Abstract
Self-ordered spatial working memory measures provide important information regarding underlying cognitive strategies, such as stereotypy. This strategy is based on repetitive sequential selection of a spatial pattern once a correct sequence has been identified. We previously reported that electroconvulsive shock (ECS) but not magnetic seizure therapy (MST) impaired performance on a spatial working memory task in a preclinical model. Here we tested the hypothesis that ECS disrupted stereotyped patterns in the selection of spatial stimuli. In a within-subject study design, we assessed the effects of ECS, MST, and sham on stereotypy and reaction time in a preclinical model. Stereotypy was assessed by the correlation of actual and predicted response patterns of spatial stimuli. Predicted patterns were based on performance during baseline sessions. ECS resulted in lower correlations between predicted and actual responses to spatial stimuli in two of the three subjects, and it also disrupted stereotypy. For one subject, there was change in the predictability of the spatial locus of responses between experimental conditions. For all three subjects, reaction time was significantly longer in ECS, relative to MST and sham. This is the first study to examine the effect of ECS, and to contrast the effects of ECS and MST, on spatial working memory component processes. Our preliminary findings show that ECS, but not MST decreased stereotypy and increased reaction time. This line of investigation may have significant implications in our understanding cognitive component processes of memory function and impairment.
Keywords: Electroconvulsive shock (ECS), magnetic seizure therapy (MST), neurocognitive, spatial working memory, stereotypy
Introduction
Experiments on spatial working memory in animals and humans use a variety of verbal and non-verbal tasks that require subjects to maintain a representation of particular locations throughout each trial. With small animals, the radial arm maze is the most widely used task to measure spatial working memory (Olton, 1985, 1987; Olton & Samuelson, 1976). Testing larger animals in a radial maze is impractical because of limitations of space.
Until recently, tests of spatial working memory were restricted to two-dimensional paper or computerized mazes, or predefined object response-tracking tasks. More recently developed tasks that have overcome these limitations allow the study of self-ordered spatial working memory (Gevins & Smith, 2000). Self-ordered spatial measures provide important information regarding a subject’s stereotypy while completing a task (Olton, 1979; Schoenfeld et al. 1950a, b; Schwartz, 1980, 1982). Stereotypy is defined as the production of a spatial pattern in a repetitive manner, once a correct sequence has been identified. It has been reported in experiments with humans (Chase et al. 2008; Owen et al. 1990) and non-human primates (Callithrix jacchus) (Collins et al. 1998). However, the latter finding has yet to be replicated.
In our previous experiments we developed a battery of computerized measures of cognition, the Columbia University Primate Cognitive Battery (CUPCB), for studying long-term memory of a single item, short-term memory of a variable item, and long-term memory for 3-item lists (Moscrip et al. 2004). New measures for working memory have been added recently ; one for spatial information, the other, for serial information (Spellman et al. 2008). The expanded CUPCB was used in two independent experiments that examined the effects of electroconvulsive shock (ECS), magnetic seizure therapy (MST), or sham intervention (anaesthesia only). MST is a novel neurostimulation treatment under development as a means of reducing the cognitive side-effects of electroconvulsive therapy (ECT) through the use of magnetic fields to induce cortically focused seizures with minimal involvement of deeper brain structures (Lisanby et al. 2001a, b, 2003a).
Both of our prior studies used a within-subject cross-over design whereby each subject served as its own control. In the first study (Moscrip et al. 2006), subjects were significantly less accurate and had longer reaction times on measures of long-term memory for a constant target, recall of a novel target, and recall of a 3-item target list following treatment by ECS (25 sessions, bilateral electrode configuration) relative to MST (25 sessions administered at 50 Hz). Similar results were obtained in a follow-up study (Spellman et al. 2008) even though the MST dose was increased to 100 Hz (high-dose MST) and up to 6× seizure threshold. Extending the cognitive profile of working memory measures showed that ECS decreased accuracy on the spatial memory task, while MST did not differ from sham (Spellman et al. 2008). That study focused on neurocognitive safety, and at the time that study was conducted, there were no methods available for examining and interpreting the effects on underlying cognitive processes (i.e. stereotypy) of spatial working memory.
Extensive research has suggested that spatial working memory in non-human primates is related to a cortical network comprising the medial-dorsolateral prefrontal cortex (M-DLPFC), medial temporal lobe structures (particularly the hippocampus), and the posterior parietal cortex (Artchakov et al. 2007; Inoue et al. 2004; Wilson et al. 1993). The M-DLPFC, particularly the principal and accurate sulci, is thought to govern the representation and manipulation of spatial stimuli (Petrides, 1995; Wilson et al. 1993). The hippocampus, specifically the dentate gyrus and the CA3 regions, is thought to recognize and temporarily store spatial patterns (Bonini et al. 2007). The posterior parietal cortex has been associated with the perception of spatial stimuli (Schwartz & Goldman-Rakic, 1984). Although Becker et al. (1980) suggest that the hippocampus is the principal component of this network, others suggest that the three cortical regions function conjointly to carry out spatial working memory functions (Carpenter et al. 2000; Goldman-Rakic, 1998; Khan & Muly, 2011).
The neural network implicated in spatial working memory may help to explain the differential effects of ECS and MST on the CUPCB spatial working memory task. Prior work with non-human primates found that the electrical current and resultant seizures induced by ECS penetrates throughout most cortical structures (Sekino & Ueno, 2002). By contrast, MST-induced currents and seizures are prominent mostly in the targeted superficial cortical regions, with little spread to deeper brain structures such as the hippocampus (Deng et al. 2009; Lisanby et al. 2003b). Thus, the seizures generated by ECS could have disrupted the spatial working memory network through effects on hippocampus and/or the prefrontal cortex (PFC), in turn negatively affecting performance. Here we examine the effects of ECS and MST on stereotypy. To this end, we evaluated performance on the CUPCB spatial working memory task in our prior investigation (Spellman et al. 2008). Based on the results of our earlier experiments, we hypothesized that ECS, but not MST would disrupt stereotypy on the spatial working memory task.
