Abstract
AIM: To determine the spectrum of pineal microstructures (solid/cystic parts) in a large clinical population using a high-resolution 3D-T2-weighted sequence.
METHODS: A total of 347 patients enrolled for cranial magnetic resonance imaging were randomly included in this study. Written informed consent was obtained from all patients. The exclusion criteria were artifacts or mass lesions prohibiting evaluation of the pineal gland in any of the sequences. True-FISP-3D-imaging (1.5-T, isotropic voxel 0.9 mm) was performed in 347 adults (55.4 ± 18.1 years). Pineal gland volume (PGV), cystic volume, and parenchyma volume (cysts excluded) were measured manually.
RESULTS: Overall, 40.3% of pineal glands were cystic. The median PGV was 54.6 mm3 (78.33 ± 89.0 mm3), the median cystic volume was 5.4 mm3 (15.8 ± 37.2 mm3), and the median parenchyma volume was 53.6 mm3 (71.9 ± 66.7 mm3). In cystic glands, the standard deviation of the PGV was substantially higher than in solid glands (98% vs 58% of the mean). PGV declined with age (r = -0.130, P = 0.016).
CONCLUSION: The high interindividual volume variation is mainly related to cysts. Pineal parenchyma volume decreased slightly with age, whereas gender-related effects appear to be negligible.
Keywords: Pineal gland volume, Pineal cyst, Magnetic resonance imaging, Etiology, Reference range
INTRODUCTION
The enormous interindividual variation of the pineal gland complicates radiological evaluations. Frequently, cases with a presumably enlarged solid or cystic pineal gland cannot be classified due to the lack of reliable data for comparison. The differential diagnosis of pineal enlargements includes pineal neoplasms, such as pineocytoma, pinealoblastoma and germinoma.
For the definition of normal pineal volume and morphometry, cross-sectional imaging studies have not yet provided sufficient data. A slice thickness of 4 mm to 10 mm is used for computed tomography, and most magnetic resonance imaging (MRI) suggest that this structure has a mean size of 6-10 mm[1]. Recently, high-resolution 3D-MRI (1 mm isotropic voxel size or less) has been applied for the characterization of the pineal gland in more detail[2-5] and for 3D-volumetry of the pineal gland[5,6]. Bumb et al[6] measured pineal volume in a clinical pediatric population, whereas a study by Sun et al[5] included young Chinese adults aged 20-30 years (n = 112) with the intention of assisting in the early diagnosis of small pineal lesions. The authors of that study concluded that the exploration of a larger age range is required to define the reference values of high resolution 3D-volumetry for clinical evaluations. Therefore, the principal goal of our study is to provide a reference range for pineal volume (and the number and size of cystic changes) in a broader clinical population ranging from young adulthood to old age.
Moreover, prior histological and high-resolution MRI studies have indicated that there is high interindividual variability in the size of the pineal gland[4,7,8]. The extent of this variability is unique among the organs of the central nervous system. Although cysts are a common finding in autoptic[7,8] and imaging studies[4], the degree to which cysts and solid parenchyma contribute to this variability is not known.
Consequently, we used high-resolution 3D-MRI to analyze in detail which morphological part of the pineal gland (cystic or solid part) contributes to the high variability. As the underlying causes of the variability are not well understood, we further investigated the effect of age and gender on the volume and microstructure of the pineal gland.
MATERIALS AND METHODS
Population and MRI protocol
A total of 347 patients enrolled for cranial MRI were randomly included in this study. Written informed consent was obtained from all patients. The exclusion criteria were artifacts or mass lesions prohibiting evaluation of the pineal gland in any of the sequences.
MRI scans were performed using two 1.5-T scanners (Siemens Sonata and Avanto, Siemens, Erlangen, Germany). The MR sequences for all patients included transversal T1-weighted spin echo (T1-SE, TR/TE 434/11), T2-weighted turbo spin echo (T2-TSE, TR/TE 3900/101), fluid-attenuated inversion recovery (TR/TI/TE 9000/2500/89, all 24 slices, 5 mm slice thickness, 20% gap, field of view 205 mm × 230 mm, matrix 448 mm × 304 mm), and true fast imaging with steady state precession (trueFISP, TR/TE 7.1/3.5, field of view 178 mm × 220 mm, matrix 212 mm × 275 mm, 36 slices, slice thickness 800 μm). A diagnosis for each patient was extracted from the medical records.
