Summary
Background
In north India, vitamin A deficiency (retinol <0·70 μmol/L) is common in pre-school children and 2–3% die at ages 1·0–6·0 years. We aimed to assess whether periodic vitamin A supplementation could reduce this mortality.
Methods
Participants in this cluster-randomised trial were pre-school children in the defined catchment areas of 8338 state-staffed village child-care centres (under-5 population 1 million) in 72 administrative blocks. Groups of four neighbouring blocks (clusters) were cluster-randomly allocated in Oxford, UK, between 6-monthly vitamin A (retinol capsule of 200 000 IU retinyl acetate in oil, to be cut and dripped into the child's mouth every 6 months), albendazole (400 mg tablet every 6 months), both, or neither (open control). Analyses of retinol effects are by block (36 vs 36 clusters). The study spanned 5 calendar years, with 11 6-monthly mass-treatment days for all children then aged 6–72 months. Annually, one centre per block was randomly selected and visited by a study team 1–5 months after any trial vitamin A to sample blood (for retinol assay, technically reliable only after mid-study), examine eyes, and interview caregivers. Separately, all 8338 centres were visited every 6 months to monitor pre-school deaths (100 000 visits, 25 000 deaths at ages 1·0–6·0 years [the primary outcome]). This trial is registered at ClinicalTrials.gov, NCT00222547.
Findings
Estimated compliance with 6-monthly retinol supplements was 86%. Among 2581 versus 2584 children surveyed during the second half of the study, mean plasma retinol was one-sixth higher (0·72 [SE 0·01] vs 0·62 [0·01] μmol/L, increase 0·10 [SE 0·01] μmol/L) and the prevalence of severe deficiency was halved (retinol <0·35 μmol/L 6% vs 13%, decrease 7% [SE 1%]), as was that of Bitot's spots (1·4% vs 3·5%, decrease 2·1% [SE 0·7%]). Comparing the 36 retinol-allocated versus 36 control blocks in analyses of the primary outcome, deaths per child-care centre at ages 1·0–6·0 years during the 5-year study were 3·01 retinol versus 3·15 control (absolute reduction 0·14 [SE 0·11], mortality ratio 0·96, 95% CI 0·89–1·03, p=0·22), suggesting absolute risks of death between ages 1·0 and 6·0 years of approximately 2·5% retinol versus 2·6% control. No specific cause of death was significantly affected.
Interpretation
DEVTA contradicts the expectation from other trials that vitamin A supplementation would reduce child mortality by 20–30%, but cannot rule out some more modest effect. Meta-analysis of DEVTA plus eight previous randomised trials of supplementation (in various different populations) yielded a weighted average mortality reduction of 11% (95% CI 5–16, p=0·00015), reliably contradicting the hypothesis of no effect.
Funding
UK Medical Research Council, USAID, World Bank (vitamin A donated by Roche).
Introduction
During the 1980s, several randomised trials were undertaken of periodic high-dose vitamin A supplementation (eg, 200 000 IU retinol every 6 months) in various populations of children with widespread biochemical vitamin A deficiency (plasma retinol <0·70 μmol/L [20 mg/dL]), severe deficiency (retinol <0·35 μmol/L [10 mg/dL]), or high child mortality from infection.1–9 Most reported significant reductions in child mortality (although two2,6 did not), and meta-analyses of all their results suggested that periodic vitamin A supplementation could reduce child mortality by 20–30%,10–14 mainly by reducing mortality from infective causes such as diarrhoea and measles.
Nevertheless, there remained some uncertainty about supplementation,15 and in many low-income populations in the 1990s few children received supplements. The policy in India was to encourage vitamin A supplementation to prevent eye disease, but in many parts of the country this was not widely implemented.16
In India, the Integrated Child Development Service (ICDS) maintains a network of child-care centres, caring for children up to age 6 years and offering the potential to deliver simple health interventions. In rural Uttar Pradesh in north India, our plans to use the ICDS infrastructure for a large cluster-randomised trial of the effects of 6-monthly deworming with albendazole on pre-school child mortality were revised into plans for a factorial trial that would also evaluate the effects on mortality of enhancing vitamin A coverage. This 5-year trial of Deworming and Enhanced Vitamin A supplementation (DEVTA) in 1 million pre-school children was larger than all other vitamin A trials combined. Its primary aim was to assess effects of a standard periodic treatment regimen on mortality at ages 1·0–6·0 years.17
Early analyses of the DEVTA vitamin A mortality findings were presented in 2007, have been available online ever since,18 and have been partly included in recent meta-analyses.19–24 Here we report final analyses of the DEVTA vitamin A findings, which are more robust than those (unpublished) early analyses and include more reliable estimates of compliance, plasma retinol concentrations, and the proportional effects on child mortality, which is the main endpoint. This report focuses on the vitamin A (ie, retinol) results. An accompanying report17 of the albendazole results includes fuller details of study logistics.
Methods
Study design
The unit of randomisation in this cluster-randomised trial was the block, a state administrative unit that generally has a rural population of more than 100 000. 36 blocks were cluster-randomly allocated retinol supplementation every 6 months for 5 years and 36 open control (figure 1). All were within a few hours of the main study centre in Lucknow, the Uttar Pradesh state capital. The study was approved by King George's Medical University ethics committee (Lucknow, India) and by the ICDS directors in each block, but no permissions were obtained from the population, except when blood samples were sought.
Figure 1.
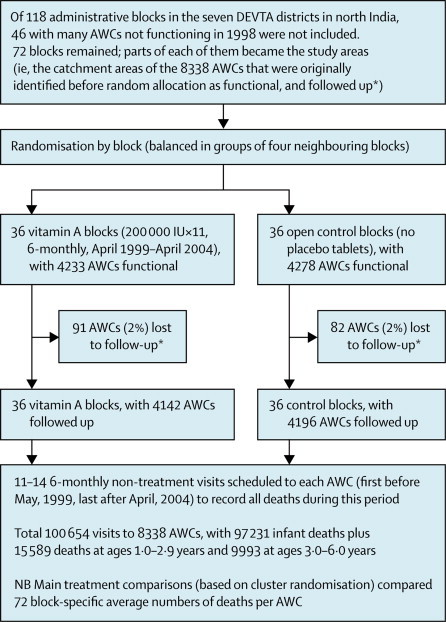
Flow diagram for the 72 mainly rural administrative blocks randomly allocated 5 years of 6-monthly vitamin A or open control
AWC: anganwadi (ie, courtyard) child-care centre. In these 72 blocks, 8338 child-care centres were followed up, with total population at ages 1·0–6·0 years 1 million and 5 million child-years at risk in the 5 years between May, 1999, and April, 2004. *AWC catchment areas correspond approximately to villages; it was determined before randomisation which AWCs were then functional, and hence potential study areas; loss of an AWC to follow-up was defined by having only 1–6 follow-up visits (mean only 3, as against 12 in included AWCs), and was generally because the AWC had ceased to function.