Materials and methods
Study design
We used a within-subject design, which allowed each subject to receive each experimental condition and serve as its own control. Each subject received 20 sessions each of electroconvulsive shock (ECS), high-dose magnetic seizure therapy (MST administered at 100 Hz), and sham (anaesthesia only), with each intervention, in a randomized order. Each intervention was followed by a recovery period to allow performance to return to baseline. Treatment order was counterbalanced between subjects (see Table 1). The methods and design of the study have been detailed elsewhere (Spellman et al. 2008). The New York State Psychiatric Institute and Columbia University’s Institutional Animal Care and Use Committee (IACUC) approved this study.
Table 1.
Condition schedule for each subject
| Subject | Condition | |||||
|---|---|---|---|---|---|---|
| Subject 1 | ECS | Recovery | MST | Recovery | Sham | Recovery |
| Subject 2 | MST | Recovery | ECS | Recovery | Sham | Recovery |
| Subject 3 | Sham | Recovery | ECS | Recovery | MST | Recovery |
ECS, Electroconvulsive shock; MST, magnetic seizure therapy; sham, anaesthesia only.
Subjects
The subjects were three pathogen-free male monkeys (Macaca mulatta) obtained from a National Institutes of Health (NIH)-sponsored breeding colony. Mean age (±s.d.) upon entering the study was 83 (±26) months, mean weight was 8 (±1) kg, and all three were past sexual maturity. The approximate age equivalent in human years is 20.8 (±6.5) yr.
ECS, MST, and sham interventions
Details of the ECS, MST, and sham interventions, including seizure threshold titration, anaesthesia, seizure monitoring, and vital-sign monitoring have previously been reported (Spellman et al. 2008). In brief, ECS was administered bilaterally with a human ECT device (MECTA Spectrum 5000Q, MECTA Corporation, USA) at 2.5× the seizure threshold. For MST, sessions were administered with a custommodified, 100 Hz Magstim magnetic stimulation device (The Magstim Co Ltd, UK) with a paediatric-sized round coil (6.2 cm diameter) placed on the vertex. MST seizure threshold was defined as the number of pulses required to elicit a tonic-clonic seizure. Subsequent MST dosage was set at 100 Hz and either 6× the seizure threshold or the maximum device output capacity (i.e. 1000 pulses at 100 Hz). For the ECS and MST sessions, seizure threshold titration occurred on day 1. Under the sham condition, anaesthesia was restricted to the same type that was administered under the ECS and the MST conditions. Each subject received a total of 20 sessions (one session per day, for 5 d/wk for 4-wk duration) of each intervention.
Neurocognitive testing
The subjects were tested individually in a distraction-free, video-monitored, acoustically shielded test chamber. Subjects responded to visually presented stimuli on a touch-sensitive monitor that was sectioned into 16 distinct response ports. A computer that was also used to administer the spatial working memory task collected all data. Other details of the cognitive testing apparatus, stimulus presentation, and training procedures have been reported in an earlier study (Moscrip et al. 2004).
We used the CUPCB, which consisted of neurocognitive measures specifically designed to assess orientation, anterograde learning and memory, retrograde memory, and working memory. Subjects completed the CUPCB after each treatment session. The CUPCB provided rapid and comprehensive cognitive testing in the immediate post-ictal period to assess acute cognitive side-effects.
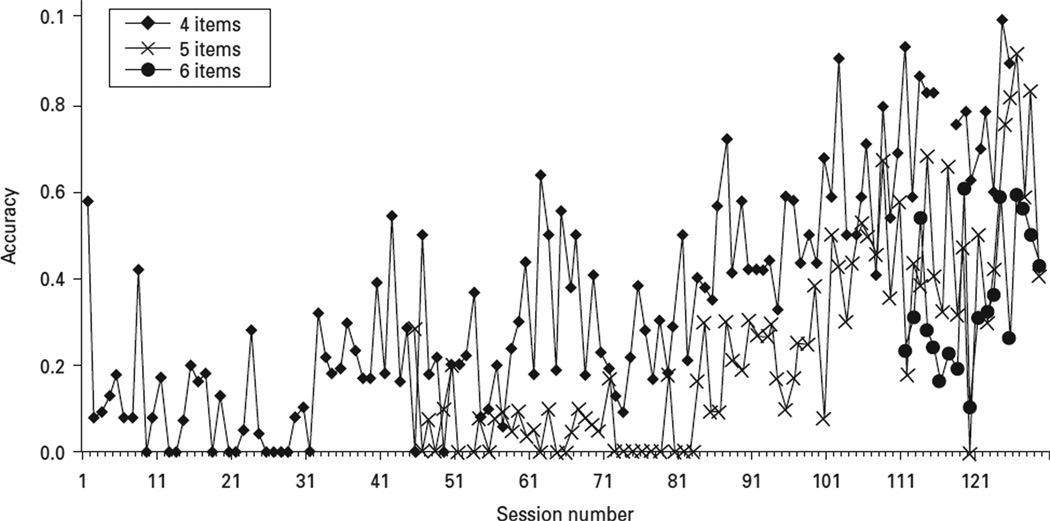
For this study, we focused on the spatial working memory task, one that was designed to measure spatial working memory. The spatial working memory task we used was modelled after the radial arm maze in which a subject begins each trial while situated on a central platform from which eight arms radiate, each separated by a 45° angle (Olton & Samuelson, 1976). Subjects could earn a pellet of food the first time they ran down each alley. Following each run, subjects returned to the central platform to ensure that the point at which they began each run was constant. A perfectly executed trial in a radial maze was defined as one, and only one, visit to each arm when standing on the central platform before each run. Before the start of this study, the spatial working memory task we used was piloted using other subjects. Each subject had no difficulty in learning the spatial task. As shown in Fig. 1, accuracy on this task was related to the number of items, as would be predicted by differences in the load on spatial working memory.
Fig. 1.
Typical learning curve on the Columbia University Primate Cognitive Battery (CUPCB) spatial working memory measure. The subjects were trained on the CUPCB spatial working memory measure with stimuli incrementally increased as mastery of the measure was obtained. This figure shows the learning curve for four, five and six spatially represented stimuli.