Evaluation
All images were evaluated digitally using OsiriX software (www.osirix-viewer.com). Two experienced neuroradiologists blinded to the clinical information evaluated the images.
Pineal gland volume (PGV) was measured as described previously[4,9]. In brief, the contour of the pineal gland was manually traced on transversal reconstructed trueFISP images using the OsiriX volume quantification tool. If cysts were detected in the trueFISP imaging, then the total cyst volume (TCV) was also measured. Pineal parenchyma volume (PPV) was defined as PGV-TCV.
To determine the intrarater variability, the same evaluator assessed all datasets a second time with a time gap of three weeks. To determine the interrater variability, a second evaluator unaware of the results of the first evaluation assessed ten randomly chosen datasets.
Ethical approval
The local ethics committee waived ethical approval of this study.
Statistical analysis
For the statistical analysis, SPSS 18.0 (IBM, IL, United States) was used. To determine intra- and interrater variability of the volume measurements, the Pearson correlation coefficient and the paired samples t test was used. Student’s t test was used to detect gender differences in PGV, PPV and TCV. The Pearson correlation coefficient was computed for the relationship between age and PPV, TCV, and number of cysts. For all tests, a significance level of 0.05 was used. Descriptive values are given as the mean ± SD if not otherwise specified.
RESULTS
Reliability of measurements
To assess intrarater variability, all 347 datasets were assessed twice by one evaluator with a time gap of three weeks (PPV: mean of first assessment 71.9 ± 66.6 mm3, mean of second assessment 72.3 ± 66.8 mm3). The Pearson correlation coefficient was 0.999 (P < 0.001). There was no difference between the evaluations (P < 0.001).
To assess interrater variability, ten of the 347 datasets were randomly chosen and assessed by a second evaluator. There was no difference between the two evaluators for these ten datasets (P < 0.05). The Pearson correlation coefficient between both evaluators was 0.829 (P = 0.003). The coefficients indicate an adequately high reliability.
Population
The study population comprised 347 individuals (55.4 ± 18.1 years, range 19-94 years; 47.0% female and 53.0% male). The diagnoses included intracranial neoplasm [glioma (12.1%), meningioma (4.3%), metastasis (5.2%), and other intracranial neoplasm (4.9%)], ischemia (25.4%), hemorrhage (3.7%), inflammatory disease of the central nervous system (3.5%), epilepsy (1.7%), hydrocephalus (9.5%), other diagnosis (13.5%), and normal findings (16.1%).
Figure 1 illustrates the smallest and largest solid pineal gland as well as the largest monocystic gland. The mean PGV, PPV and TCV values are shown in Table 1. Of the 347 pineal glands, 40.3% contained cysts. More than 10% were multicystic (29.1% with one cyst, 9.5% with two cysts, 1.2% with three cysts, 0.3% with four cysts and 0.3% with five cysts).
Figure 1.
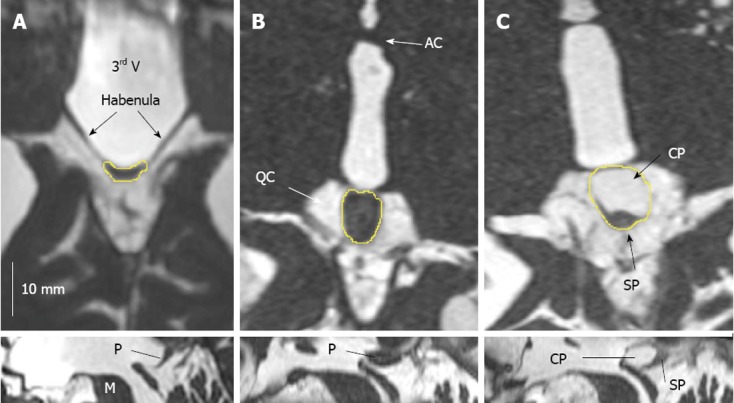
TrueFISP imaging of the pineal region illustrating the spectrum of pineal volumes and cysts. A, B: Solid glands. Smallest pineal parenchyma volume (PPV), 5 mm3, the small pineal gland can hardly be demarcated from the habenula (A); largest PPV, 228 mm3 (B); C: Largest single cyst, 189 mm3. The pineal gland was axially reformatted along its long axis. the cystic part (CP) of the pineal gland is larger than the solid part (SP) and accounts for most of the pineal volume. Upper row: Axial reconstruction; Lower row: Sagittal reconstruction. 3rd V: Third ventricle; AC: Anterior commissure; M: Mesencephalon; P: Pineal gland; QC: Quadrigeminal cistern.