Within the participating blocks, the study population was all young children living in the defined catchment areas of 8338 village anganwadi child-care centres (AWCs; anganwadi means courtyard) that the ICDS regarded as functional (except in one block17) (figure 1). A typical rural AWC employs an ICDS-funded AWC child-care worker and serves a village with a population of about 1000 (with 10–15% aged 1·0–6·0 years); large villages can have more AWCs. AWCs register about two-thirds of the children of age 1·0–5·0 years (and one-third of 5-year-olds) for possible nutritional supplementation.
In a typical block these study catchment areas included about 10 000–20 000 pre-school children of age 1·0–6·0 years, so at any one time the study included 1 million such children. As the study began it had a million participants, and during it another million 1-year-olds joined it and a million 6-year-olds left (figure 2), so at one time or another it included about 2 million children. The study continued for 5 years (5 million child-years), and recorded 25 000 child deaths at ages 1·0–6·0 years.
Figure 2.
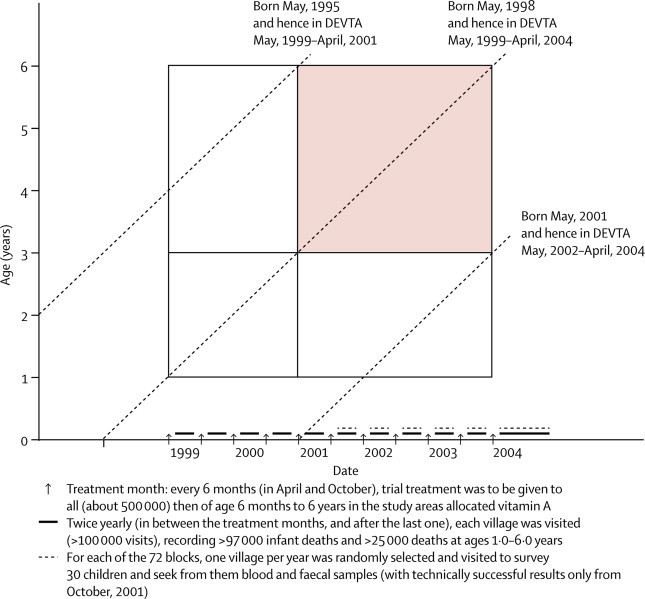
Times when treatment was to be given and mortality monitored, and range of ages (1·0–6·0 years) and dates (May, 1999–April, 2004) for inclusion in main analyses of child mortality
Shaded area indicates more than 2 years’ treatment already received. Diagonal lines describe involvement of children born on May 1 in 1995, 1998, and 2001. With 1 million children of age 1·0–6·0 years at any one time, 5 million child-years at risk are included.
Randomisation and masking
Neighbouring blocks (clusters), in groups of four (where possible in the same district), were randomly allocated in Oxford, UK, using a factorial design, to: (1) usual care; (2) 6-monthly vitamin A; (3) 6-monthly albendazole; or (4) both. No placebos were used; the control was open. Apart from the district each block was in, no relevant details of it were known to those generating the random allocation. This report compares retinol (36 blocks) versus not (36 blocks), largely ignoring albendazole allocation and stratum.
Procedures
The Sight and Life Foundation, funded by Roche (Basel, Switzerland), donated standard Roche capsules of 200 000 IU retinyl acetate in oil, to be cut and dripped into the child's mouth. Dosage was independent of age. When treatment became due (in April or October), in each treatment-allocated block a mass-treatment day was selected on which all children of apparent age 6–72 months in all study AWCs in that block would be given the trial treatment by their AWC worker. Those treated were checked off on the AWC lists, and the few children missed were often found by the AWC worker and treated soon afterwards.
On all mass-treatment days, unannounced visits were made to every fourth AWC on a numbered list to check that distribution had started. During the third study year, monitors abstracted from local records name, sex, age, and father's name for all (just over 1 million) pre-school children in the study areas (logistic details in accompanying report17). This mid-study census was used before each subsequent mass-treatment day to list all in 25 AWCs per block with predicted age 12–70 months (ie, well within the range to be treated); monitors established soon after whether those listed had actually been treated, and whether they were currently registered with the AWC.
Annually, Oxford randomly selected one AWC per block for fieldwork teams to survey 30 pre-school children (six per year of age) 1–5 months after a mass-treatment day, seeking from them blood and faecal samples. In practice, full information with complete assay results was obtained for about 24 children per block. The children surveyed were not randomly chosen and were often taken from AWC lists, hence mainly registered with the AWC. Caregivers were asked whether the previous mass treatment had been received (and, if so, whether any acute illness followed) and about recent (past 4 weeks) illnesses.
Fieldworker teams were trained to seek Bitot's spots and measure finger-prick haemoglobin. Venous blood in 5 mL EDTA vacutainers was transported chilled to Lucknow, separated next morning (15 min at 3500 rpm), stored at −20°C then flown on dry ice to Oxford for storage at −;80°C and retinol assay (appendix). Despite low retinol concentrations, individual measurements were reproducible, so the difference in plasma retinol with treatment allocation could be measured reliably. Retinol assay results were, however, available only after mid-study because earlier samples were biologically contaminated in storage and were unmeasurable.
Deaths were recorded by 18 full-time motorcycle village-to-village monitors.17 Monitors covered four neighbouring blocks at a time, one in each treatment group, and within 6 months went to all (roughly 500) study AWCs in those blocks to identify and visit all households where in the past year a liveborn infant or child younger than 10 years had died, recording age (often given only approximately, so our analyses of mortality at 1–5 completed years of age include all deaths at recorded ages 1·0–6·0 years), sex, name, parental names, and (by simple verbal autopsy) likely cause.
6 months later a different monitor visited the same blocks, again to record all deaths in the past year, so each death should have been reported twice, independently. Monitors knew supervisors would, with 5% probability, randomly re-study selected villages shortly afterwards to ensure relevant households had all been visited (and to help to consolidate monitors’ discipline and methodology).
Statistical analysis
The prespecified primary analysis was of pre-school child mortality (appendix), operationalised because of digit preference in stating ages to mortality at reported ages 1·0–6·0 years during the 5-year study period (secondarily subdivided into ages 1·0–2·9 and 3·0–6·0 years, and into study years 1–2 and 3–5). Analyses of effects of vitamin A allocation on any category of mortality (or on any other outcomes) derived only from the 72 block averages for the outcome or explanatory factors of interest, giving all blocks equal weight because they were of similar size.
Mortality analyses calculated for each block the mean number of child deaths recorded per study AWC and the mean number of infant deaths recorded per study AWC. Analyses of effects on child mortality (reflecting the cluster-randomised design) then regressed child deaths per AWC (72 values, one per block) on retinol allocation (0/1), with simultaneous adjustment for albendazole allocation (also 0/1), an interaction term (±1, included only in simultaneous analyses of both treatments) and infant deaths per AWC (an important explanatory factor that cannot have been materially affected by trial treatment and varied two-fold between blocks). Sensitivity analyses explored the relevance of not adjusting for infant mortality or of additional adjustment for district (by which randomisation had been stratified). All these analyses did not depend on the mid-study census, but further sensitivity analyses explored the effect of adjusting for the mid-study census estimate of child population per AWC (an unimportant factor, as it varied little between blocks).
