts by a pathologist but can also alter mRNA expression level of many genes.16, 17, 18, 19 Owing to the variability of all of these factors, it is not surprising that there is less than perfect correlation between Oncotype DX recurrence score and the Magee equations that we have created for estimating recurrence score.
Decades of prior work has shown the importance of tumor grade, tumor size, lymph node status, hormone receptors and HER2 status in determining patient prognosis and prediction of response to hormonal therapy and systemic chemotherapy. Our equations use all of these factors and we have demonstrated that they can be useful in estimating the recurrence score. Risk prediction models such as the St Gallen, Adjuvant! Online, and the Nottingham Prognostic Index use some of these factors to predict outcome, and have been used by clinicians to guide therapy.20, 21, 22, 23 Each of these systems, however, have some shortcomings. The St Gallen system does not include HER2 status in prognostication (although accepted HER2-positive status to assign trastuzumab in 2007) and owing to overlapping features in some cases, risk categorization could be difficult. Nottingham Prognostic Index fails to include receptor status for prognostication. Adjuvant! Online does not include HER2 status in the equation and also does not account for tumor hormone receptor content (quantification) for prognostication and prediction of chemotherapy effectiveness. The oncotype DX include expression levels of genes that are critical in determining prognosis and effectiveness of chemotherapy, but this test also has several shortcomings as discussed above. Therefore, all of these predictive models and assays should be considered as estimates and clinicians should use all available information rather than result of one assay.
Our study reinforces the findings in our pilot study, and prior studies that show correlation between standard pathology findings and the Oncotype DX recurrence score.7, 24, 25, 26 In a study of 77 cases, Tang and co-workers27 showed the correlation between aggressive morphologic features and lack of PR expression with high Oncotype DX recurrence score. The importance of PR semiquantitative score was also shown by Clark et al,28 who identified an inverse relationship between PR expression level and Oncotype DX recurrence score, which was independent of tumor grade. Recently, one large study has shown the prognostic significance of semiquantitative ER, PR, HER2, and Ki-67 immunohistochemical results to be similar to the Oncotype DX recurrence score. The study also showed that combining the immunohistochemical results with clinical parameters provided superior prognostic information than Oncotype DX alone.11 In another large recent study, Tang et al29 integrated the recurrence score with clinical and pathologic factors and reported a better assessment of distant recurrence. The follow-up time for patients in our study (the 255 validation set cases from 2010 to 2011) is short. By following clinical outcomes, we hope to verify the utility of our findings, and reinforce the importance of standard morphoimmunohistologic variables.
The equations we have created are user-friendly and available free of any cost to the user. By using the Microsoft Excel worksheets we have provided (see Supplementary Data), pathologists and oncologists can easily calculate estimated recurrence score themselves and compare the result to the actual recurrence score. If an estimated recurrence score falls clearly in the high-risk or low-risk category, then oncologists should not expect a dramatically different result from Oncotype DX. In these cases, the use of Oncotype DX may be avoided. In cases where Oncotype DX is performed, a result dramatically different from the estimated recurrence score should be thoroughly investigated by the pathologist in concert with the clinical team, to ensure that the Oncotype DX result is accurate in the sense that it is representative of the tumor that is properly microdissected.
Acknowledgments
We thank Ms Diane Bell for secretarial assistance. This project used the UPCI Biostatistics Facility and was supported in part by award P30CA047904.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Modern Pathology website (http://www.nature.com/modpathol)
Supplementary Material
References
- Pereira H, Pinder SE, Sibbering DM, et al. Pathological prognostic factors in breast cancer. IV: Should you be a typer or a grader? A comparative study of two histological prognostic features in operable breast carcinoma. Histopathology. 1995;27:219–226. doi: 10.1111/j.1365-2559.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Pinder SE, Ellis IO, Elston CW. Prognostic factors in primary breast carcinoma. J Clin Pathol. 1995;48:981–983. doi: 10.1136/jcp.48.11.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Pinder SE, Wencyk P, Sibbering DM, et al. Assessment of the new proliferation marker MIB1 in breast carcinoma using image analysis: associations with other prognostic factors and survival. Br J Cancer. 1995;71:146–149. doi: 10.1038/bjc.1995.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- Flanagan MB, Dabbs DJ, Brufsky AM, et al. Histopathologic variables predict Oncotype DX recurrence score. Mod Pathol. 2008;21:1255–1261. doi: 10.1038/modpathol.2008.54. [DOI] [PubMed] [Google Scholar]