Table 1.
Morphometric characteristics of 347 pineal glands
| Gender | F/M | F | F | F | F | F | M | M | M | M | M |
| Age (yr) | > 18 (n = 347) | > 18 (n = 163) | 18-39 (n = 28) | 40-59 (n = 63) | 60-79 (n = 57) | > 80 (n = 15) | > 18 (n = 184) | 18-39 (n = 41) | 40-59 (n = 59) | 60-79 (n = 79) | > 80 (n = 5) |
| PGV (mL) | |||||||||||
| Median | 54.6 | 54.6 | 73.0 | 54.6 | 50.1 | 54.9 | 54.6 | 77.8 | 58.0 | 51.3 | 27.2 |
| mean ± SD | 78.3 ± 89.0 | 82.0 ± 107.8 | 118.5 ± 191.7 | 78.8 ± 78.7 | 72.3 ± 88.9 | 64.3 ± 31.9 | 75 ± 68.3 | 102.7 ± 99.9 | 56.2 ± 31.1 | 77.8 ± 65.5 | 26.1 ± 7.1 |
| PPV (mL) | |||||||||||
| Median | 53.6 | 53.6 | 69.8 | 53.6 | 48.4 | 54.9 | 53.5 | 73.3 | 58.0 | 51.3 | 27.2 |
| mean ± SD | 71.9 ± 66.7 | 74.0 ± 78.1 | 100.8 ± 133.4 | 72.1 ± 60.0 | 66.3 ± 66.5 | 61.4 ± 27.1 | 70.1 ± 55.0 | 92.3 ± 74.5 | 54.6 ± 28.0 | 72.9 ± 55.5 | 25.9 ± 6.9 |
| TCV (mL) | |||||||||||
| Median | 5.4 | 5.3 | 6.3 | 6.3 | 4.7 | 3.2 | 5.4 | 5.3 | 2.5 | 8.4 | 1.0 |
| mean ± SD | 15.8 ± 37.2 | 18.6 ± 46.7 | 30.9 ± 77.8 | 15.6 ± 29.3 | 16.6 ± 40.7 | 7.3 ± 8.3 | 12.9 ± 24.3 | 20.2 ± 36.4 | 5.7 ± 7.4 | 12.3 ± 19.3 | 1.0 (n = 1) |
| Number of cysts (in cystic glands) | 1.3 ± 0.6 | 1.3 ± 0.6 | 1.3 ± 0.4 | 1.4 ± 0.6 | 1.2 ± 0.5 | 1.3 ± 0.5 | 1.4 ± 0.7 | 1.2 ± 0.4 | 1.5 ± 1.2 | 1.4 ± 0.6 | 1 (n = 1) |
| Cystic glands | 40.3% | 42.9% | 57.1% | 42.9% | 36.8% | 40.0% | 38.0% | 51.2% | 28.8% | 39.2% | 20.0% |
| PPVsolid | |||||||||||
| Median | 42.7 | 42.1 | 38.2 | 43.7 | 37.3 | 48.5 | 43.4 | 46.6 | 45.4 | 42.9 | 24.2 |
| mean ± SD | 46.6 ± 26.8 | 45.0 ± 23.0 | 41.9 ± 22.0 | 43.7 ± 19.0 | 45.4 ± 27.5 | 52.6 ± 20.9 | 47.9 ± 29.6 | 52.5 ± 29.2 | 47.7 ± 24.4 | 48.2 ± 34.4 | 25.0 ± 7.7 |
F: Female; M: Male; PGV: Pineal gland volume; PPV: Pineal parenchyma volume; TCV: Total cyst volume.
Influence of gender
The differences in the median PGV, PPV, and TCV between females and males were subtle (Table 1). The median PGV was equivalent in females and males. The median PPV was slightly higher in females, whereas the median TCV was higher in males.
The mean PGV and PPV were slightly higher in females than in males, but the difference was far from significant (P = 0.464 and P = 0.586, respectively) (Table 1 and Figure 2A and B).
Figure 2.