Further logistic and statistical details are described elsewhere, including power calculations;17 if, as suggested by previous trials, treatment reduced mortality by at least 20% there was 95% chance of achieving p<0·01. SAS version 9.1 was used. This trial is registered at ClinicalTrials.gov, NCT00222547.
Role of the funding sources
Study sponsors had no role in design, conduct, analysis, or interpretation. SA, RP, DB, and SR had full data access and entirely controlled submission for publication.
Results
Compliance with treatment allocation was good. Unannounced visits to a quarter of the AWCs in vitamin A blocks on or just after each mass-treatment day found 99% (10 925/11 090 visits) were distributing treatment. Independently, enquiries a week after mass-treatment days about lists of named children from the mid-study census confirmed supplementation of 96% (133 602/138 966) of those registered with the AWC and 72% (80 048/111 227) of those not, largely independent of age (data not shown). Because two-thirds of under-5s and one-third of 5-year-olds were AWC-registered, overall compliance was about 86% (not 91%, as previously estimated18). Consistent with this finding, the biomedical surveys of randomly chosen AWCs, which tended to over-sample AWC-registered children, found caregivers reporting 91% (2337/2581) had received retinol on the previous mass-treatment day. On one occasion in mid-study, polio vaccine plus retinol was supposed to be given to all children in the study districts; other than this event, there was little non-trial retinol supplementation. Loss to follow-up was similarly uncommon in both treatment arms (figure 1), and was generally due to AWC closures.
Allocation to retinol halved the prevalence of severe deficiency 1–5 months after mass-treatment days. Retinol assays were available (only during the second half of the study) for 5165 children with complete information on all assays and questionnaire replies, 2581 in retinol-allocated versus 2584 in control blocks (table 1). Among them, mean retinol was 0·72 (SE 0·01) μmol/L versus 0·62 (0·01) μmol/L, a 16% increase (p<0·00001). These retinol values are both 1·2 times higher (see appendix) than the uncalibrated values reported previously.18 Since compliance with the previous mass supplementation was 91% in children who gave blood, but only 86% overall, the proportional increase in plasma retinol in the whole study population in retinol-allocated blocks was about 15%. Among those whose blood was tested, the prevalence of severe vitamin A deficiency (retinol <0·35 μmol/L) was 6% versus 13%. Haemoglobin, weight, and height were not significantly affected.
Table 1.
Effects of vitamin A allocation on plasma retinol, haemoglobin, weight, height, Bitot's spots, and recent ill health (generally as reported by the child's guardian) in a subsample from each of the 72 blocks during the second half of the study, by age
|
Ages 1·0–2·9 years |
Ages 3·0–6·0 years |
Ages 1·0–6·0 years |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin A (n=810) | Control (n=775) | 95% CI for difference | Vitamin A (n=1771) | Control (n=1809) | 95% CI for difference | Vitamin A (n=2581) | Control (n=2584) | 95% CI for difference | ||
| Biomedical measurements | ||||||||||
| Retinol | ||||||||||
| All participants (μmol/L) | 0·70* | 0·63 | −0·11 to −0·04 | 0·73† | 0·61 | −0·15 to −0·09 | 0·72† | 0·62 | −0·13 to −0·08 | |
| Boys (μmol/L) | 0·71‡ | 0·63 | −0·12 to −0·03 | 0·73† | 0·62 | −0·15 to–0·08 | 0·72† | 0·62 | −0·14 to −0·07 | |
| Girls (μmol/L) | 0·70‡ | 0·64 | −0·11 to −0·02 | 0·74† | 0·61 | −0·15 to–0·09 | 0·72† | 0·62 | −0·13 to −0·08 | |
| Retinol <0·35 μmol/L (%) | 6·9%§ | 11·9% | 1·2 to 8·7 | 5·7%† | 13·6% | 5·1 to10·7 | 6·4%† | 13·3% | 4·2 to 9·6 | |
| Retinol <0·70 μmol/L (%) | 50·9%§ | 61·9% | 4·7 to 17·5 | 47·0%† | 65·6% | 13·4 to 23·7 | 48·7%† | 64·8% | 11·4 to 20·9 | |
| Haemoglobin (g/L) | 95·4 | 94·9 | −2·4 to 1·5 | 103·6 | 103·3 | −1·9 to 1·3 | 99·7 | 99·3 | −1·8 to 1·0 | |
| Weight (kg) | 9·39 | 9·47 | −0·10 to 0·26 | 12·68 | 12·72 | −0·14 to 0·21 | 11·04 | 11·09 | −0·09 to 0·19 | |
| Height (cm) | 74·5 | 74·3 | −0·8 to 0·4 | 88·7 | 88·7 | −0·7 to 0·6 | 81·7 | 81·5 | −0·7 to 0·4 | |
| BMI (kg/m2) | 16·9 | 17·1 | −0·1 to 0·6 | 16·1 | 16·1 | −0·2 to 0·2 | 16·5 | 16·6 | −0·1 to 0·3 | |
| Eye problems (% prevalence) | ||||||||||
| Bitot's spots | 1·0% | 1·8% | −0·5 to 2·1 | 2·2%‡ | 4·8% | 0·9 to 4·3 | 1·4%‡ | 3·5% | 0·8 to 3·4 | |
| Bitot's spots or night blindness | 1·0% | 1·9% | −0·5 to 2·1 | 2·3%‡ | 5·0% | 0·9 to 4·3 | 1·5%‡ | 3·6% | 0·8 to 3·4 | |
| Boys | 1·8% | 1·7% | −1·7 to 1·7 | 3·8% | 6·0% | −0·4 to 4·9 | 2·7% | 4·5% | −0·2 to 3·7 | |
| Girls | 0·6% | 2·4% | −0·5 to 4·2 | 1·0%‡ | 3·8% | 0·9 to 4·6 | 0·4%‡ | 2·9% | 1·0 to 3·9 | |
| Bitot's spots, night blindness, or conjunctivitis in past 4 weeks | 2·3% | 4·6% | 0·5 to 4·1 | 3·9%‡ | 7·4% | 1·4 to 5·6 | 3·0%* | 6·2% | 1·4 to 4·9 | |
| Illness in past 4 weeks (% prevalence) | ||||||||||
| Conjunctivitis | 1·3% | 2·9% | 0·1 to 3·1 | 1·6% | 2·7% | −0·1 to 2·4 | 1·5%§ | 2·8% | 0·2 to 2·4 | |
| Diarrhoea | 39·0% | 44·1% | −1·8 to 12·0 | 32·2% | 34·5% | −2·2 to 6·9 | 36·1% | 39·0% | −1·3 to 7·1 | |