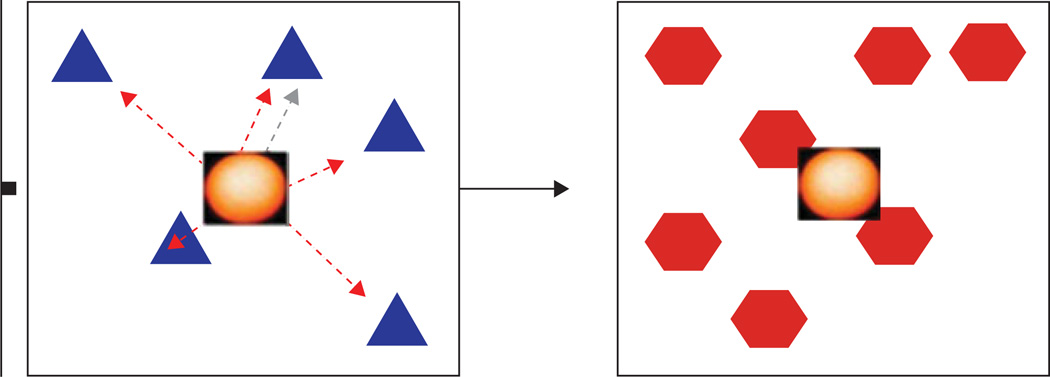
Subjects were required to touch all of the stimuli that were displayed on the touch-sensitive monitor in any order. An array of four, five, or six identical targets was presented on the subject’s monitor in spatial configurations that were changed randomly between trials. Because the locations of the stimuli that were presented on a given trial were physically identical, they could only be differentiated by their position on the screen. All stimuli remained on the screen until the end of the trial. In order to simulate a radial arm maze in which the subject is required to return to the centre of the maze, the subjects were required to touch an icon in the centre of the stimuli between each response (see Fig. 2). The inclusion of the central icon made all stimuli equally accessible before each response, and prevented cuing based on paw position.
Fig. 2.
Columbia University Primate Cognitive Battery (CUPCB) spatial working memory task. A number of identical geometrical shapes were presented simultaneously on the monitor. Subjects touched each spatial position once to complete the trial. Successive touches to a position that differed from the current position produced positive visual/auditory feedback. Touches that were not directed at a near position resulted in negative visual/auditory feedback. Items could be touched in any order. A new set of objects, in new locations that were randomly selected, were presented on each trial. The subject had to select each stimulus in turn without repeating an earlier selection. Between selections, the subject had to return to the centre and select a ‘reset’ stimulus.
Subjects were required to select each stimulus only once, in whatever sequence they preferred. If a subject responded to each of the stimuli without repeating any response, a food reward was delivered at the end of the trial and a 3-s intertrial interval (ITI) followed. If the subject returned to a previously selected stimulus, the trial terminated immediately, the video screen turned black, and the duration of the ITI was increased to 6 s. Subjects were tested on this task following each ECS, MST, or sham session. Baseline data were obtained during the 2-wk period that preceded the first treatment.
Data analysis
To measure stereotypy, it is necessary to quantify the consistency of a subject’s spatial preference. We did that by rank ordering each of the 16 response ports for each subject, where the ranks were based on the mean ordinal position of responses made at that port during the baseline trials. The response port that, on average, was touched first during a given trial was assigned the rank 1. The port touched last was assigned the rank 16. Ranks, which were obtained during baseline (predicted locations), were compared with those following each treatment (obtained locations) and Kendall’s tau correlation coefficient was calculated for each experimental condition as a measure of stereotypy. The more consistent the spatial pattern of responses during baseline and following treatment, the higher the correlation coefficient. Thus, the rank correlation between the predicted and the actual ordinal position of the ports serves as a quantitative measure of stereotypy. Confidence intervals were obtained (Efron, 1987) by resampling the data from each condition with replacement to create 1000 new datasets for each condition. Kendall’s tau correlation coefficients were calculated for each new dataset and were used to determine 95% confidence intervals around the correlation coefficient. Correlations were interpreted using standard guidelines (Nunnally & Bernstein, 1994).
We also assessed the effects of each condition on reaction time (RT), the time that elapsed between a response to the central icon and a response to one of the ports, with analysis of variance (ANOVA). Significant main effects were followed with post-hoc comparisons that were adjusted for multiple comparisons (Tukey’s range test).Wepresent the estimated difference and 95% confidence intervals. Data were analysed using SPSS version 15 for Windows (SPSS Inc., USA). All missing variables were excluded from analyses; no data were imputed. Statistical significance was determined as a two-sided p value of <0.01.
Results
Performance at baseline
During baseline, RTs were short (mean=0.80) on both correct and incorrect trials, but there was greater variability in performance on incorrect trials. Associations between predicted and actual responding of spatial stimuli were moderate and ranged between correlations of r=0.31–0.43.
Correlation between actual and predicted ordinal position within condition
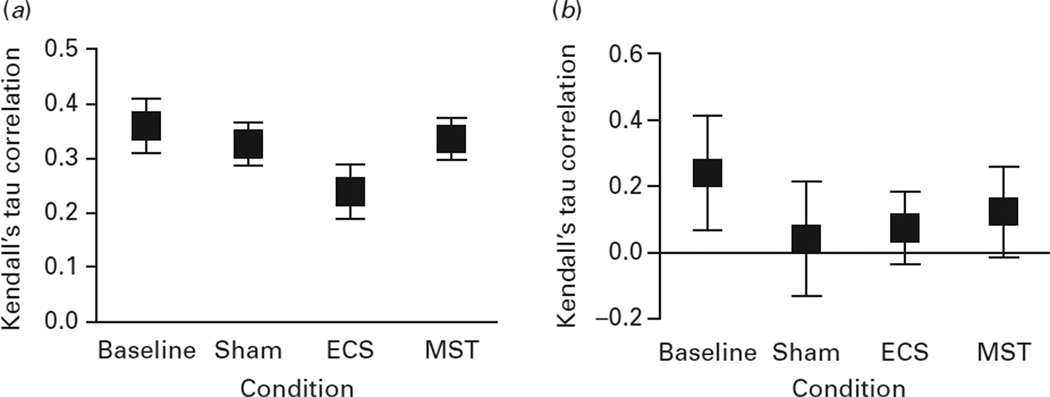
Across all trials, associations between the predicted and actual ordinal position of response ports were lower in the ECS condition (r=0.20, 95% CI 0.19–0.22) compared to baseline (r=0.31, 95% CI 0.29–0.33), sham (r=0.30, 95% CI 0.28–0.31), and MST (r=0.31, 95% CI 0.29–0.32) conditions, showing that ECS disrupted stereotypy. As shown in Fig. 3, a similar pattern was observed in the case of incorrect trials, but correlations were lower than the correlations on correct trials. No stereotypy was observed on incorrect trials.