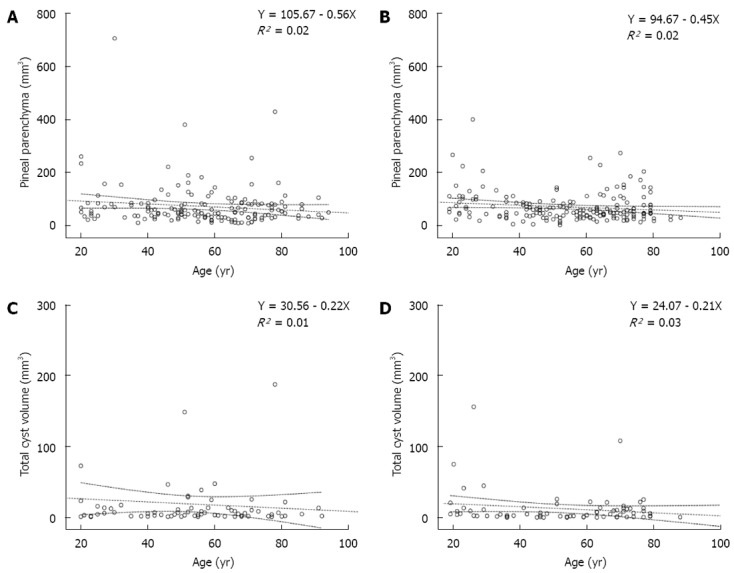
Regression diagram of pineal parenchyma and age. A, B: In both females (A) and males (B), the mean pineal parenchyma volume decreased slightly with age; C, D: In both females (C) and males (D), the total cystic volume of the pineal gland decreased slightly with age. Dotted line: Linear regression and 95% mean prediction interval.
Females displayed almost 6% more cystic glands than males. However, females had a smaller mean TCV, although this difference was not significant (Table 1 and Figure 2C and D).
There was no difference with respect to the number of cysts (P = 0.516) (Table 1).
In both females and males, PGV, PPV, and TCV had high standard deviations (Table 1), indicating high interindividual variations.
To focus on the relationship between pineal parenchyma and gender, we factored out any possible effect of the cysts and analyzed only the solid glands (PPVsolid in Table 1). Interestingly, even in this case, the difference between the sexes was still negligible.
Females: In women (n = 163), the mean PPV decreased with age (Figure 2A) (r = -0.13, P = 0.097). Among the cysts in females, TCV exhibited a weakly negative correlation with age (Figure 2C). TCV and number of cysts showed no significant correlation with age (P = 0.455). TCV showed a strong positive correlation with PPV (P < 0.001, Pearson correlation coefficient 0.934).
Males: In men (n = 184), the mean PPV exhibited a weakly negative correlation with age (P = 0.044, r = -0.149) (Table 1 and Figure 2B). Regarding cysts in males, TCV showed a weakly negative correlation with age, although this correlation was not significant (Figure 2D). The number of cysts showed no significant correlation with age (P = 0.744, r = -0.024). Similar to women, TCV was strongly correlated with PPV in men (P < 0.001, Pearson correlation coefficient 0.846).
Influence of age
PPV declined with age (r = -0.135, P = 0.012). PGV also declined with age (r = -0.130, P = 0.016).
There were no significant correlations between age and the number of pineal cysts (P = 0.242) and between age and the TCV (P = 0.190).
To exclude a potential effect of the cysts on the volume measuring procedure, we analyzed the solid glands separately (n = 207). Here, the correlation with age was slightly worse (R2 = 0.003, Y = 51.13 - 0.079X), which highlights that the effect of age on the pineal volume is minor.
Interindividual variation: Secondary to the solid or cystic component?
The median and mean PGV, PPV, TCV, and the PPVcystic/solid values are shown in Table 1. The expected high interindividual variability of PGV is reflected by a standard deviation of 114% of the mean PGV (PGV 78.3 ± 89.0 mm3).
To answer the question of whether the interindividual variation of the PGV is secondary to the solid or cystic component, the standard deviations of the solid and cystic glands were compared. The standard deviation of the PGVsolid was 58% of the mean (46.6 ± 26.8 mm3). This variation suggests that substantial variation is secondary to the solid parenchyma. The standard deviation of PGVcystic was 98% of the mean (125.2 ± 122.2 mm3), emphasizing the high influence of the cystic component. Accordingly, TCV shows a high variation. Taken together, the variation in the cystic component of the pineal gland adds more to the variation than the solid component.
