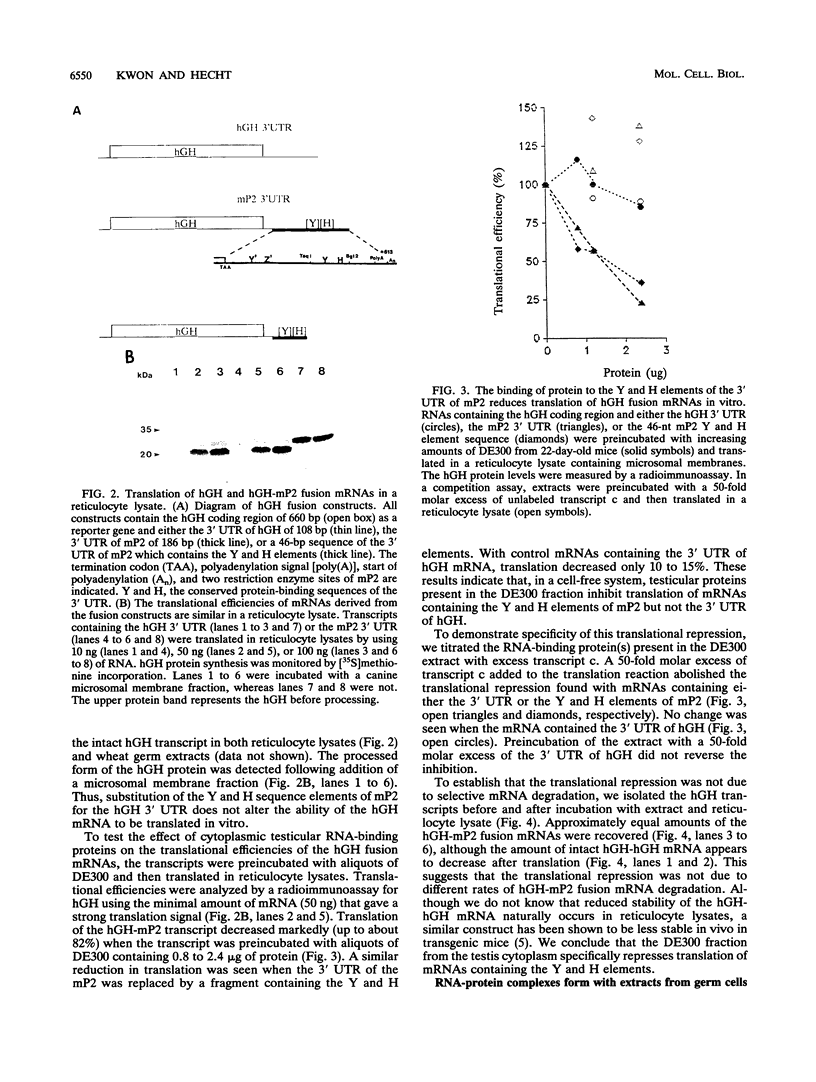
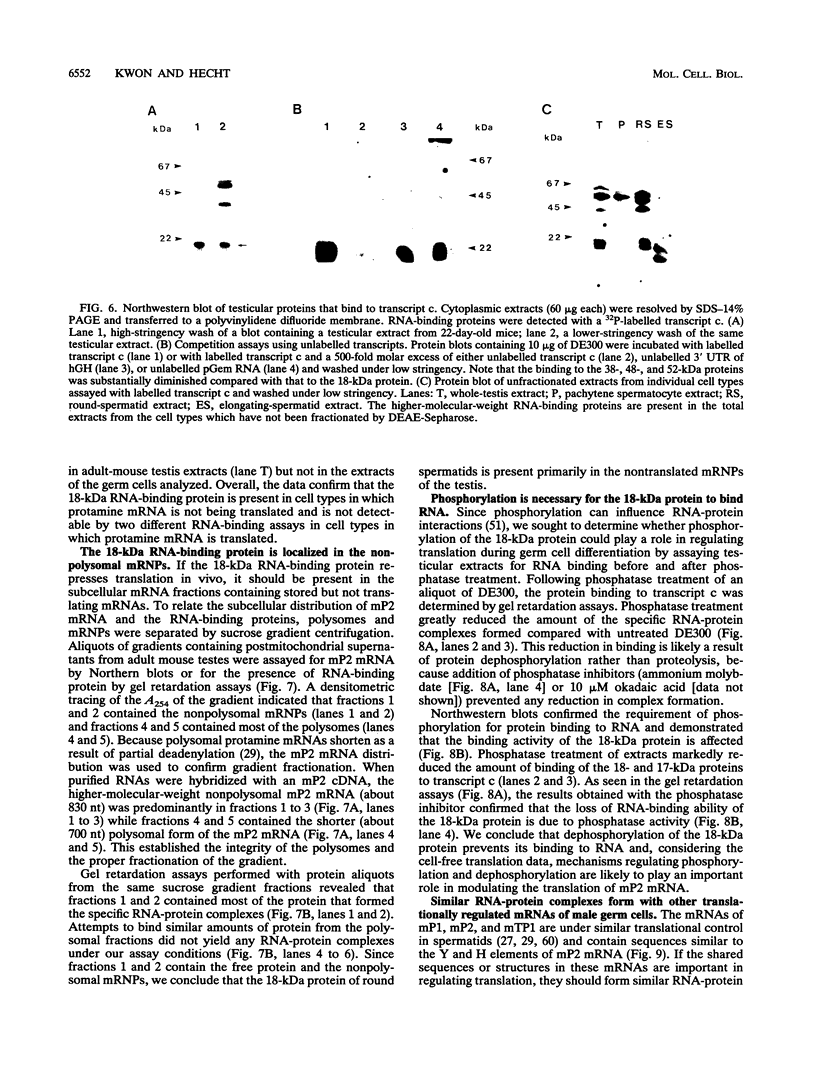
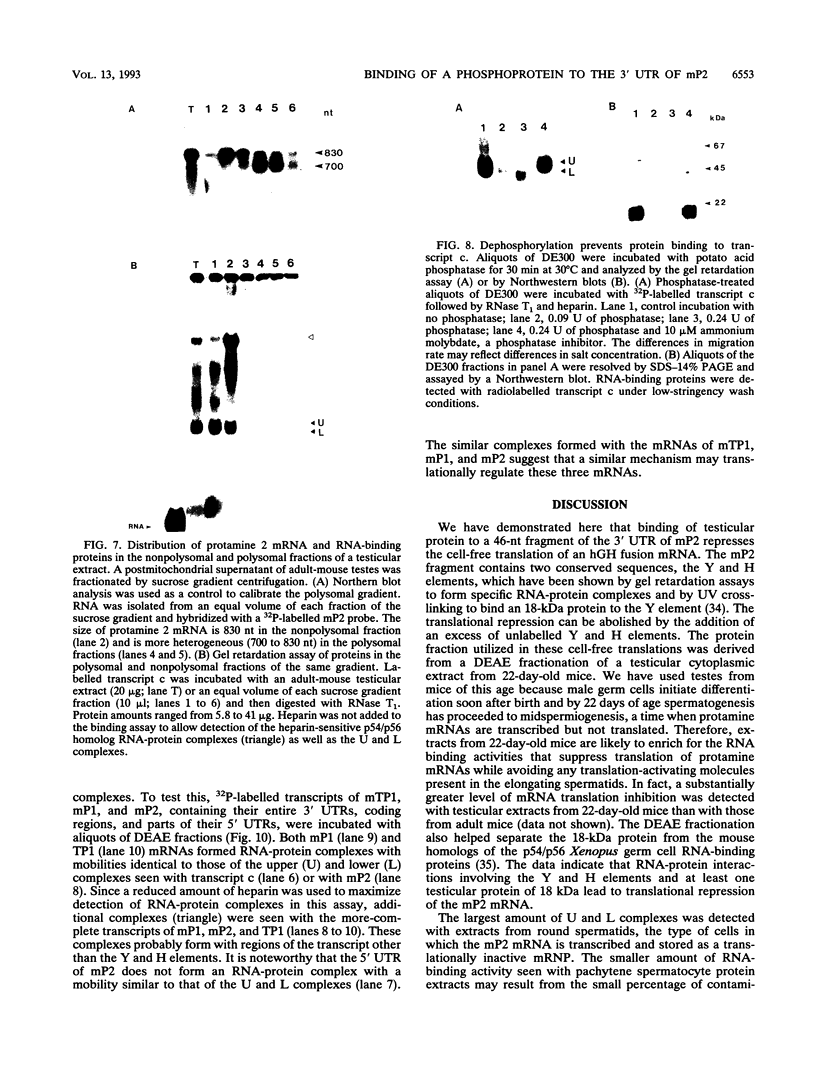
Abstract
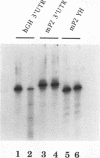
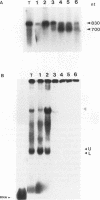
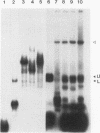
The synthesis of the protamines, the predominant nuclear proteins of mammalian spermatozoa, is regulated during germ cell development by mRNA storage for about 7 days in the cytoplasm of differentiating spermatids. Two highly conserved sequences, the Y and H elements present in the 3' untranslated regions (UTRs) of all known mammalian protamine mRNAs, form RNA-protein complexes and specifically bind a protein of 18 kDa. Here, we show that translation of fusion mRNAs was markedly repressed in reticulocyte lysates supplemented with a mouse testis extract enriched for the 18-kDa protein when the mRNAs contained the 3' UTR of mouse protamine 2 (mP2) or the Y and H elements of mP2. No significant decrease was seen when the fusion mRNAs contained the 3' UTR of human growth hormone. The 18-kDa protein is developmentally regulated in male germ cells, requires phosphorylation for RNA binding, and is found in the ribonucleoprotein particle fractions of a testicular postmitochondrial supernatant. We propose that a phosphorylated 18-kDa protein plays a primary role in repressing translation of mP2 mRNA by interaction with the highly conserved Y and H elements. At a later stage of male gamete differentiation, the 18-kDa protein no longer binds to the mRNA, likely as a result of dephosphorylation, enabling the protamine mRNA to be translated.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahringer J., Kimble J. Control of the sperm-oocyte switch in Caenorhabditis elegans hermaphrodites by the fem-3 3' untranslated region. Nature. 1991 Jan 24;349(6307):346–348. doi: 10.1038/349346a0. [DOI] [PubMed] [Google Scholar]
- Alcivar A. A., Hake L. E., Millette C. F., Trasler J. M., Hecht N. B. Mitochondrial gene expression in male germ cells of the mouse. Dev Biol. 1989 Oct;135(2):263–271. doi: 10.1016/0012-1606(89)90178-4. [DOI] [PubMed] [Google Scholar]
- Bellvé A. R., Cavicchia J. C., Millette C. F., O'Brien D. A., Bhatnagar Y. M., Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977 Jul;74(1):68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R. E., Peschon J. J., Behringer R. R., Brinster R. L., Palmiter R. D. Protamine 3'-untranslated sequences regulate temporal translational control and subcellular localization of growth hormone in spermatids of transgenic mice. Genes Dev. 1989 Jun;3(6):793–802. doi: 10.1101/gad.3.6.793. [DOI] [PubMed] [Google Scholar]