Fig. 3.
Kendal’s tau correlation rank coefficients between actual and predicted touch for correct and incorrect trials. This figure represents the correlation coefficient (squares) and the 95% confidence intervals (bars) for (a) correct and (b) incorrect trials. For correct trials, the correlations were significantly lower in the ECS condition relative to baseline, MST, and sham. For incorrect trials, there were no significant differences between the conditions. ECS, Electroconvulsive shock; MST, magnetic seizure therapy; sham, anaesthesia only.
Stereotypic patterns of movement
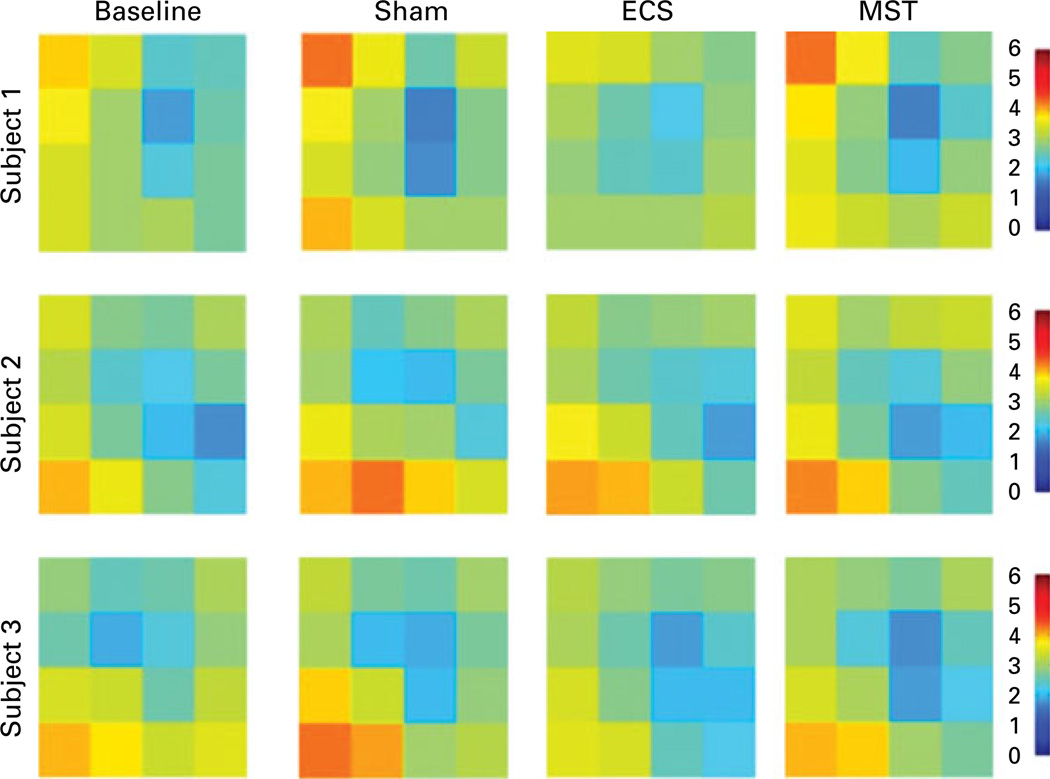
Each subject had a preferential starting point and a predictable pattern of response locations on the spatial working memory task (see Fig. 4). Subject 1 started in the upper right portion of the screen and moved clockwise to the bottom right, bottom left, and finally to the top left most response ports. Subject 2 started to respond on the right side of the screen, then the middle, and finally on the left side of the screen. Subject 3 started responding at the top of the screen and then moved gradually towards the bottom left-hand corner of the screen. A preferential starting point and stereotypic movement was observed during the sham (subject 1: r=0.32, 95% CI 0.29–0.35 ; subject 3: r=0.31, 95% CI 0.28–0.33) and MST (subject 1: r=0.33, 95% CI 0.31–0.36 ; subject 3: r=0.23, 95% CI 0.20–0.21) conditions, but decreased during the ECS condition for subject 1 (r=0.13, 95% CI 0.10–0.16) and subject 3 (r=0.11, 95% CI 0.08–0.14). Minimal differences in stereotypy were observed for subject 2 between conditions (sham: r=0.27, 95% CI 0.24–0.30; MST: r=0.35, 95% CI 0.32–0.38 ; ECS: r=0.31, 95% CI 0.29–0.34).
Fig. 4.
Stereotypic first touch on the spatial working memory measure. This figure represents the pattern of the first touch for each subject when completing the spatial working memory measure. The orange regions show the preference for starting in the same region whereas the blue regions show the lack of preference for starting in the same region. ECS, Electroconvulsive shock; MST, magnetic seizure therapy; sham, anaesthesia only.