DISCUSSION
The pineal gland is an important neuro-endocrine organ located in the center of the brain. It is one of the circumventricular organs lacking a blood-brain barrier[10]. Its major function is circadian production of the hormone melatonin, the most important hormone in chronobiology[11].
The distinction between normal pineal tissue and a small tumor can be problematic. In T1- and T2-weighted imaging, the signal characteristics are similar[12,13]. As the pineal gland lacks a blood-brain barrier, a distinction by simple enhancement differences is not possible. Therefore, the establishment of normal reference values of the pineal volume is important for their evaluation.
The development of new techniques has improved the radiological precision of pineal imaging. Before the introduction of high-resolution 3D-sequences, PGV could only be estimated indirectly from two-dimensional measurements. This estimation included the measurement of up to three diameters and a geometric model (for example: globe). However, the shape of the pineal gland is known to be highly variable[14], and consequently, there is only a very weak correlation between the estimated volume and the true volumetric value[5]. Until now, no study has directly quantified volume in adults older than thirty years. Two studies used direct volume quantification for pediatric patients[6] and for young male adults up to 30 years of age[5]. For clinical practice, however, a much broader age range is necessary[5].
For the first time, we present precise volumetric data covering the whole spectrum of adulthood. For young adults[5], a mean pineal volume of 94 mm³ was found. In our study, the mean for this age group (18-39 years) was higher (109 mm³). Although differences in the population may play a role, the choice of the volumetric sequence may also be influential [Sun et al[5] used non-isotropic T1-weighted 3D-sequence (fast spoiled gradient echo)]. Because the contrast of a small structure surrounded by the cerebrospinal fluid is obviously higher in heavily T2-weighted imaging than in T1-weighted imaging, we opted for the trueFISP sequence, an isotropic heavily T2-weighted 3D-sequence. As a consequence, the observed organ border volumes were more precise, the partial volume effects were minimized, and the volumes measured were more valid.
Sequence choice was also the reason for the higher prevalence of pineal cysts in our analysis. Pineal cysts were detected in 40.3% of the participants in the present study, which is in good agreement with histological studies reporting prevalences of 39.1%[7]. Similar prevalences were reported for trueFISP imaging by Nolte et al[4] and Bumb et al[6] (35.1% in adults and 57.4% in children). The studies using T1-weighted 3D-sequences by Pu et al[2] and Sun et al[5] found prevalences of only 25% and 23%, respectively. Previous MRI studies using 2D-sequences with a slice thickness of more than 3 mm reported substantially lower prevalences of 0.14%-4.3%[15-20]. Obviously, both the chosen sequence and the resolution may influence the sensitivity.
Theories concerning the pathogenesis of pineal cysts include a dysontogenetic residual of the embryonic pineal diverticulum and a degeneration within a glial plaque or within a cluster of pinealocytes[16,21-23].
A significant effect of the degenerative hypothesis would entail more and possibly larger cysts with increasing age. We found no correlation either between age and TCV or between age and the number of cysts. This result is a strong argument against a significant influence of age on most pineal cysts. The stable TCV and the stable number of cysts support the assumption that the dysontogenetic mechanism is predominant for the formation of pineal cysts.
Some authors suggest that pineal cysts are subject to hormonal influence[16,24]. In our study, we analyzed the prevalence, number of cysts, and cystic pineal volume but did not find significant gender differences. Other high-resolution MRI studies analyzing cyst prevalence reported that these values were slightly higher in females[2] or that there was no difference between males and females[5]. Nevertheless, longitudinal studies are necessary to fully answer this question with respect to hypothetical volume changes during the menstrual cycle.
Phylogenetically, the pineal gland is a very old structure. It is present not only in almost all mammals but also in a number of lizards, fishes, reptiles (but not in crocodiles), birds, and even probably in certain dinosaurs (brachiosaurus, among others)[25]. An interesting finding is that across the different species, the volume appears to be dependent on both the degree of latitude (the more distant from the equator, the larger the volume) and nocturnality and diurnality (e.g., a smaller volume in owls and a larger volume in horses).