- Cummings A., Sommerville J. Protein kinase activity associated with stored messenger ribonucleoprotein particles of Xenopus oocytes. J Cell Biol. 1988 Jul;107(1):45–56. doi: 10.1083/jcb.107.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearsly A. L., Johnson R. M., Barrett P., Sommerville J. Identification of a 60-kDa phosphoprotein that binds stored messenger RNA of Xenopus oocytes. Eur J Biochem. 1985 Jul 1;150(1):95–103. doi: 10.1111/j.1432-1033.1985.tb08993.x. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenjoud L., Fronia C., Uhde F., Engel W. Sequence of human protamine 2 cDNA. Nucleic Acids Res. 1988 Aug 11;16(15):7733–7733. doi: 10.1093/nar/16.15.7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsevier S. M. Messenger RNA encoding basic chromosomal proteins of mouse testis. Dev Biol. 1982 Mar;90(1):1–12. doi: 10.1016/0012-1606(82)90205-6. [DOI] [PubMed] [Google Scholar]
- Erickson R. P., Kramer J. M., Rittenhouse J., Salkeld A. Quantitation of mRNAs during mouse spermatogenesis: protamine-like histone and phosphoglycerate kinase-2 mRNAs increase after meiosis. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6086–6090. doi: 10.1073/pnas.77.10.6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. A., Sheets M. D., Wickens M. P. Poly(A) addition during maturation of frog oocytes: distinct nuclear and cytoplasmic activities and regulation by the sequence UUUUUAU. Genes Dev. 1989 Dec;3(12B):2151–2162. doi: 10.1101/gad.3.12b.2151. [DOI] [PubMed] [Google Scholar]
- Galili G., Kawata E. E., Smith L. D., Larkins B. A. Role of the 3'-poly(A) sequence in translational regulation of mRNAs in Xenopus laevis oocytes. J Biol Chem. 1988 Apr 25;263(12):5764–5770. [PubMed] [Google Scholar]
- Goessling L. S., Daniels-McQueen S., Bhattacharyya-Pakrasi M., Lin J. J., Thach R. E. Enhanced degradation of the ferritin repressor protein during induction of ferritin messenger RNA translation. Science. 1992 May 1;256(5057):670–673. doi: 10.1126/science.1316633. [DOI] [PubMed] [Google Scholar]
- Hake L. E., Alcivar A. A., Hecht N. B. Changes in mRNA length accompany translational regulation of the somatic and testis-specific cytochrome c genes during spermatogenesis in the mouse. Development. 1990 Sep;110(1):249–257. doi: 10.1242/dev.110.1.249. [DOI] [PubMed] [Google Scholar]