Reaction time
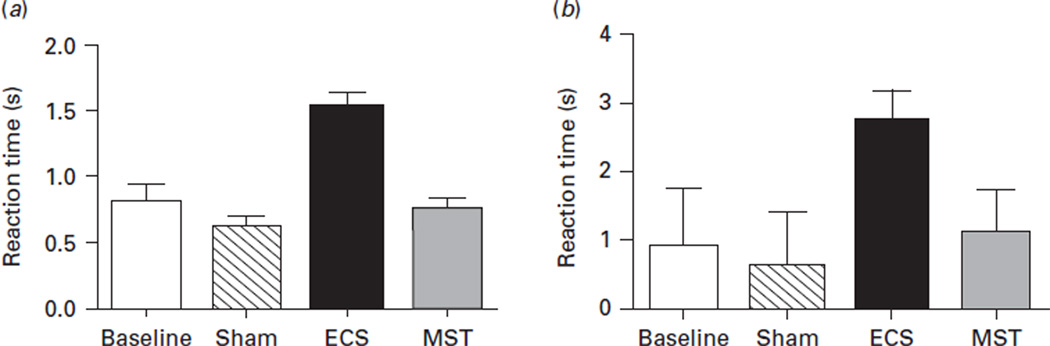
On correct trials, analyses of RT showed a significant interaction between condition and the number of items on the list (F1,6=3.0, p=0.006), but only a significant main effect of condition (F1,3=169.8, p<0.0001), and no main effect for number of items (F1,2=0.3, p=0.76). Reaction times were significantly longer following ECS treatments relative to baseline (estimated difference 0.72, 95% CI 0.31–1.13), sham (estimated difference 0.92, 95% CI 0.60–1.25), and MST (estimated difference 0.78, 95% CI 0.44–1.11) conditions. There were no differences in RT between baseline, sham, or MST (see Fig. 5). Although a similar pattern was observed in the case of errors (longer RTs during the ECS condition), that effect was only marginally significant (F1,3=2.62, p=0.05).
Fig. 5.
Reaction time on the spatial working memory measure. This figure represents the reaction time (in seconds; mean and error bar) on the spatial working memory task for the subjects for each condition, and by (a) correct or (b) incorrect trials. For correct and incorrect trials, reaction time was significantly longer in the ECS condition relative to baseline, MST, and sham. There were no other significant differences. ECS, Electroconvulsive shock; MST, magnetic seizure therapy; sham, anaesthesia only.
Discussion
To our knowledge, this is the first study to report an effect of ECS on stereotypy, a widely used measure of spatial working memory. We also showed that stereotypy is preserved following treatment with MST, compared to treatment following ECS. This could be attributed to the more focal effects of MST, which spares the hippocampus. This study also replicated the finding that non-human primates demonstrate stereotypy to solve a self-ordered spatial working memory task (Collins et al. 1998). Our findings show that ECS, but not MST or anaesthesia-alone sham, has an adverse effect on the cognitive mechanism that gives rise to stereotypy. It also helps to explain our prior finding that ECS decreases accuracy on a spatial working memory task (Spellman et al. 2008). Last, but not least in importance, is the usefulness of stereotypy as a computerized measure of self-ordered spatial working memory.
Stereotypy is dependent upon medial temporal lobe structures (particularly the hippocampus) that are used to respond correctly on spatial working memory tasks (Dale & Innis, 1986; Mumby et al. 2002). The majority of spatial working memory research examining the effects of ECS in rodent models (Bohbot et al. 1996; Maki, 1985) has not reported a disruption in stereotypy or RT. Thus, this is one of the first reports to find that ECS impacts stereotypy and decreases efficiency of processing spatial information. Following ECS, subjects were unable to retrieve their stereotypic pattern of responding that they acquired during baseline training, and thus were more prone to error during the task. This can be attributed to the retrograde amnesia that is a well known side-effect of ECS (Sobin et al. 1995). Intact storage and retrieval of correct sequencing strategies (Owen et al. 1990; Winters et al. 2010) on spatial working memory tasks are a prerequisite for working memory because these data show how decreased stereotypy is and are useful indicators of impaired spatial working memory. These findings may be of clinical importance with respect to the impact of ECT on driving and the risk of falls following ECT, for which the field lacks valid predictors.
The hippocampus is thought to play a role in stereotypy (Owen et al. 1996), thus the differential effects of ECS and MST on this component process has implications for the differential impact on hippocampal function. ECS has been found to cause structural and physiological changes (e.g. mossy fibre sprouting) in the hippocampus (Barnes et al. 1994; Chen et al. 2001), which could explain the disruption in stereotypy. The finding that ECS disrupts spatial working memory in rodent models has been replicated in many studies (Beatty et al. 1985; Holzhauer & Bures, 1986; Maki, 1985; Popik et al. 1994; Shavalia et al. 1981), and has been reported in one human study (Falconer et al. 2010).
The finding that non-human primates perform a spatial task in an organized manner was reported by Collins et al. (1998). In that study, subjects would start the task in a variety of positions, but they navigated through the stimuli in either a clockwise or counterclockwise direction. In our study, subjects navigated through the spatial stimuli in a clockwise or counterclockwise direction and they had a preferential starting point. This is consistent with the strategy of stereotypy, which allows a subject to develop an efficient navigational map of spatial configurations (Olton, 1979) in order to respond correctly on a cognitively demanding spatial working memory task (Miller, 1956; Schwartz, 1980, 1982). An important methodological difference between our study and Collins et al.’s (1998) study is that the spatial working memory task in our study required subjects to return to a central point on each trial, and thus prevented cuing based on paw position. This is important because paw cuing could mitigate the findings on a spatial working memory task as it primes the subject to the previously pressed stimuli and the subsequent stimuli to press.
An important line of investigation stemming from this study is the development of specific neurocognitive measures that are able to discern cognitive component processes, such as the task presented here that is able to elucidate the specific role of the component of stereotypy within the larger context of spatial working memory. Designing an animal model of memory impairment may be a useful strategy to study intricate component cognitive processes (Olton, 1985) and has been exemplified in the work of Squire et al. (1988), and more recently with the Cognitive Neuroscience Treatment Research to Improve Schizophrenia Initiative (CNTRICS) (Barch & Smith, 2008; Carter et al. 2008).
Researchers have attempted to modify the classic radial arm maze to accommodate humans and non-human primates by use of computer rendered three-dimensional (3D) environments. Humans are able to master complex environments (Owen et al. 1998), and it has been demonstrated that rhesus macaques are also capable of rudimentary spatial navigation in 3D spatial environments (Washburn & Astur, 2003). These novel neurocognitive measures of spatial working memory are useful analogues to the classic radial arm maze and we suggest that our spatial working memory measure may prove to be a useful supplement to this work, as it requires less training and energy expenditure by the subject and, importantly, it does not require a joystick for task completion. This is advantageous as our measure is also able to assess real-time port presses to inform the cognitive component processes (i.e. stereotypy) that underscore spatial working memory.