In humans, the variation in brain structures is relatively small; for example, the hippocampus volume of young and healthy adults varies by approximately < 10% of the mean (SD of the mean)[26]. In contrast, the pineal weight has been reported to vary approximately 50% in an autopsy study, with an estimated volume range from 20 to 330 mm3[7]. A study of young Chinese adults (20 to 30 years) reported a mean pineal volume of 95 ± 31 mm3[27]. Despite the observation that pineal cysts are very common (13%-57% in high resolution 3D-imaging, depending on the sequence, resolution and minimal diameter[2,4-6]), until now, whether there is “true” variation in the hormone-producing parenchyma or whether the cysts are the cause of the high variability in humans was not clear.
To clarify whether the variation in PGV is secondary to the cystic and/or solid compartments, we determined that the volume variation in cystic glands was much higher (almost twice) than in purely solid glands (as a percent of the mean). This finding is contrary to the study by Sun et al[5], who reported higher variations in solid than in “macrocystic” glands (standard deviation 29 mm3 vs 20 mm3). In this study, however, the small sample size of the macrocystic group (n = 7) might explain the contra-intuitive finding.
Although the high variation of cystic glands may not be surprising, the observation that the variation in the solid glands was considerably high in both women and men throughout the different age groups is important for the evaluation of the pineal gland and has not been reported so far.
Similar striking interindividual differences have been reported for the production of melatonin[28-30]. In a pilot study, Nolte et al[3] indicated that the production of melatonin was linked to the volume of the solid pineal tissue. Interestingly, the high variability of the solid pineal volume reported here appears to mirror the highly varying melatonin production in adults.
The reason for the high interindividual variability of the pineal volume is not clear. By studying this variability with respect to age, we found a weakly significant negative correlation (r = -0.130). In other words, only approximately 1.69% (R2) of the variance can be explained by the influence of age, indicating that there is only a slight decrease in the pineal volume with age. Whether genetic factors are involved remains to be elucidated.
Herein, we report directly measured pineal volume parameters in a large clinical population. The results can be used as a reference for clinical research and radiological evaluations. The high interindividual variability of the pineal volume is secondary mainly to the variability of the cystic compartment and, to a lesser degree, to the solid compartment. Our study suggests that PPV and TCV decrease slightly with age and that there is no substantial effect of gender.
COMMENTS
Background
The knowledge regarding pineal gland volume (PGV) and microstructure is fragmentary only. Cross-sectional imaging studies have not yet provided sufficient data on the definition of normal pineal volume and morphometry. Consequently, the enormous interindividual variation of the pineal gland frequently complicates the radiological evaluation in radiological practice. Especially, large solid or cystic pineal glands cannot be satisfactory classified. Therefore, their study was designed to determine and accurately describe the spectrum of pineal volume and microstructures (solid/cystic parts) in a large clinical population using a high-resolution 3D-T2-weighted sequence.
Research frontiers
The development of new techniques has improved the radiological precision of pineal imaging. Up to now, PGV was estimated indirectly from two-dimensional measurements. These estimations were based on the measurement of up to three diameters and a geometric model (for example: globe). However, there is only a very weak correlation between the estimated volume and the true volumetric value. Furthermore, the relatively high slice thicknesses precluded an accurate measurement of the pineal volume. Therefore authors used a high-resolution 3D-sequence to determine the pineal volume.
Innovations and breakthroughs
For the first time, authors present precise volumetric data covering the whole spectrum of adulthood in a huge clinical sample. Previous studies were based on much smaller populations. Authors used the true fast imaging with steady state precession (trueFISP) sequence, an isotropic heavily T2-weighted 3D-sequence, because of the superior contrast for the definition of the small pineal gland against the cerebrospinal fluid in comparison to the frequently used 3D-T1-weighted sequences. Thereby, the organ borders are more precisely delineated and partial volume effects are minimized, resulting in more valid volumes measurements.
Applications
Authors obtained precise information about pineal volume and microstructure in a large population. These data can be used as reference values for radiological comparison and reflect an enormous spectrum of morphological variability. The results suggest that most of the high interindividual volume variation is secondary to cysts. Pineal parenchyma volume decreased slightly with age, whereas gender-related effects appear to be negligible.
Terminology
The pineal gland is an endocrine gland localized in the diencephalon of the human brain. Its major task is the synthesis and release of melatonin, a very versatile hormone regulating many physiological body functions. The pineal gland is localized at the dorsal wall of the third ventricle, in front of the superior colliculi of the quadrigeminal plate and under the splenium of the corpus callosum. The habenulae connect the pineal gland to the thalamus.
