Abstract
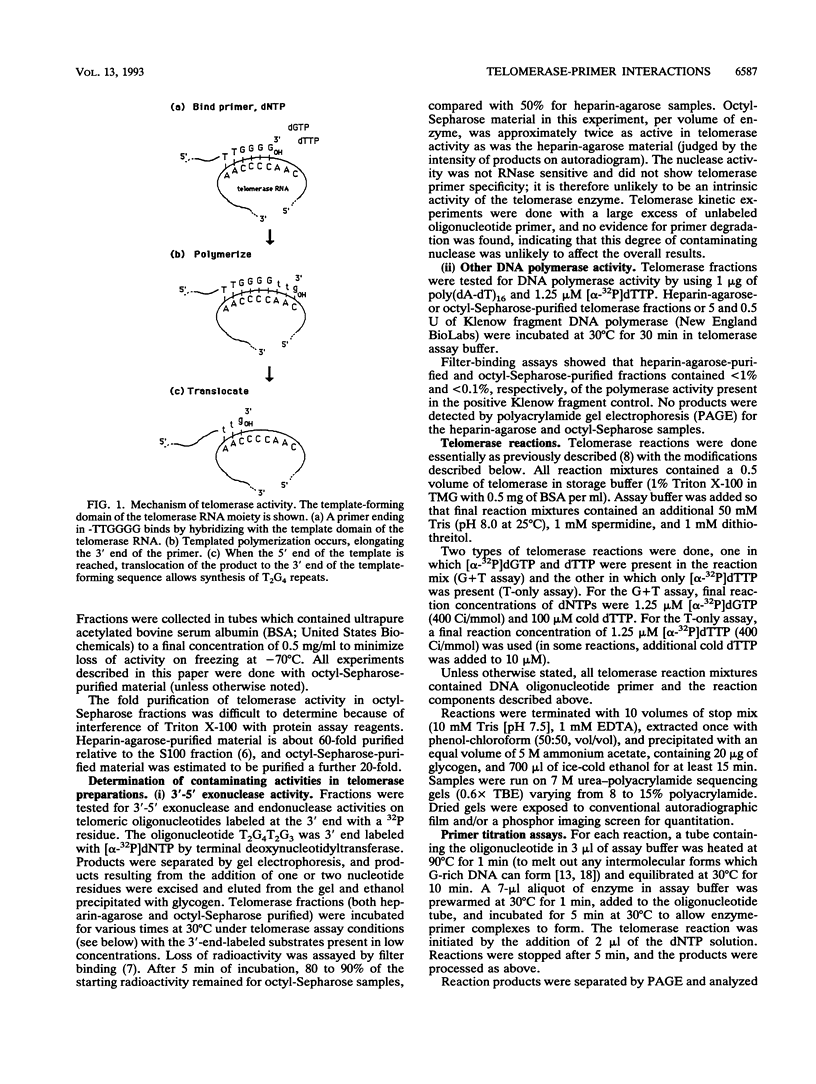
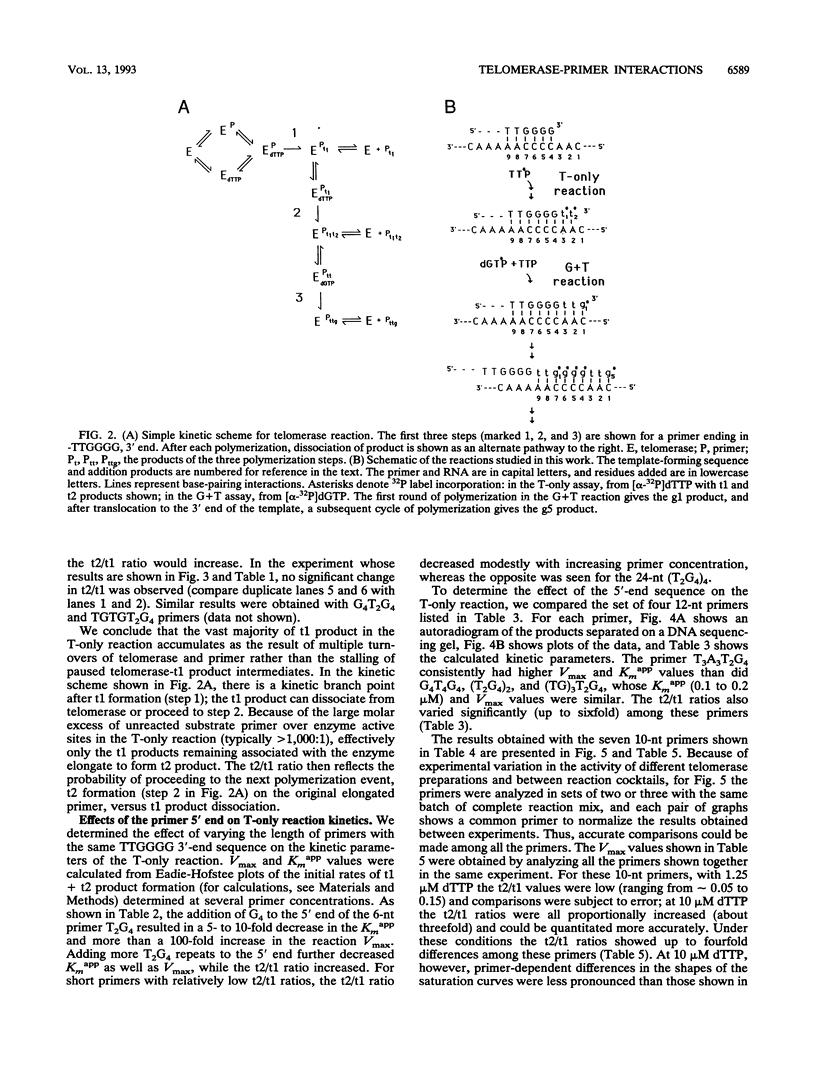
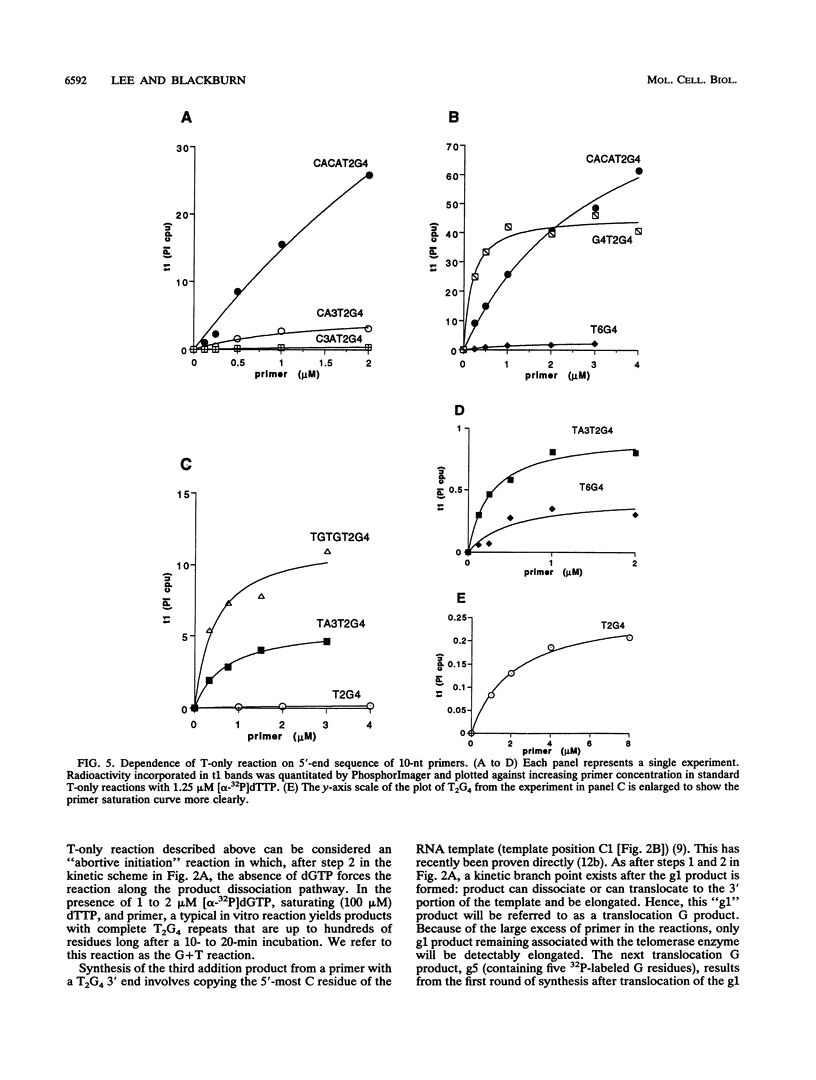
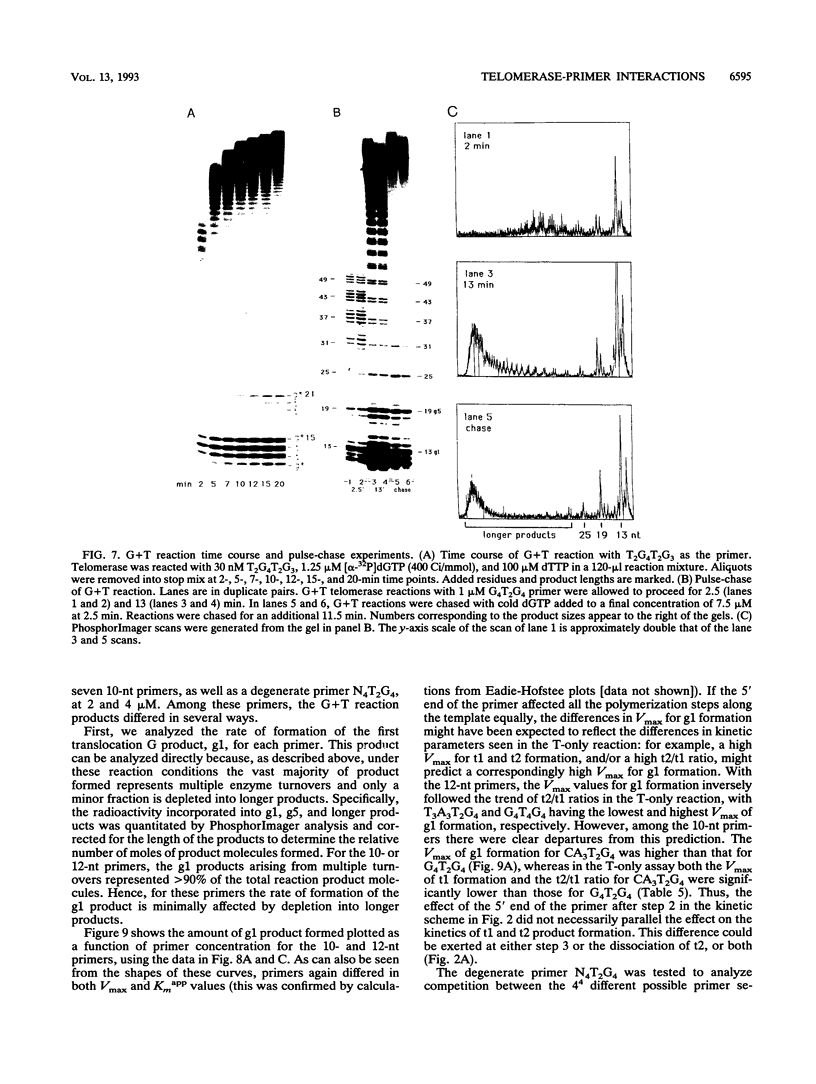
The ribonucleoprotein enzyme telomerase synthesizes one strand of telomeric DNA by copying a template sequence within the RNA moiety of the enzyme. Kinetic studies of this polymerization reaction were used to analyze the mechanism and properties of the telomerase from Tetrahymena thermophila. This enzyme synthesizes TTGGGG repeats, the telomeric DNA sequence of this species, by elongating a DNA primer whose 3' end base pairs with the template-forming domain of the RNA. The enzyme was found to act nonprocessively with short (10- to 12-nucleotide) primers but to become processive as TTGGGG repeats were added. Variation of the 5' sequences of short primers with a common 3' end identified sequence-specific effects which are distinct from those involving base pairing of the 3' end of the primer with the RNA template and which can markedly induce enzyme activity by increasing the catalytic rate of the telomerase polymerization reaction. These results identify an additional mechanistic basis for telomere and DNA end recognition by telomerase in vivo.