| Cough | 17·1% | 20·5% | −1·3 to 8·1 | 17·5% | 18·0% | −2·8 to 3·8 | 17·5% | 19·0% | −1·8 to 4·7 | |
| Runny nose | 13·8% | 13·6% | −5·5 to 5·2 | 10·4% | 11·7% | −1·9 to 4·5 | 11·5% | 12·6% | −2·1 to 4·1 | |
| Fast breathing | 3·7% | 5·0% | −1·1 to 3·7 | 2·3%‡ | 4·1% | 0·6 to 3·1 | 2·8%§ | 4·4% | 0·4 to 2·8 | |
| Difficult breathing | 3·6% | 4·5% | −1·3 to 3·1 | 2·3% | 3·5% | 0·0 to 2·4 | 2·9% | 3·9% | −0·2 to 2·2 | |
| Noisy breathing | 2·7% | 3·0% | −1·7 to 2·3 | 1·9% | 2·3% | −0·7 to 1·5 | 2·2% | 2·7% | −0·6 to 1·5 | |
| Measles | 1·8% | 1·4% | −1·6 to 0·9 | 1·3% | 0·6% | −1·6 to 0·3 | 1·5% | 1·0% | −1·4 to 0·3 | |
| Fever | 34·1% | 35·3% | −4·2 to 6·6 | 28·0% | 28·6% | −3·3 to 4·6 | 30·4% | 31·3% | −2·5 to 4·3 | |
| Skin infection | 14·0% | 14·6% | −3·4 to 4·5 | 12·4% | 15·0% | −1·1 to 6·3 | 13·2% | 15·1% | −1·5 to 5·3 | |
Biomedical visit to one random village per block per year (from mid-study): 5165 children with no data missing. Data from examination of children and caregiver interviews. Each entry is the mean of 36 block-specific values. The 95% CIs reflect possible effects of randomly choosing 36 out of the 72 block-specific values, ignoring the stratification. Results are standardised to ages 2·0, 4·0, or 3·0 years, respectively, for ages 1·0–2·9, 3·0–6·0, or 1·0–6·0 years, and to 50:50 averages for half-year season (dry/wet). Height, weight, and body-mass index (BMI) are further standardised for study half-years. Except where indicated, results are standardised 50:50 for sex. Compliance with previous vitamin A dosage was 90% in these children, but about 86% in all children allocated vitamin A.
p<0·001.
p<0·00001.
p<0·01.
Two-sided p<0·05.
The prevalence of Bitot's spots (1·4% vs 3·5%) was also halved, as was that of conjunctivitis within the past 4 weeks. Night blindness is difficult to assess in young children, and was uncommon in the absence of Bitot's spots. The proportion with any of these eye problems was 3·0% versus 6·2% (p<0·001). Of other indices of recent ill health, only chest infection with fast breathing was significantly reduced (2·8% vs 4·4%, p=0·03 [not significant with Bonferroni correction for multiple comparisons]).
During the entire study, monitors recorded 97 231 liveborn infant deaths (86 084 before age 6 months) and 25 582 child deaths at ages 1·0–6·0 years (figure 1). This figure includes any extra deaths identified by a special mid-study retrospective enquiry (which found similar numbers of missed child deaths in the two treatment groups, suggesting little ascertainment bias). Numbers of infant and child deaths per AWC in different blocks are plotted against each other in figure 3. The mean number of infant deaths per AWC was 11·7 (SD 1·8), the mean number of child deaths per AWC was 3·1 (SD 0·7), and there was a strong (0·69) correlation between the numbers of infant and child deaths per AWC in different blocks.
Figure 3.
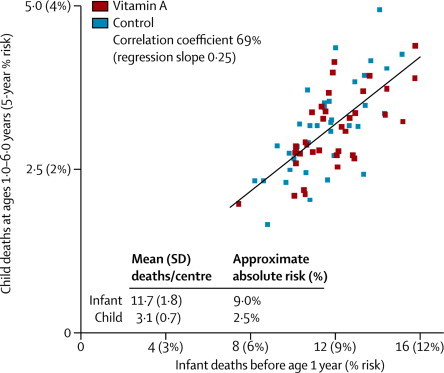
Correlation between 72 block-specific average numbers of infant and child deaths per child-care centre (AWC) during the entire study
The inter-block correlation (illustrated here) between numbers of infant and child deaths per AWC was 68·7% ignoring trial treatment allocation (or 68·4% given the four-way allocation to albendazole, retinol, both or neither), and ranged from 66–71% within the four treatment groups. Mortality at ages 0–6 months had correlation 99·3% with infant and 68·2% with child mortality.
Because any trial treatment in infancy began at around 9 months of age and most infant deaths occur much earlier, infant mortality cannot have been materially affected by it. Hence, this strong correlation reflects differences between low-risk and high-risk blocks that have nothing to do with trial treatment and that substantially affect both infant and child mortality (confirming this conclusion, the correlation between infant and child mortality was equally strong among blocks that had all had the same treatment; figure 3 legend). We therefore used the number of infant deaths as an explanatory factor to reduce chance variation in our main analyses of the effects of treatment allocation on the number of child deaths (see Methods; mortality at ages 0–6 months had correlation 99·3% with infant and 68·2% with child mortality).
Table 2 shows the findings for child mortality at ages 1·0–2·9 years, 3·0–6·0 years, and 1·0–6·0 years, along with the relative risk for the age range 1·0–6·0 years. Deaths per child-care centre at ages 1·0–6·0 years during the 5-year study (the primary trial endpoint) were 3·01 retinol versus 3·15 control (absolute reduction 0·14 [SE 0·11], mortality rate ratio [RR] 0·96, 95% CI 0·89–1·03, p=0·22), suggesting absolute risks of death between ages 1·0 and 6·0 years of approximately 2·5% retinol versus 2·6% control. Although this finding suggests that overall child mortality was 4% lower in vitamin A than in control blocks, this 4% reduction includes the possibility of no benefit and the possibility of appreciable benefit (95% confidence limit for reduction 11%). For, there is two-fold variation in block mortality rates (figure 3), and randomisation was by block rather than by AWC or by individual.
Table 2.