Peer review
The authors reported their results of measured pineal volume parameters using a high-resolution 3D-T2-weighted trueFISP-3D-imaging sequence. The study is done in a large clinical population (347 adults). The conclusion is that most of the high pineal volume variation is secondary to cysts. This group is a very large population compared to that reported in literatures. Therefore, the results are more convincing and provide useful reference for evaluation of the pineal volume clinically.
Footnotes
P- Reviewer Chen F S- Editor Gou SX L- Editor Webster JR E- Editor Xiong L
References
- 1.Schmitz SA, Platzek I, Kunz D, Mahlberg R, Wolf KJ, Heidenreich JO. Computed tomography of the human pineal gland for study of the sleep-wake rhythm: reproducibility of a semi-quantitative approach. Acta Radiol. 2006;47:865–871. doi: 10.1080/02841850600827585. [DOI] [PubMed] [Google Scholar]
- 2.Pu Y, Mahankali S, Hou J, Li J, Lancaster JL, Gao JH, Appelbaum DE, Fox PT. High prevalence of pineal cysts in healthy adults demonstrated by high-resolution, noncontrast brain MR imaging. AJNR Am J Neuroradiol. 2007;28:1706–1709. doi: 10.3174/ajnr.A0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nolte I, Lütkhoff AT, Stuck BA, Lemmer B, Schredl M, Findeisen P, Groden C. Pineal volume and circadian melatonin profile in healthy volunteers: an interdisciplinary approach. J Magn Reson Imaging. 2009;30:499–505. doi: 10.1002/jmri.21872. [DOI] [PubMed] [Google Scholar]
- 4.Nolte I, Brockmann MA, Gerigk L, Groden C, Scharf J. TrueFISP imaging of the pineal gland: more cysts and more abnormalities. Clin Neurol Neurosurg. 2010;112:204–208. doi: 10.1016/j.clineuro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Sun B, Wang D, Tang Y, Fan L, Lin X, Yu T, Qi H, Li Z, Liu S. The pineal volume: a three-dimensional volumetric study in healthy young adults using 3.0 T MR data. Int J Dev Neurosci. 2009;27:655–660. doi: 10.1016/j.ijdevneu.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Bumb JM, Brockmann MA, Groden C, Al-Zghloul M, Nölte I. TrueFISP of the pediatric pineal gland: volumetric and microstructural analysis. Clin Neuroradiol. 2012;22:69–77. doi: 10.1007/s00062-011-0110-5. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa A, Ohtsubo K, Mori W. Pineal gland in old age; quantitative and qualitative morphological study of 168 human autopsy cases. Brain Res. 1987;409:343–349. doi: 10.1016/0006-8993(87)90720-7. [DOI] [PubMed] [Google Scholar]
- 8.Golan J, Torres K, Staśkiewicz GJ, Opielak G, Maciejewski R. Morphometric parameters of the human pineal gland in relation to age, body weight and height. Folia Morphol (Warsz) 2002;61:111–113. [PubMed] [Google Scholar]
- 9.Brzozowski T, Zwirska-Korczala K, Konturek PC, Konturek SJ, Sliwowski Z, Pawlik M, Kwiecien S, Drozdowicz D, Mazurkiewicz-Janik M, Bielanski W, et al. Role of circadian rhythm and endogenous melatonin in pathogenesis of acute gastric bleeding erosions induced by stress. J Physiol Pharmacol. 2007;58 Suppl 6:53–64. [PubMed] [Google Scholar]
- 10.Duvernoy HM, Risold PY. The circumventricular organs: an atlas of comparative anatomy and vascularization. Brain Res Rev. 2007;56:119–147. doi: 10.1016/j.brainresrev.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Haus E. Chronobiology in the endocrine system. Adv Drug Deliv Rev. 2007;59:985–1014. doi: 10.1016/j.addr.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Hayakawa K, Konishi Y, Matsuda T, Kuriyama M, Konishi K, Yamashita K, Okumura R, Hamanaka D. Development and aging of brain midline structures: assessment with MR imaging. Radiology. 1989;172:171–177. doi: 10.1148/radiology.172.1.2740500. [DOI] [PubMed] [Google Scholar]