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn E. H., Greider C. W., Henderson E., Lee M. S., Shampay J., Shippen-Lentz D. Recognition and elongation of telomeres by telomerase. Genome. 1989;31(2):553–560. doi: 10.1139/g89-104. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomerases. Annu Rev Biochem. 1992;61:113–129. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985 Dec;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987 Dec 24;51(6):887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- Greider C. W. Telomerase is processive. Mol Cell Biol. 1991 Sep;11(9):4572–4580. doi: 10.1128/mcb.11.9.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L. A., Greider C. W. Telomerase primer specificity and chromosome healing. Nature. 1991 Oct 3;353(6343):451–454. doi: 10.1038/353451a0. [DOI] [PubMed] [Google Scholar]
- Henderson E. R., Blackburn E. H. An overhanging 3' terminus is a conserved feature of telomeres. Mol Cell Biol. 1989 Jan;9(1):345–348. doi: 10.1128/mcb.9.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschlag D., Cech T. R. Catalysis of RNA cleavage by the Tetrahymena thermophila ribozyme. 2. Kinetic description of the reaction of an RNA substrate that forms a mismatch at the active site. Biochemistry. 1990 Nov 6;29(44):10172–10180. doi: 10.1021/bi00496a004. [DOI] [PubMed] [Google Scholar]
- Morin G. B. Recognition of a chromosome truncation site associated with alpha-thalassaemia by human telomerase. Nature. 1991 Oct 3;353(6343):454–456. doi: 10.1038/353454a0. [DOI] [PubMed] [Google Scholar]
- Raghuraman M. K., Dunn C. J., Hicke B. J., Cech T. R. Oxytricha telomeric nucleoprotein complexes reconstituted with synthetic DNA. Nucleic Acids Res. 1989 Jun 12;17(11):4235–4253. doi: 10.1093/nar/17.11.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon J. E., Miller W. H. Human immunodeficiency virus reverse transcriptase. Substrate and inhibitor kinetics with thymidine 5'-triphosphate and 3'-azido-3'-deoxythymidine 5'-triphosphate. J Biol Chem. 1990 Nov 25;265(33):20302–20307. [PubMed] [Google Scholar]
- Shippen-Lentz D., Blackburn E. H. Functional evidence for an RNA template in telomerase. Science. 1990 Feb 2;247(4942):546–552. doi: 10.1126/science.1689074. [DOI] [PubMed] [Google Scholar]
- Shippen-Lentz D., Blackburn E. H. Telomere terminal transferase activity from Euplotes crassus adds large numbers of TTTTGGGG repeats onto telomeric primers. Mol Cell Biol. 1989 Jun;9(6):2761–2764. doi: 10.1128/mcb.9.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. L., Blackburn E. H. Developmentally programmed healing of chromosomes by telomerase in Tetrahymena. Cell. 1991 Nov 15;67(4):823–832. doi: 10.1016/0092-8674(91)90077-c. [DOI] [PubMed] [Google Scholar]
- Yu G. L., Bradley J. D., Attardi L. D., Blackburn E. H. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990 Mar 8;344(6262):126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Williamson J. R., Cech T. R., Prescott D. M. Inhibition of telomerase by G-quartet DNA structures. Nature. 1991 Apr 25;350(6320):718–720. doi: 10.1038/350718a0. [DOI] [PubMed] [Google Scholar]
- Zakian V. A. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]