- Heidaran M. A., Kozak C. A., Kistler W. S. Nucleotide sequence of the Stp-1 gene coding for rat spermatid nuclear transition protein 1 (TP1): homology with protamine P1 and assignment of the mouse Stp-1 gene to chromosome 1. Gene. 1989 Jan 30;75(1):39–46. doi: 10.1016/0378-1119(89)90381-8. [DOI] [PubMed] [Google Scholar]
- Heidaran M. A., Showman R. M., Kistler W. S. A cytochemical study of the transcriptional and translational regulation of nuclear transition protein 1 (TP1), a major chromosomal protein of mammalian spermatids. J Cell Biol. 1988 May;106(5):1427–1433. doi: 10.1083/jcb.106.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heus H. A., Pardi A. Structural features that give rise to the unusual stability of RNA hairpins containing GNRA loops. Science. 1991 Jul 12;253(5016):191–194. doi: 10.1126/science.1712983. [DOI] [PubMed] [Google Scholar]
- Houman F., Diaz-Torres M. R., Wright A. Transcriptional antitermination in the bgl operon of E. coli is modulated by a specific RNA binding protein. Cell. 1990 Sep 21;62(6):1153–1163. doi: 10.1016/0092-8674(90)90392-r. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Standart N. Do the poly(A) tail and 3' untranslated region control mRNA translation? Cell. 1990 Jul 13;62(1):15–24. doi: 10.1016/0092-8674(90)90235-7. [DOI] [PubMed] [Google Scholar]
- Kick D., Barrett P., Cummings A., Sommerville J. Phosphorylation of a 60 kDa polypeptide from Xenopus oocytes blocks messenger RNA translation. Nucleic Acids Res. 1987 May 26;15(10):4099–4109. doi: 10.1093/nar/15.10.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Waters S. H., Hake L. E., Hecht N. B. Identification and developmental expression of a smooth-muscle gamma-actin in postmeiotic male germ cells of mice. Mol Cell Biol. 1989 May;9(5):1875–1881. doi: 10.1128/mcb.9.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Cheong C., Moore P. B. Tetramerization of an RNA oligonucleotide containing a GGGG sequence. Nature. 1991 May 23;351(6324):331–332. doi: 10.1038/351331a0. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Harford J. B. cis-trans models for post-transcriptional gene regulation. Science. 1989 Nov 17;246(4932):870–872. doi: 10.1126/science.2683086. [DOI] [PubMed] [Google Scholar]