Our measure of spatial working memory differs from the radial arm maze. One difference between the two measures involves the provision of a reward to the subject. In the radial arm maze, reward is provided during the trial, whereas the reward in the CUPCB spatial working memory task is provided only at the end of a trial. This feature of the radial maze was necessary to prevent subject satiation. In our investigation, the subjects learned to make multiple presses in order to obtain the reward, and lack of motivation did not seem to be an issue. Another way in which the CUPCB spatial working memory measure differs from the radial arm maze is that a trial ended as soon as a subject made a mistake whereas in the radial arm maze, the task is continued until all arms have been explored. A significant difference between these two measures that has direct clinical relevance is that our task is a touch-sensitive computerized measure. This mode of test administration may ease the adaptation of this measure for use in neurocognitive studies with humans. An adapted human neurocognitive measure, predicated on cognitive science and linked to neuroanatomical correlates, could provide unique information (Buffalo et al. 2006) in clinical settings. For example, this advanced neurocognitive measure may be able to discern the impact of clinical confounds (i.e. mood) and clinical interventions (i.e. ECT) on cognitive components of working memory.
This study was limited by small sample size, risk of carry-over effects between interventions, and the possibility of practice effects. Carry-over effects should be negligible because we used only performance on the CUPCB before any intervention as the baseline in order to prevent carry-over effects, and subjects had to return to baseline cognitive performance levels before switching to the next intervention. Practice effects were minimized as the computer stimuli allowed for a wide range of spatial configurations on subsequent testing sessions. The decreased accuracy and increased RT on the spatial working memory test during the ECS condition suggests that there were little to no practice effects. Moreover, the generalization of these findings into clinical practice may be limited by the study design. Specifically, ECS was provided on a daily basis with bilateral electrode configuration and brief pulsewidth. However, in clinical practice, certain strategies including the use of right unilateral electrode configuration, ultra-brief pulsewidth, and the provision of ECT only two or three times per week are used to minimize adverse cognitive effects of ECT (Prudic, 2008). As with other basic science neurocognitive investigations (Roberts, 1996; Sarter, 2004; Squire et al. 1988), it is unclear at this time how our findings will translate into humans, particularly patients treated with ECT. For instance, most patients treated with ECT have an underlying neuropsychiatric disease (e.g. major depressive disorder), which may be associated with neurocognitive function, and thus mediate the neurocognitive effects of the treatment (Semkovska & McLoughlin, 2010).
Overall, this study found that ECS relative to MST or sham disrupted stereotypy and decreased efficiency of processing spatial information, suggesting that unique convulsive modalities differentially affect spatial working memory. Future work is required to confirm the effects of ECS and MST on stereotypy and to explore if these preliminary findings are replicated in other basic science models, particularly ones that integrate the neurocognitive effects of neuropsychiatric disease. Ultimately, we expect that this line of research will have significant implications in helping to design more definitive investigations to better understand the factors underlying the amnestic syndrome secondary to ECT.
Acknowledgements
This work was supported in part by National Institute of Mental Health (NIMH) R01 MH060884 (PI : S. H. Lisanby) and K23 MH087739 (PI : S. M. McClintock). Support for the development of magnetic seizure therapy has also come from the Stanley Medical Research Foundation, American Federation for Aging Research/Beeson Scholars Program, and the National Alliance for Research on Schizophrenia and Depression (NARSAD). We thank Dr Haley Speed, Mr Niko Reyes, Ms Elisabeth Bernhardt, and members of the Columbia University Primate Cognitive Laboratory.
Statement of Interest
Dr McClintock has received research funding from the National Institutes of Health (NIH), National Center for Research Resources (NCRR), and the National Alliance for Research on Schizophrenia and Depression (NARSAD). Dr Husain has received research funding from the National Institutes of Health (NIH), Stanley Medical Research Foundation, Neuronetics, Cyberonics, Advanced Neuromodulation Systems, and Brainsway. Dr Rowny has received research funding from the National Institutes of Health (NIH) and the National Alliance for Research on Schizophrenia and Depression (NARSAD). Dr Terrace has received research funding from the National Institutes of Health (NIH). Dr Lisanby has received research funding from the National Institutes of Health (NIH), the National Alliance for Research on Schizophrenia and Depression (NARSAD), DARPA, Stanley Medical Research Foundation, American Federation for Aging Research/Beeson Scholars Program, Neuronetics, Cyberonics, Advanced Neuromodulation Systems, and Brainsway. She has received equipment support from Magstim and Magventures. She formerly chaired a Data Safety and Monitoring Board for a study sponsored by Northstar Neuroscience.
Footnotes
Parts of this work were presented at the 2010 Annual Meetings of the International Neuropsychological Society (INS) and the American College of Neuropsychopharmacology (ACNP).