Effects of albendazole allocation on pre-school child mortality: absolute numbers of deaths per anganwadi child-care centre (AWC) by allocated treatment, albendazole versus control (A vs C), and, from these, mortality rate ratio (A/C) and approximate absolute risk of death from age 1·0 to 6·0 years
|
Number of child deaths recorded per AWC during the whole study, vitamin A versus control (A vs C) and 95% CI for the difference (C–A)* |
Mortality rate ratio (RR=A/C), age 1·0–6·0 years |
Absolute risk of death from age 1·0–6·0 years (%) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Age 1·0–2·9 years |
Age 3·0–6·0 years |
Age 1·0–6·0 years |
RR | 95% CI | Vitamin A | Control | ||||||||
| A | C | CI for (C–A) | A | C | CI for (C–A) | A | C | CI for (C–A) | ||||||
| Cause of death | ||||||||||||||
| Diarrhoea | 0·54 | 0·56 | −0·04 to 0·09 | 0·29 | 0·33 | −0·02 to 0·09 | 0·83 | 0·89 | −0·05 to 0·16 | 0·94 | 0·83 to 1·06 | 0·70% | 0·75% | |
| Pneumonia | 0·33 | 0·32 | −0·05 to 0·04 | 0·12 | 0·12 | −0·03 to 0·02 | 0·45 | 0·44 | −0·07 to 0·05 | 1·02 | 0·89 to 1·18 | 0·38% | 0·37% | |
| Measles | 0·10 | 0·13 | −0·01 to 0·06 | 0·09 | 0·08 | −0·04 to 0·02 | 0·19 | 0·21 | −0·04 to 0·07 | 0·91 | 0·69 to 1·19 | 0·16% | 0·18% | |
| Other infection/unknown | 0·57 | 0·61 | −0·05 to 0·12 | 0·42 | 0·46 | −0·03 to 0·10 | 0·99 | 1·07 | −0·06 to 0·21 | 0·93 | 0·82 to 1·06 | 0·83% | 0·90% | |
| Malnutrition | 0·19 | 0·18 | −0·05 to 0·03 | 0·05 | 0·06 | −0·01 to 0·02 | 0·24 | 0·24 | −0·06 to 0·05 | 1·02 | 0·82 to 1·27 | 0·21% | 0·20% | |
| Other or external | 0·11 | 0·11 | −0·03 to 0·02 | 0·18 | 0·19 | −0·02 to 0·04 | 0·30 | 0·30 | −0·04 to 0·05 | 0·98 | 0·84 to 1·15 | 0·25% | 0·26% | |
| All causes, by subgroup | ||||||||||||||
| Boys | 0·85 | 0·85 | −0·08 to 0·07 | 0·58 | 0·60 | −0·05 to 0·09 | 1·43 | 1·44 | −0·10 to 0·14 | 0·99 | 0·91 to 1·07 | 2·20% | 2·22% | |
| Girls | 0·99 | 1·06 | −0·02 to 0·16 | 0·59 | 0·64 | −0·00 to 0·11 | 1·58 | 1·70 | −0·00 to 0·24 | 0·93 | 0·86 to 1·00 | 2·92% | 3·15% | |
| May, 1999–April, 2001 (2 years) | 0·84 | 0·87 | −0·05 to 0·11 | 0·55 | 0·58 | −0·02 to 0·10 | 1·39 | 1·46 | −0·05 to 0·18 | 0·96 | 0·88 to 1·03 | 2·92% | 3·06% | |
| May, 2001–April, 2004 (3 years) | 1·00 | 1·04 | −0·06 to 0·14 | 0·62 | 0·65 | −0·05 to 0·12 | 1·62 | 1·69 | −0·10 to 0·25 | 0·96 | 0·86 to 1·06 | 2·26% | 2·37% | |
| Trial albendazole | 1·77 | 1·93 | −0·03 to 0·36 | 1·15 | 1·15 | −0·17 to 0·16 | 2·92 | 3·08 | −0·15 to 0·48 | 0·95 | 0·85 to 1·05† | 2·45% | 2·59% | |
| No trial albendazole | 1·92 | 1·89 | −0·23 to 0·17 | 1·18 | 1·32 | −0·02 to 0·31 | 3·10 | 3·21 | −0·20 to 0·43 | 0·96 | 0·87 to 1·06† | 2·60% | 2·70% | |
| All causes, total‡ | 1·84 | 1·91 | −0·07 to 0·21 | 1·16 | 1·24 | −0·05 to 0·19 | 3·01 | 3·15 | −0·08 to 0·36 | 0·96 | 0·89 to 1·03§ | 2·53% | 2·64% | |
Reduction (C–A) in number of child deaths per AWC and its standard error, s, were calculated by regression of 72 block-specific numbers of child deaths per AWC on vitamin A allocation (0/1), albendazole allocation (also 0/1), and on the block-specific numbers of infant deaths per AWC (to help to correct for any pre-existing variation in prognosis. RR is then A/C with 95% CI (A – x)/(C + x) to (A + x)/(C – x), where x=1·96s/2. Assuming approximately 119 (65 male, 54 female) children per AWC at ages 1·0–6·0 years, approximate absolute 5-year risks were calculated as 5 times (annual deaths per AWC)/(119, 65, or 54, as appropriate). Sensitivity analyses: further inclusion of district (as six indicators) or child population per AWC (which varied little) had no material effect.
Interaction p=0·83.
Mortality at ages 1–6 months had correlation 99·3% with infant and 68·2% with child mortality, so results were unchanged if it was used instead of infant mortality to correct for initial variation in prognosis; without either correction, numbers of child deaths per AWC at ages 1·0–2·9, 3·0–6·0, and 1·0–6·0 years would have been, respectively, 1·87 versus 1·88 (p=0·96), 1·18 versus 1·22 (p=0·50), and 3·05 versus 3·10 (p=0·74); RR 0·98 (0·89–1·08); absolute risks 2·56% versus 2·61%.
2-sided p=0·22.
Sensitivity analyses (table 2) showed that the RR had been little changed (although its CI was narrowed) by the adjustment for infant deaths, and would be little changed by further adjustment for district or for mean number of children per AWC. The RR for the apparent effects of retinol on child mortality was much the same if neither group got albendazole as it was if both groups got albendazole (interaction p=0·83). So, the RR was little changed (although its CI was widened) by restricting attention to 18 retinol-plus-albendazole versus 18 albendazole-alone blocks, where monitoring could not be biased by one group getting no treatment.
For no specific disease category was the RR significantly affected by the vitamin A allocation, although again the findings include the possibility of appreciable benefit and of no benefit. For diarrhoeal mortality (28% of all deaths) the RR was 0·94 (95% CI 0·83–1·06) and for measles mortality (6% of all deaths), which varied particularly widely between blocks, the RR was 0·91 (0·70–1·19). The annual number of deaths per AWC was greater at ages 1·0–2·9 than 3·0–6·0 years (particularly for diarrhoea, pneumonia, and malnutrition), greater in girls than in boys, and greater during the earlier than the later study years. For no category of disease, age, sex, or time period, however, was there a clearly significant effect of vitamin A allocation on child mortality (table 2). Even for those on treatment more than 2 years (ages 3·0–6·0 years in last 3 study years; figure 2) there was no significant difference in mortality between blocks allocated vitamin A and control.
Our mid-study census enumerated about 119 children of age 1·0–6·0 years per AWC (65 male, 54 female), with little variation between blocks (coefficient of variation 4·6%). This suggests absolute risks of death between ages 1·0 and 6·0 years of 2·5% retinol versus 2·6% control. If our census under-enumerated children, all the absolute risks in the last column of table 2 should be increased by a common factor, without affecting the ratios of the absolute risks to each other. In table 2 the overall numbers of child deaths per AWC and the mortality rate ratios are completely independent of our mid-study census of the child population per AWC, the findings from which affect only the estimates of absolute risk in the right-hand column.