- Kleene K. C., Borzorgzadeh A., Flynn J. F., Yelick P. C., Hecht N. B. Nucleotide sequence of a cDNA clone encoding mouse transition protein 1. Biochim Biophys Acta. 1988 Jul 13;950(2):215–220. doi: 10.1016/0167-4781(88)90013-9. [DOI] [PubMed] [Google Scholar]
- Kleene K. C., Distel R. J., Hecht N. B. Translational regulation and deadenylation of a protamine mRNA during spermiogenesis in the mouse. Dev Biol. 1984 Sep;105(1):71–79. doi: 10.1016/0012-1606(84)90262-8. [DOI] [PubMed] [Google Scholar]
- Kleene K. C., Flynn J. Translation of mouse testis poly(A)+ mRNAs for testis-specific protein, protamine 1, and the precursor for protamine 2. Dev Biol. 1987 Sep;123(1):125–135. doi: 10.1016/0012-1606(87)90434-9. [DOI] [PubMed] [Google Scholar]
- Kleene K. C. Poly(A) shortening accompanies the activation of translation of five mRNAs during spermiogenesis in the mouse. Development. 1989 Jun;106(2):367–373. doi: 10.1242/dev.106.2.367. [DOI] [PubMed] [Google Scholar]
- Klemm U., Lee C. H., Burfeind P., Hake S., Engel W. Nucleotide sequence of a cDNA encoding rat protamine and the haploid expression of the gene during rat spermatogenesis. Biol Chem Hoppe Seyler. 1989 Apr;370(4):293–301. doi: 10.1515/bchm3.1989.370.1.293. [DOI] [PubMed] [Google Scholar]
- Krawetz S. A., Connor W., Dixon G. H. Bovine protamine genes contain a single intron. The structures of the two alleles. J Biol Chem. 1988 Jan 5;263(1):321–326. [PubMed] [Google Scholar]
- Krawetz S. A., Dixon G. H. Isolation and in vitro translation of a mammalian protamine mRNA. Biosci Rep. 1984 Jul;4(7):593–603. doi: 10.1007/BF01121917. [DOI] [PubMed] [Google Scholar]
- Kwon Y. K., Hecht N. B. Cytoplasmic protein binding to highly conserved sequences in the 3' untranslated region of mouse protamine 2 mRNA, a translationally regulated transcript of male germ cells. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3584–3588. doi: 10.1073/pnas.88.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y. K., Murray M. T., Hecht N. B. Proteins homologous to the Xenopus germ cell-specific RNA-binding proteins p54/p56 are temporally expressed in mouse male germ cells. Dev Biol. 1993 Jul;158(1):99–100. doi: 10.1006/dbio.1993.1170. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Hoyer-Fender S., Engel W. The nucleotide sequence of a human protamine 1 cDNA. Nucleic Acids Res. 1987 Sep 25;15(18):7639–7639. doi: 10.1093/nar/15.18.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luerssen H., Hoyer-Fender S., Engel W. The nucleotide sequence of human transition protein 1 cDNA. Nucleic Acids Res. 1988 Aug 11;16(15):7723–7723. doi: 10.1093/nar/16.15.