- McCarty KS, Miller LS, Cox EB, et al. Estrogen receptor analyses. correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109:716–721. [PubMed] [Google Scholar]
- Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- Dabbs DJ, Klein ME, Mohsin SK, et al. High false-negative rate of HER2 quantitative reverse transcription polymerase chain reaction of the Oncotype DX test: an independent quality assurance study. J Clin Oncol. 2011;29:4279–4285. doi: 10.1200/JCO.2011.34.7963. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Dowsett M, Pineda S, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol. 2011;29:4273–4278. doi: 10.1200/JCO.2010.31.2835. [DOI] [PubMed] [Google Scholar]
- Gwin K, Pinto M, Tavassoli FA. Complementary value of the Ki-67 proliferation index to the oncotype DX recurrence score. Int J Surg Pathol. 2009;17:303–310. doi: 10.1177/1066896909340274. [DOI] [PubMed] [Google Scholar]
- Sahebjam S, Aloyz R, Pilavdzic D, et al. Ki 67 is a major, but not the sole determinant of Oncotype Dx recurrence score. Br J Cancer. 2011;105:1342–1345. doi: 10.1038/bjc.2011.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acs G, Esposito NN, Kiluk J, et al. A mitotically active, cellular tumor stroma and/or inflammatory cells associated with tumor cells may contribute to intermediate or high Oncotype DX recurrence scores in low-grade invasive breast carcinomas. Mod Pathol. 2012;25:556–566. doi: 10.1038/modpathol.2011.194. [DOI] [PubMed] [Google Scholar]
- Cronin M, Sangli C, Liu ML, et al. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin Chem. 2007;53:1084–1091. doi: 10.1373/clinchem.2006.076497. [DOI] [PubMed] [Google Scholar]
- Bergers E, Jannink I, van Diest PI, et al. The influence of fixation delay on mitotic activity and flow cytometric cell cycle variables. Hum Pathol. 1997;28:95–100. doi: 10.1016/s0046-8177(97)90286-0. [DOI] [PubMed] [Google Scholar]
- De Cecco L, Musella V, Veneroni S, et al. Impact of biospecimens handling on biomarker research in breast cancer. BMC Cancer. 2009;9:409. doi: 10.1186/1471-2407-9-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donhuijsen K, Schmidt U, Hirche H, et al. Changes in mitotic rate and cell cycle fractions caused by delayed fixation. Hum Pathol. 1990;21:709–714. doi: 10.1016/0046-8177(90)90030-9. [DOI] [PubMed] [Google Scholar]
- Yildiz-Aktas IZ, Dabbs DJ, Bhargava R. The effect of cold ischemic time on the immunohistochemical evaluation of estrogen receptor, progesterone receptor, and HER2 expression in invasive breast carcinoma. Mod Pathol. 2012;25:1098–1105. doi: 10.1038/modpathol.2012.59. [DOI] [PubMed] [Google Scholar]
- Galea MH, Blamey RW, Elston CE, et al. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat. 1992;22:207–219. doi: 10.1007/BF01840834. [DOI] [PubMed] [Google Scholar]
- Goldhirsch A, Wood WC, Gelber RD, et al. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–1144. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- Ravdin PM. A computer based program to assist in adjuvant therapy decisions for individual breast cancer patients. Bull Cancer. 1995;82 (Suppl 5:561s–564s. [PubMed] [Google Scholar]
- Ravdin PM. A computer program to assist in making breast cancer adjuvant therapy decisions. Semin Oncol. 1996;23:43–50. [PubMed] [Google Scholar]
- Allison KH, Kandalaft PL, Sitlani CM, et al. Routine pathologic parameters can predict Oncotype DX recurrence scores in subsets of ER positive patients: who does not always need testing. Breast Cancer Res Treat. 2012;131:413–424. doi: 10.1007/s10549-011-1416-3. [DOI] [PubMed] [Google Scholar]
- Geradts J, Bean SM, Bentley RC, et al. The oncotype DX recurrence score is correlated with a composite index including routinely reported pathobiologic features. Cancer Invest. 2010;28:969–977. doi: 10.3109/07357907.2010.512600. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Shen J. Is the Oncotype DX assay necessary in strongly estrogen receptor-positive breast cancers. Am Surg. 2011;77:1364–1367. [PubMed] [Google Scholar]
- Tang P, Wang J, Hicks DG, et al. A lower Allred score for progesterone receptor is strongly associated with a higher recurrence score of 21-gene assay in breast cancer. Cancer Invest. 2010;28:978–982. doi: 10.3109/07357907.2010.496754. [DOI] [PubMed] [Google Scholar]
- Clark BZ, Dabbs DJ, Cooper KL, et al. Impact of progesterone receptor semiquantitative immunohistochemical result on oncotype DX recurrence score: a quality assurance study of 1074 Cases Appl Immunohistochem Mol Morphol 2012. PMID: 23060300; e-pub ahead of print. [DOI] [PubMed]
- Tang G, Cuzick J, Costantino JP, et al. Risk of recurrence and chemotherapy benefit for patients with node-negative, estrogen receptor-positive breast cancer: recurrence score alone and integrated with pathologic and clinical factors. J Clin Oncol. 2011;29:4365–4372. doi: 10.1200/JCO.2011.35.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.