Retinol administration was not associated with any immediate serious adverse effects. In the 36 retinol-allocated blocks, 356 children died during the 7 days before mass-treatment was due and 354 during the next 7 days (rate 0·4% per year, suggesting no immediate hazard). Caregivers were asked at biomedical surveys whether the previous treatment had been closely followed by any apparent illness; no material differences in vomiting, diarrhoea, or fever were seen, after allowing for the clustering of childhood infection outbreaks.17
Discussion
If considered in isolation from the previous community-based trials of vitamin A and child mortality, the present analyses of DEVTA yield only a non-significant mortality reduction. This result was unexpected, given that several1,3–5,7,8 of the previous trials had reported a significant mortality reduction. In interpretation of DEVTA, however, the key finding is not the mortality rate ratio of RR 0·96, but its 99% CI (0·87–1·05; figure 4). This CI is readily compatible both with zero effect and with prevention of somewhat more than 10% of all child mortality, but excludes prevention of much more than 13% of child mortality (panel).
Figure 4.
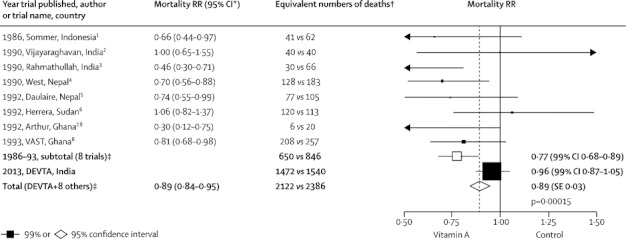
Published results from the eight large previous trials of regular vitamin A supplementation and child mortality, DEVTA results, and weighted averages of results from the other eight trials and from all nine trials
Heterogeneity between eight previous trials p=0·010; heterogeneity between DEVTA and subtotal of eight previous trials p=0·0010. Methods as in appendix p 9. Calculation of a weighted average does not assume the mortality rate ratios (RRs) in different studies are the same. The equivalent numbers of deaths are approximately additive when calculating weighted averages, but are not used in calculations. *95% CI to 2 dp derived from study publications. †Number of deaths (vitamin A vs control) in a large, 50:50 individually randomised trial that would yield the same RR and CI. Trials were excluded if they had a total of fewer than 20 such deaths, recruited patients with disease, or gave single-dose treatment. ‡From the inverse-variance-weighted average of log RR in each separate trial.
Panel. Research in context.
Meta-analyses of the previous trials21,22 of periodic vitamin A supplementation in developing countries had suggested that supplementation could generally reduce childhood mortality by about 20–30%. DEVTA shows that the mortality reduction is not as great as previous trials had suggested, but does not show it is zero. (It also shows retinol supplementation is not followed by any acute increase in the risk of death.) An updated meta-analysis, based on an appropriately weighted average of the present and previous trials, now suggests that where vitamin A deficiency is widespread, but xerophthalmia is rare, the reduction in childhood mortality from full compliance with supplementation is likely to be about 5–15%, only about a quarter or half as great as previously suggested.
DEVTA (1 million participants at any one time, 2 million ever) was larger than all the other trials combined and it was reliably conducted, with an average of about 86% compliance with 6-monthly supplementation and careful, unbiased ascertainment of child mortality. Furthermore, conventional indications for supplementation were present, since the control group had mean retinol 0·62 μmol/L and appreciable prevalences of severe biochemical vitamin A deficiency (retinol <0·35 μmol/L) and of Bitot's spots, which were both halved by allocation to vitamin A. Overall, mean plasma retinol increased by about 15% in children allocated vitamin A, an increase about as great as in the largest previous trial.7,8 Higher or more frequent doses might have had greater effects, but the regimen we tested is the one that is currently recommended internationally.
Initial analyses, presented orally in 2007,18 had also indicated a mortality ratio of 0·96, but were based on the estimated death rate in each block, which depended on our mid-study estimate of the number of children. In unpublished discussions of those initial analyses it was noted that errors in census methods might affect this denominator. We therefore undertook extensive further data checks—thereby eliminating a few duplicate records—and the mortality ratios in table 2 are now robustly based on the average number of deaths per AWC in each of the 72 blocks, which does not depend at all on knowledge of the AWC populations.
Single trial results should generally be considered not on their own, but together with all other randomised evidence.25,26 The most appropriate method of combining evidence from different trials is generally to take a weighted average of their results that gives proportionately more weight to those that are more reliable. To minimise the effects of chance on the weighted average, the weight given to each result should be inversely proportional to the variance of that result (yielding the inverse-variance-weighted average). Figure 4 shows the published results from the eight other trials1–8 (with the 99% CI for each calculated from the 95% CI), and gives an inverse-variance-weighted average of these eight results (RR 0·77, 99% CI 0·68–0·89; appendix p 9). The CIs generally1,3–8 allowed for any clustering. A recent Cochrane review of these eight previous trials plus the single-dose and smaller trials yielded a pooled RR of 0·76.21,22
Figure 4 also shows the result for DEVTA (RR 0·96) and for the aggregate of all nine trials (RR 0·89; appendix p 9). Calculation of a weighted average of several results does not implicitly assume that the real treatment effect in all those trials is the same25,26 (hence, although the term fixed-effect meta-analysis is sometimes used to describe such a weighted average, this usage is misleading). Nevertheless, the apparent discrepancy between DEVTA and the weighted average of the other eight trial results is significant (p=0·001) and was unexpected, since there is no obvious reason to expect the treatment effect to be much smaller in DEVTA than in most other trials. These apparently discrepant results need cautious interpretation, especially in view of the current controversies about vitamin A supplementation.23,24
It would not be appropriate to dismiss the previous trials and use DEVTA alone to argue that vitamin A supplementation confers no net benefit in India; DEVTA does not exclude a reduction of more than 10% in child mortality, which would be an important benefit, and there is no good reason to ignore the definite evidence of benefit from the aggregated results of the other eight trials.
Conversely, it would not be appropriate to dismiss the DEVTA result, or to conceal the reliability of it by a random effects meta-analysis (which, when trials are of very different sizes, implicitly gives undue weight to small trials and insufficient weight to large trials25,26). DEVTA contributes substantially more statistical information than all previous trials put together, is directly relevant to current supplementation strategies, and its 99% confidence limits exclude mortality reductions greater than 13%.
For illustrative purposes only, figure 4 and appendix p 9 give, along with each result, the equivalent number of deaths (vitamin A vs control) that would, if seen in a large, individually randomised (50:50) trial, yield the same RR and CI. Because the weights used were chosen to minimise the effects of chance on the weighted average, these equivalent numbers of deaths are approximately additive when information from different trials is combined, which helps to clarify how much each trial contributes to the weighted average. (These illustrative numbers were, however, not used in any calculations.)