References
- Artchakov D, Tikhonravov D, Vuontela V, Linnankoski I, et al. Processing of auditory and visual location information in the monkey prefrontal cortex. Experimental Brain Research. 2007;180:469–479. doi: 10.1007/s00221-007-0873-8. [DOI] [PubMed] [Google Scholar]
- Barch DM, Smith E. The cognitive neuroscience of working memory: relevance to CNTRICS and Schizophrenia. Biological Psychiatry. 2008;64:11–17. doi: 10.1016/j.biopsych.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Jung MW, McNaughton BL, Korol DL, et al. LTP saturation and spatial learning disruption: effects of task variables and saturation. Journal of Neuroscience. 1994;14:5793–5806. doi: 10.1523/JNEUROSCI.14-10-05793.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Bierley RA, Rush JR. Spatial memory in rats : electroconvulsive shock selectively disrupts working memory but spares reference memory. Behavioral and Neural Biology. 1985;44:403–414. doi: 10.1016/s0163-1047(85)90760-5. [DOI] [PubMed] [Google Scholar]
- Becker JT, Walker JA, Olton DS. Neuroanatomical bases of spatial memory. Brain Research. 1980;200:307–320. doi: 10.1016/0006-8993(80)90922-1. [DOI] [PubMed] [Google Scholar]
- Bohbot V, Otahal P, Liu Z, Nadel L, et al. Electroconvulsive shock and lidocaine reveal rapid consolidation of spatial working memory in the water maze. Proceedings of the National Academy of Sciences USA. 1996;93:4016–4019. doi: 10.1073/pnas.93.9.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini JS, Da Silva WC, Bevilaqua LRM, Medina JH, et al. On the participation of hippocampal PKC in acquisition, consolidation and reconsolidation of spatial memory. Neuroscience. 2007;147:37–45. doi: 10.1016/j.neuroscience.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Bellgowan PS, Martin A. Distinct roles for medial temporal lobe structures in memory for objects and their locations. Learning & Memory. 2006;13:638–643. doi: 10.1101/lm.251906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Reichle ED. Working memory and executive function: evidence from neuroimaging. Current Opinion in Psychiatry. 2000;10:195–199. doi: 10.1016/s0959-4388(00)00074-x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Barch DM, Buchanan RW, Bullmore E, et al. Identifying cognitive mechanisms targeted for treatment development in schizophrenia : an overview of the first meeting of the cognitive neuroscience treatment research to improve cognition in schizophrenia initiative. Biological Psychiatry. 2008;64:4–10. doi: 10.1016/j.biopsych.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Clark L, Sahakian BJ, Bullmore ET, et al. Dissociable roles of prefrontal subregions in self-ordered working memory performance. Neuropsychologia. 2008;46:2650–2661. doi: 10.1016/j.neuropsychologia.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Chen AC, Shin KH, Duman RS, Sanacora G. ECS-induced mossy fiber sprouting and BDNF expression are attenuated by ketamine pretreatment. Journal of ECT. 2001;17:27–32. doi: 10.1097/00124509-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Collins P, Roberts AC, Dias R, Everitt BJ, et al. Perseveration and strategy in novel spatial self-ordered sequencing task for nonhuman primates : effects of excitotoxic lesions and dopamine depletions of the prefrontal cortex. Journal of Cognitive Neuroscience. 1998;10:332–354. doi: 10.1162/089892998562771. [DOI] [PubMed] [Google Scholar]
- Dale RHI, Innis NK. Interactions between response stereotypy and memory strategies on the eight-arm radial maze. Behavioural Brain Research. 1986;19:17–25. doi: 10.1016/0166-4328(86)90043-4. [DOI] [PubMed] [Google Scholar]
- Deng ZD, Lisanby SH, Peterchev AV. Effect of anatomical variability on neural stimulation strength and focality in electroconvulsive therapy (ECT) and magnetic seizure therapy (MST) Proceedings of the Conference on IEEE Engineering in Medicine and Biological Society. 2009;2009:682–688. doi: 10.1109/IEMBS.2009.5334091. [DOI] [PubMed] [Google Scholar]
- Efron B. Better bootstrap confidence intervals. Journal of the American Statistical Association. 1987;82:171–185. [Google Scholar]
- Falconer DW, Cleland J, Fielding S, Reid IC. Using the Cambridge Neuropsychological Test Automated Battery (CANTAB) to assess the cognitive impact of electroconvulsive therapy on visual and visualspatial memory. Psychological Medicine. 2010;40:1017–1025. doi: 10.1017/S0033291709991243. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME. Neurophysiological measurs of working memory and individual differences in cognitive ability and cognitive style. Cerebral Cortex. 2000;10:829–839. doi: 10.1093/cercor/10.9.829. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and central executive. In: Roberts AC, Robbins TW, Weiskrantz L, editors. The Prefrontal Cortex : Executive and Cognitive Functions. New York: Oxford University Press; 1998. pp. 87–102. [Google Scholar]
- Holzhauer MS, Bures J. Influence of electroconvulsive shock and naloxone on acquistion and retention of a spatial navigation task in rats. Physiology and Behavior. 1986;38:551–556. doi: 10.1016/0031-9384(86)90424-5. [DOI] [PubMed] [Google Scholar]
- Inoue M, Mikami A, Ando I, Tsukada H. Functional brain mapping of the macaque related to spatial working memory as revealed by PET. Cerebral Cortex. 2004;14:106–119. doi: 10.1093/cercor/bhg109. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Muly EC. Molecular mechanisms of working memory. Behavioural Brain Research. 2011;219:329–341. doi: 10.1016/j.bbr.2010.12.039. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Luber B, Finck AD, Schroeder C, et al. Deliberate seizure induction with repetitive transcranial magnetic stimulation in nonhuman primates. Archives of General Psychiatry. 2001a;58:199–200. doi: 10.1001/archpsyc.58.2.199. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Luber B, Schlaepfer TE, Sackeim HA. Safety and feasibility of magnetic seizure therapy (MST) in major depression: randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharmacology. 2003a;28:1852–1865. doi: 10.1038/sj.npp.1300229. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Moscrip T, Morales O, Luber B, et al. Neurophysiological characterization of magnetic seizure therapy (MST) in non-human primates. Supplements to Clinical Neurophysiology. 2003b;56:81–99. doi: 10.1016/s1567-424x(09)70212-0. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Schlaepfer TE, Fisch H-U, Sackeim HA. Magnetic seizure therapy of major depression. Archives of General Psychiatry. 2001b;58:303–305. doi: 10.1001/archpsyc.58.3.303. [DOI] [PubMed] [Google Scholar]