- 13.Tien RD, Barkovich AJ, Edwards MS. MR imaging of pineal tumors. AJR Am J Roentgenol. 1990;155:143–151. doi: 10.2214/ajr.155.1.2162137. [DOI] [PubMed] [Google Scholar]
- 14.Sener RN. The pineal gland: a comparative MR imaging study in children and adults with respect to normal anatomical variations and pineal cysts. Pediatr Radiol. 1995;25:245–248. doi: 10.1007/BF02011087. [DOI] [PubMed] [Google Scholar]
- 15.Lum GB, Williams JP, Machen BC, Akkaraju V. Benign cystic pineal lesions by magnetic resonance imaging. J Comput Tomogr. 1987;11:228–235. doi: 10.1016/0149-936x(87)90087-7. [DOI] [PubMed] [Google Scholar]
- 16.Sawamura Y, Ikeda J, Ozawa M, Minoshima Y, Saito H, Abe H. Magnetic resonance images reveal a high incidence of asymptomatic pineal cysts in young women. Neurosurgery. 1995;37:11–15; discussion 15-16. doi: 10.1227/00006123-199507000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Petitcolin V, Garcier JM, Mohammedi R, Ravel A, Mofid R, Viallet JF, Vanneuville G, Boyer L. [Prevalence and morphology of pineal cysts discovered at pituitary MRI: review of 1844 examinations] J Radiol. 2002;83:141–145. [PubMed] [Google Scholar]
- 18.Mamourian A, Towfighi J. MR of pineal cysts. AJNR Am J Neuroradiol. 1994;15:1796–1797. [PMC free article] [PubMed] [Google Scholar]
- 19.Katzman GL, Dagher AP, Patronas NJ. Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. JAMA. 1999;282:36–39. doi: 10.1001/jama.282.1.36. [DOI] [PubMed] [Google Scholar]
- 20.Caldas JG, Doyon D, Lederman H, Carlier R. [Magnetic resonance study of the pineal region. Normal pineal gland and simple cysts] Arq Neuropsiquiatr. 1998;56:237–244. doi: 10.1590/s0004-282x1998000200012. [DOI] [PubMed] [Google Scholar]
- 21.Krabbe KH. Histologische und embryologische Untersuchungen über die Zirbel-drüse des Menschen. Anat Hefte. 1916;163:187. [Google Scholar]
- 22.Osborn AG, Preece MT. Intracranial cysts: radiologic-pathologic correlation and imaging approach. Radiology. 2006;239:650–664. doi: 10.1148/radiol.2393050823. [DOI] [PubMed] [Google Scholar]
- 23.Crosby EC, Humphrey T, Lauer EW, editors . Correlative Anatomy of the Nervous System. New York: MacMillan; 1962. [Google Scholar]
- 24.Klein P, Rubinstein LJ. Benign symptomatic glial cysts of the pineal gland: a report of seven cases and review of the literature. J Neurol Neurosurg Psychiatry. 1989;52:991–995. doi: 10.1136/jnnp.52.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ralph CL. The pineal gland and geographical distribution of animals. Int J Biometeorol. 1975;19:289–303. doi: 10.1007/BF01451040. [DOI] [PubMed] [Google Scholar]
- 26.Lupien SJ, Evans A, Lord C, Miles J, Pruessner M, Pike B, Pruessner JC. Hippocampal volume is as variable in young as in older adults: implications for the notion of hippocampal atrophy in humans. Neuroimage. 2007;34:479–485. doi: 10.1016/j.neuroimage.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal M, Brahmanday G, Bajaj SK, Ravikrishnan KP, Wong CY. Revisiting the prognostic value of preoperative (18)F-fluoro-2-deoxyglucose ( (18)F-FDG) positron emission tomography (PET) in early-stage (I & amp; II) non-small cell lung cancers (NSCLC) Eur J Nucl Med Mol Imaging. 2010;37:691–698. doi: 10.1007/s00259-009-1291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arendt J. Melatonin and human rhythms. Chronobiol Int. 2006;23:21–37. doi: 10.1080/07420520500464361. [DOI] [PubMed] [Google Scholar]
- 29.Bergiannaki JD, Soldatos CR, Paparrigopoulos TJ, Syrengelas M, Stefanis CN. Low and high melatonin excretors among healthy individuals. J Pineal Res. 1995;18:159–164. doi: 10.1111/j.1600-079x.1995.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 30.Touitou Y. Human aging and melatonin. Clinical relevance. Exp Gerontol. 2001;36:1083–1100. doi: 10.1016/s0531-5565(01)00120-6. [DOI] [PubMed] [Google Scholar]