The weighted average of all nine results yields a RR of 0·89 with 95% CI 0·84–0·95, p=0·00015. Thus, even if the benefits of vitamin A supplementation were by chance exaggerated in the previous trial results, they are not zero. To interpret the randomised evidence, it is appropriate to bear in mind the apparent discrepancy between the results from DEVTA and from the average of the other eight trials, but also to bear in mind the overall result obtained by combining both (yielding a weighted average of all nine results). This finding might well be the best guide to the proportional mortality reduction that should be expected in the future from reasonably good compliance with a programme of supplementation in populations with widespread vitamin A deficiency that is not extreme enough to cause much risk of xerophthalmia.
In such moderately deficient populations, the corresponding absolute risk reduction (and hence the number needed to treat for 5 years to prevent one death) depends on the child mortality rate, which varies substantially from one such population to another and from one decade to another, so no estimate of it is given. Provisional acceptance of a weighted average of all the trial results might offer an evidence-based way forward from current arguments about whether the effects of vitamin A supplementation on child mortality are substantial or negligible.23,24
The identical numbers of child deaths in DEVTA in the week before mass treatment and the next week is important evidence of safety, and suggests that the widely publicised observation from Assam27 of a number of deaths just after widespread retinol dosage was misleading.
Considering all of the randomised trial results, both the suggestion of no mortality reduction and the expectation of a large proportional mortality reduction are contradicted. The proportional reduction in child mortality achievable in such populations by good compliance with a programme of pre-school vitamin A supplementation is not zero, but it appears to be only about a quarter or half as great as the previous trials had suggested (perhaps 5–15%, to judge from the confidence interval for the weighted average of all trial results, rather than 20–30%). This, rather than the result of DEVTA alone or the results from the other eight trials without DEVTA, provides the most appropriate summary of the randomised evidence on the effect of vitamin A on child mortality in this and many other populations.
Acknowledgments
Acknowledgments
This report is dedicated to Martin Frigg (1943–201028). Our chief acknowledgment is to the anganwadi centre (AWC) workers, communities, and children in the 8338 participating AWCs; the seven district and 72 ICDS officials in the following districts and blocks (Lucknow: Mall, Malihabad, Bakshi Ka Talab, Chinhat, Sarojini Nagar, Kakori, Mohan Lal Ganj, Gosai Ganj; Unnao: S Karan, S Sirosi, Hasan Ganj, Miyan Ganj, Asoha, Auras, Safipur, Bangarmau, Fatehpur Chourasi, Bichhiya, Sumerpur, Bighapur, Purva, Ganjmuradabad; Kanpur: Kanpur Nagar I, Kanpur Nagar II, Sarsoul, Bilhour, Patara, Vidhnu, Choubepur, Kalyanpur; Sitapur: Mishrikh, Sakaran, Khairabad, Godlamau, Hargaon, Kasmanda, Machrehta, Biswan, Pahla; Lakhimpur Kheeri: Lakhimpur Kheeri, Nidyasan, Vijuva, Ishanagar, Dharora, Pasgawan, Mohammadi, Golagokaran Nath, Bakey Ganj, Mitouli, Paliya Kala, Nakha, Phool Behad, Behjam; Raibareli: Singhpur, Dalmau, Unchahar, Maharajganj, Bahadurpur, Salon, Harchanpur, Bachrawana, Khero, Tiloi; Hardoi: Kachhouna, Kothawa, Bilgram, Hariyawan, Pihani, Tadiawan, Sursa, Ahirori, Behinder); the Government of Uttar Pradesh Department of Health and Department of Woman and Child Welfare (with ICDS); and the Vice-Chancellor of King George's Medical University, M Bhandari. Rajiv Awasthi, Jill Boreham, Frances Davidson, Nilanthi de Silva, Martin Frigg, Andrew Hall, John Horton, Trudie Lang, Penny Nestel, Hongchao Pan, Lorenzo Savioli, and Sarman Singh helped to plan, execute, or comment on the study. Funding was received from USAID OMNI project, World Bank, and UK Medical Research Council (via CTSU). Albendazole (Zentel) was donated by SmithKlineBeecham (now GlaxoSmithKline), and vitamin A by Hoffman-La Roche via the Sight and Life Program. We acknowledge the CTSU's computing and other support in Oxford, including funds from the 1991 Helmut Horten cancer research award to Richard Peto and Richard Doll (1912–200529).
Contributors
SA, RP, and DB contributed to the design. SA and VP were responsible for organisation and conduct. SR and SC undertook data management. RP and SR were responsible for the analysis. RP, SR, and DB drafted the report. All authors contributed to redrafting.
DEVTA staff
Computing Lakshmi Ayyar, Atul Chandra, Vipin Chandra Joshi, Nisha Narang, Hasibur Rehman, Nikhil Saxena, Naveen Prakash, Anuradha Sharma, Ms Monika Sharma, Manish Tripathi, Deepak Kumar Upreti; Documentation Rohini Das, Anupama Lal, Tuhina Rastogi; Finance and accounts S S Mani, Dheeraj Chitransh, Lalit Pandey, Amit Tandon; Laboratory Durgesh Bajpai, Umesh Chandra, Arunesh Dwivedi, P K Pant, Vivekanand Shukla; Motorcycle monitors Amir Ahmad, Hafeez Ahmad, Jitendra Bahadur, Harish Chandra, Ramesh Chandra, Anil Chaturvedi, Shailesh Dwivedi, Amar Kumar, Digant Kumar, Mahesh Kumar, Neelu Kumar, Rajesh Kumar, Sudheer Kumar, Sunil Kumar I (who died while working for the study), Sunil Kumar II, Chandra Pal, Mahesh Prasad, Rakesh Kumar I, Rakesh Kumar II, Zafar Rashid, Hitler Singh, Hanslal Shukla, Kunj Bihari Shukla, Shiv Shanker Shukla, Vipin Bihari Shukla, Sangram Singh, Satyawan Singh, Shiv Shankar Verma, Shiv Singh Verma; Drivers: Ramesh Chandra, Inamul Haq, Mohd Kazi, Ajay Kumar, Keshav Kumar, Manoj Kumar, Sharawan Kumar, Bansi Lal, Ashok Kumar Tiwari; Office and fieldwork management Shrawan Awasthi, Alpana Maseeh, Safia Najeeb, Lalji Neetu, Shahnaaz Parween, B Rai, Tuhina Rastogi, Ajay Sharma, Reetu Shukla, Sudha Shukla, Lalji Shukla, Anuj Srivastava, Vinay Kumar Srivastava.
Conflicts of interest
We declare that we have no conflicts of interest.