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W. M., Adham I., Klemm U., Engel W. The nucleotide sequence of a boar protamine 1 cDNA. Nucleic Acids Res. 1988 Dec 23;16(24):11826–11826. doi: 10.1093/nar/16.24.11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew L. L., Dworkin-Rastl E., Dworkin M. B., Richter J. D. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 1989 Jun;3(6):803–815. doi: 10.1101/gad.3.6.803. [DOI] [PubMed] [Google Scholar]
- Morales C. R., Kwon Y. K., Hecht N. B. Cytoplasmic localization during storage and translation of the mRNAs of transition protein 1 and protamine 1, two translationally regulated transcripts of the mammalian testis. J Cell Sci. 1991 Sep;100(Pt 1):119–131. doi: 10.1242/jcs.100.1.119. [DOI] [PubMed] [Google Scholar]
- Munro H. N. Iron regulation of ferritin gene expression. J Cell Biochem. 1990 Oct;44(2):107–115. doi: 10.1002/jcb.240440205. [DOI] [PubMed] [Google Scholar]
- Murray M. T., Krohne G., Franke W. W. Different forms of soluble cytoplasmic mRNA binding proteins and particles in Xenopus laevis oocytes and embryos. J Cell Biol. 1991 Jan;112(1):1–11. doi: 10.1083/jcb.112.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J., Swenson K., Piwnica-Worms H., Richter J. D. Maturation-specific polyadenylation: in vitro activation by p34cdc2 and phosphorylation of a 58-kD CPE-binding protein. Genes Dev. 1991 Sep;5(9):1697–1708. doi: 10.1101/gad.5.9.1697. [DOI] [PubMed] [Google Scholar]
- Richter J. D. Information relay from gene to protein: the mRNP connection. Trends Biochem Sci. 1988 Dec;13(12):483–486. doi: 10.1016/0968-0004(88)90236-8. [DOI] [PubMed] [Google Scholar]
- Richter J. D., Smith L. D. Reversible inhibition of translation by Xenopus oocyte-specific proteins. Nature. 1984 May 24;309(5966):378–380. doi: 10.1038/309378a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. T., Hunt T., Ruderman J. V. Selective translation of mRNA controls the pattern of protein synthesis during early development of the surf clam, Spisula solidissima. Cell. 1980 Jun;20(2):487–494. doi: 10.1016/0092-8674(80)90635-2. [DOI] [PubMed] [Google Scholar]
- Sachs A. B., Davis R. W. The poly(A) binding protein is required for poly(A) shortening and 60S ribosomal subunit-dependent translation initiation. Cell. 1989 Sep 8;58(5):857–867. doi: 10.1016/0092-8674(89)90938-0. [DOI] [PubMed] [Google Scholar]