- Maki WS. Differential effects of electroconvulsive shock on concurrent spatial memories: ‘old’ memories are impaired while ‘new’ memories are spared. Behavioral and Neural Biology. 1985;43:162–177. doi: 10.1016/s0163-1047(85)91347-0. [DOI] [PubMed] [Google Scholar]
- Miller GA. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychological Review. 1956;63:81–97. [PubMed] [Google Scholar]
- Moscrip TD, Terrace HS, Sackeim HA, Lisanby SH. A primate model of anterograde and retrograde amnesia produced by convulsive treatment. Journal of ECT. 2004;20:26–36. doi: 10.1097/00124509-200403000-00007. [DOI] [PubMed] [Google Scholar]
- Moscrip TD, Terrace HS, Sackeim HA, Lisanby SH. Randomized controlled trial of the cognitive side-effects of magnetic seizure therapy (MST) and electroconvulsive shock (ECS) International Journal of Neuropsychopharmacology. 2006;9:1–11. doi: 10.1017/S146114570500578X. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, et al. Hippocampal damage and exploratory preferences in rats : memory for objects, places, and contexts. Learning & Memory. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnally JC, Bernstein IH. Psychometric Theory. New York: McGraw-Hill; 1994. [Google Scholar]
- Olton DS. Mazes, maps, and memory. American Psychologist. 1979;34:583–596. doi: 10.1037//0003-066x.34.7.583. [DOI] [PubMed] [Google Scholar]
- Olton DS. Strategies for the development of animal models of human memory impairment. Annals of the New York Academy of Sciences. 1985;444:113–121. doi: 10.1111/j.1749-6632.1985.tb37583.x. [DOI] [PubMed] [Google Scholar]
- Olton DS. The radial arm maze as a tool in behavioral pharmacology. Physiology and Behavior. 1987;40:793–797. doi: 10.1016/0031-9384(87)90286-1. [DOI] [PubMed] [Google Scholar]
- Olton DS, Samuelson RJ. Remembrance of places passed: spatial memory in rats. Journal of Experimental Psychology: Animal Behavior Processes. 1976;2:97–116. [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, et al. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Owen AM, Morris RG, Sahakian BJ, Polkey CE, et al. Double dissociations of memory and executive functions in working memory tasks following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Brain. 1996;119:1597–1615. doi: 10.1093/brain/119.5.1597. [DOI] [PubMed] [Google Scholar]
- Owen AM, Stern CE, Look RB, Tracey I, et al. Functional organization of spatial and nonspatial working memory processing within the human lateral frontal cortex. Proceedings of the National Academy of Sciences USA. 1998;95:7721–7726. doi: 10.1073/pnas.95.13.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Impairments on nonspatial self-ordered and externally ordered working memory tasks after lesions of the mid-dorsal part of the lateral frontal cortex in the monkey. Journal of Neuroscience. 1995;15:359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik P, Mamczarz J, Vetulani J. The effect of electroconvulsive shock and nifedipine on spatial learning and memory in rats. Biological Psychiatry. 1994;35:864–869. doi: 10.1016/0006-3223(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Prudic J. Strategies to minimize cognitive side effects with ECT: aspects of ECT technique. Journal of ECT. 2008;24:46–51. doi: 10.1097/YCT.0b013e31815ef238. [DOI] [PubMed] [Google Scholar]
- Roberts AC. Comparison of cognitive function in human and non-human primates. Cognitive Brain Research. 1996;3:319–327. doi: 10.1016/0926-6410(96)00017-1. [DOI] [PubMed] [Google Scholar]
- Sarter M. Animal cognition : defining the issues. Neuroscience & Biobehavioral Reviews. 2004;28:645–650. doi: 10.1016/j.neubiorev.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Schoenfeld WN, Antonitis JJ, Bersh PJ. A preliminary study of training conditions necessary for secondary reinforcement. Journal of Experimental Psychology. 1950a;40:40–45. [Google Scholar]
- Schoenfeld WN, Antonitis JJ, Bersh PJ. Unconditioned reponse rate of the white rat in a bar-pressing apparatus. Journal of Comparative and Physiological Psychology. 1950b;43:41–48. doi: 10.1037/h0059309. [DOI] [PubMed] [Google Scholar]
- Schwartz B. Development of complex, stereotyped behavior in pigeons. Journal of the Experimental Analysis of Behavior. 1980;33:153–166. doi: 10.1901/jeab.1980.33-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B. Failure to produce response variability with reinforcement. Journal of the Experimental Analysis of Behavior. 1982;37:171–181. doi: 10.1901/jeab.1982.37-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ML, Goldman-Rakic PS. Callosal and intrahemispheric connectivity of the prefrontal association cortex in rhesus monkey: relation between intraparietal and principal sulcal cortex. Journal of Comparative Neurology. 1984;226:403–420. doi: 10.1002/cne.902260309. [DOI] [PubMed] [Google Scholar]
- Sekino M, Ueno S. Comparison of current distributions in electroconvulsive therapy and transcranial magnetic stimulation. Journal of Applied Physiology. 2002;91:8730–8732. [Google Scholar]
- Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biological Psychiatry. 2010;68:568–577. doi: 10.1016/j.biopsych.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Shavalia DA, Dodge AM, Beatty WW. Time-dependent effects of ECS on spatial memory in rats. Behavioral and Neural Biology. 1981;31:261–273. doi: 10.1016/s0163-1047(81)91274-7. [DOI] [PubMed] [Google Scholar]
- Sobin C, Sackeim HA, Prudic J, Devanand DP, et al. Predictors of retrograde amnesia following ECT. American Journal of Psychiatry. 1995;152:995–1001. doi: 10.1176/ajp.152.7.995. [DOI] [PubMed] [Google Scholar]
- Spellman T, McClintock SM, Terrace H, Luber B, et al. Differential effects of high-dose magnetic seizure therapy and electroconvulsive shock on cognitive function. Biological Psychiatry. 2008;63:1163–1170. doi: 10.1016/j.biopsych.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S, Chen KS. Human amnesia and animal models of amnesia: performance of amnesic patients on tests designed for the monkey. Behavioral Neuroscience. 1988;102:210–221. doi: 10.1037//0735-7044.102.2.210. [DOI] [PubMed] [Google Scholar]
- Washburn DA, Astur RS. Exploration of virtual mazes by rhesus monkeys. Animal Cognition. 2003;6:161–168. doi: 10.1007/s10071-003-0173-z. [DOI] [PubMed] [Google Scholar]
- Wilson FAW, O’Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Implications of animal object memory research for human amnesia. Neuropsychologia. 2010;48:2251–2261. doi: 10.1016/j.neuropsychologia.2010.01.023. [DOI] [PubMed] [Google Scholar]