Supplementary Material
References
- 1.Sommer A, Tarwotjo I, Djunaedi E. The Aceh Study Group. Impact of vitamin A supplementation on childhood mortality: a randomised controlled community trial. Lancet. 1986;1:1169–1173. doi: 10.1016/s0140-6736(86)91157-8. [DOI] [PubMed] [Google Scholar]
- 2.Vijayaraghavan K, Radhaiah G, Prakasam BS, Sarma KVR, Reddy V. Effect of massive dose vitamin A on morbidity and mortality in Indian children. Lancet. 1990;336:1342–1345. doi: 10.1016/0140-6736(90)92895-o. [DOI] [PubMed] [Google Scholar]
- 3.Rahmathullah L, Underwood BA, Thulasiraj RD. Reduced mortality among children in southern India receiving a small weekly dose of vitamin A. N Engl J Med. 1990;323:929–935. doi: 10.1056/NEJM199010043231401. [DOI] [PubMed] [Google Scholar]
- 4.West KP, Jr, Pokhrel RP, Katz J. Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet. 1991;338:67–71. doi: 10.1016/0140-6736(91)90070-6. [DOI] [PubMed] [Google Scholar]
- 5.Daulaire NMP, Starabuck ES, Houston RM, Church MS, Stukel TA, Pandey MR. Childhood mortality after a high dose of vitamin A in a high risk population. BMJ. 1992;304:207–210. doi: 10.1136/bmj.304.6821.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrera MG, Nestel P, El Amin A, Fawzi WW, Mohamed KA, Weld L. Vitamin A supplementation and child survival. Lancet. 1992;340:267–271. doi: 10.1016/0140-6736(92)92357-l. [DOI] [PubMed] [Google Scholar]
- 7.Arthur P, Kirkwood B, Ross D. Impact of vitamin A supplementation on childhood morbidity in northern Ghana. Lancet. 1992;339:361–362. doi: 10.1016/0140-6736(92)91677-z. [DOI] [PubMed] [Google Scholar]
- 8.Ghana VAST Study Team Vitamin A supplementation in northern Ghana: effects on clinic attendances, hospital admissions, and child mortality. Lancet. 1993;342:7–12. [PubMed] [Google Scholar]
- 9.Muhilal. Permeisih D, Idjradinata YR, Muherdiyantiningsih. Karyadi D. Vitamin A-fortified monosodium glutamate and health, growth, and survival of children: a controlled field trial. Am J Clin Nutr. 1988;48:1271–1276. doi: 10.1093/ajcn/48.5.1271. [DOI] [PubMed] [Google Scholar]
- 10.Tonascia JA. Public health significance of vitamin A deficiency and its control—proceedings of the Bellagio meeting on vitamin A deficiency and childhood mortality. Helen Keller International; New York: 1993. Meta-analysis of published community trials: impact of vitamin A on mortality. [Google Scholar]
- 11.Beaton GH, Martorell R, Aronson KA. Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. Final report of the Canadian International Development Agency. University of Toronto; Toronto: 1993. [Google Scholar]
- 12.Beaton GH, Martorell R, Aronson KA, et al. Effectiveness of Vitamin A supplementation in the control of young child morbidity and mortality in developing countries. ACC/SCN State of the Art Series, Nutrition Policy Discussion United Nations, Paper No. 13, 1993.
- 13.Fawzi WW, Chalmers TC, Herrera G, Mosteller F. Vitamin A supplementation and child mortality: a meta-analysis. JAMA. 1993;269:898–903. [PubMed] [Google Scholar]
- 14.Glasziou PP, Mackerras DEM. Vitamin A supplementation in infectious diseases: a meta-analysis. BMJ. 1993;306:366–370. doi: 10.1136/bmj.306.6874.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conclusions and Recommendations. Ministry of Health & Family Welfare; New Delhi, India: 2000. A Report on the National Consultation on the Benefits and Safety of Vitamin A Administration to Pre-school Children and Pregnant and Lactating Women. [Google Scholar]
- 16.Gragnolati M, Shekar M, Das Gupta M, Bredenkamp C, Lee Y-K. India's undernourished children: a call for reform and action. Health, Nutrition and Population Division, World Bank, Washington, DC, 2006. https://openknowledge.worldbank.org/bitstream/handle/10986/7241/368050REVISED0101OFFICIAL0USE0ONLY1.pdf?sequence=1 (accessed July 13, 2012).
- 17.Awasthi S, Peto R, Read S, Richards SM, Pande V, Bundy D, the DEVTA (Deworming and Enhanced Vitamin A) team Population deworming every 6 months with albendazole in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial. Lancet. 2013 doi: 10.1016/S0140-6736(12)62126-6. http://dx.doi.org/10.1016/S0140-6736(12)62126-6 published online March 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Awasthi S, Peto R, Read S, Bundy D. Six-monthly vitamin A from 1 to 6 years of age—DEVTA: cluster randomised trial in 1 million children in North India. Powerpoints and abstract of a talk presentated at 2007 micronutrient forum, Istanbul http://www.micronutrientforum.org/meeting2007/Tuesday/T1445%20Awasthi.pdf (accessed July 13, 2012), plus short 1998 DEVTA protocol. http://www.ctsu.ox.ac.uk/research/research-archive/devta-de-worming-and-enhanced-vitamin-a (accessed July 13, 2012).
- 19.Gogia S, Sachdev HS. Neonatal vitamin A supplementation for prevention of mortality and morbidity in infancy: systematic review of randomised controlled trials. BMJ. 2009;338:b919. doi: 10.1136/bmj.b919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371:417–440. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 21.Imdad A, Herzer K, Mayo-Wilson E, Yakoob MY, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age. Cochrane Database Syst Rev. 2010;12:CD008524. doi: 10.1002/14651858.CD008524.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Imdad A, Yakoob MW, Sudfeld C, Haider BA, Black RE, Bhutta ZA. Impact of vitamin A supplementation on infant and childhood mortality. BMC Public Health. 2011;11(suppl 3):S20. doi: 10.1186/1471-2458-11-S3-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latham M. The great vitamin A fiasco. World Nutrition. 2010;1:12–45. [Google Scholar]
- 24.West KP, Jr, Klemm RDW, Sommer A. Vitamin A saves lives. Sound science, sound policy. World Nutrition. 2010;1:211–229. [Google Scholar]
- 25.Early Breast Cancer Trialists' Collaborative Group . Treatment of early breast cancer: worldwide evidence, 1985–1990. Oxford University Press; Oxford: 1990. Introduction and methods available at http://www.ctsu.ox.ac.uk/research/meta-trials/ebctcg/original-methods-for-ebctcg-meta-analyses (accessed July 13, 2012). [Google Scholar]
- 26.Baigent C, Peto R, Gray R, Parish S, Collins R. Large-scale randomized evidence: trials and meta-analyses of trials. In: Warrell DA, Cox TM, Firth JD, editors. Oxford Textbook of Medicine. 5th edn. Oxford University Press; Oxford: 2010. pp. 31–45. [Google Scholar]
- 27.Kapil U. Update on vitamin A-related deaths in Assam, India. Am J Clin Nutr. 2004;80:1082–1083. doi: 10.1093/ajcn/80.4.1082. [DOI] [PubMed] [Google Scholar]
- 28.Anon In memoriam: Martin Frigg, 1943–2010. Sight and Life. 2010;3:64–66. [Google Scholar]
- 29.Peto R, Beral V. Sir Richard Doll CH OBE 28 October 1912–24 July 2005. Biogr Mems Fell R Soc. 2010;56:63–83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