- Sinclair G. D., Dixon G. H. Purification and characterization of cytoplasmic protamine messenger ribonucleoprotein particles from rainbow trout testis cells. Biochemistry. 1982 Apr 13;21(8):1869–1877. doi: 10.1021/bi00537a026. [DOI] [PubMed] [Google Scholar]
- Sommerville J. RNA-binding proteins: masking proteins revealed. Bioessays. 1992 May;14(5):337–339. doi: 10.1002/bies.950140509. [DOI] [PubMed] [Google Scholar]
- Standart N., Dale M., Stewart E., Hunt T. Maternal mRNA from clam oocytes can be specifically unmasked in vitro by antisense RNA complementary to the 3'-untranslated region. Genes Dev. 1990 Dec;4(12A):2157–2168. doi: 10.1101/gad.4.12a.2157. [DOI] [PubMed] [Google Scholar]
- Standart N., Hunt T. Control of translation of masked mRNAs in clam oocytes. Enzyme. 1990;44(1-4):106–119. doi: 10.1159/000468751. [DOI] [PubMed] [Google Scholar]
- Tanhauser S. M., Hecht N. B. Nucleotide sequence of the rat protamine 2 gene. Nucleic Acids Res. 1989 Jun 12;17(11):4395–4395. doi: 10.1093/nar/17.11.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C. Tetraloops and RNA folding. Nature. 1990 Aug 16;346(6285):613–614. doi: 10.1038/346613a0. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Huarte J., Belin D., Gubler P., Vassalli A., O'Connell M. L., Parton L. A., Rickles R. J., Strickland S. Regulated polyadenylation controls mRNA translation during meiotic maturation of mouse oocytes. Genes Dev. 1989 Dec;3(12B):2163–2171. doi: 10.1101/gad.3.12b.2163. [DOI] [PubMed] [Google Scholar]
- Wightman B., Bürglin T. R., Gatto J., Arasu P., Ruvkun G. Negative regulatory sequences in the lin-14 3'-untranslated region are necessary to generate a temporal switch during Caenorhabditis elegans development. Genes Dev. 1991 Oct;5(10):1813–1824. doi: 10.1101/gad.5.10.1813. [DOI] [PubMed] [Google Scholar]
- Yelick P. C., Balhorn R., Johnson P. A., Corzett M., Mazrimas J. A., Kleene K. C., Hecht N. B. Mouse protamine 2 is synthesized as a precursor whereas mouse protamine 1 is not. Mol Cell Biol. 1987 Jun;7(6):2173–2179. doi: 10.1128/mcb.7.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelick P. C., Kwon Y. H., Flynn J. F., Borzorgzadeh A., Kleene K. C., Hecht N. B. Mouse transition protein 1 is translationally regulated during the postmeiotic stages of spermatogenesis. Mol Reprod Dev. 1989;1(3):193–200. doi: 10.1002/mrd.1080010307. [DOI] [PubMed] [Google Scholar]