Despite the serious risk that live yellow fever virus (YFV) vaccination poses for immunocompromised individuals, inadvertent immunization does occur (1–3). We present a case study in which we measured the antiviral immune response of a kidney transplant recipient who received intravenous immunoglobulin (IVIG) as a precautionary step to reduce the risk of vaccine-associated viscerotropic disease following YFV vaccination.
Clinical History
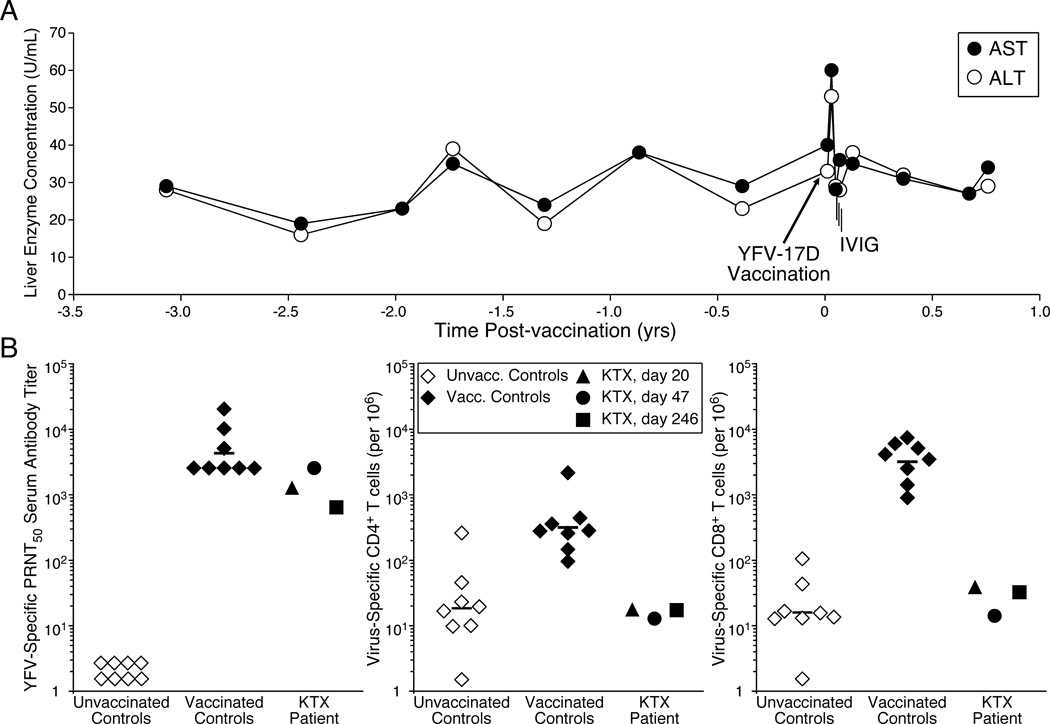
In 2007, a 55-year old Caucasian male kidney transplant (KTX) patient received live attenuated YFV-17D vaccine (YF-VAX®, Sanofi–Pasteur), in addition to concurrent immunization with an inactivated hepatitis A vaccine in preparation for a trip to South America. At the time of vaccination, the subject was 19 years post deceased donor kidney transplant and receiving maintenance immune suppression with cyclosporine (100 mg, twice daily) and mycophenolic acid (360 mg, twice daily). Shortly after immunization, the patient reviewed the vaccine contraindications and realizing the risks associated with his vaccination, he contacted his nephrologist who then contacted OHSU Transplant Infectious Disease for further consultation. Since viremia could not be monitored in real-time, liver enzymes were followed as a marker for yellow fever associated viscerotropic disease (YEL-AVD), a potentially life threatening condition that results in virus dissemination and multi-organ failure (4). Prior to vaccination, the subject had average AST values of 28 U/mL (range = 19–38) and ALT levels of 27 U/mL (range = 16–27) (Figure 1A). By 11 days post-vaccination, the patient did not report having a fever but AST and ALT levels had increased to 60 U/mL and 53 U/mL, respectively. IVIG was administered on days 20, 21 and 22 post-immunization, with a daily dose of 500 mg/kg. AST and ALT levels had returned to normal by day 18 post-vaccination but total bilirubin was elevated (2.4 Units/mL) at day 25 post-vaccination. On day 26 (i.e., four days following final IVIG administration), the patient reported what may have been an urticarial or eczematous response on his face that resolved within five days. Total bilirubin, AST, and ALT levels were within normal range when examined at day 47 post-vaccination. Follow-up with the patient at approximately eight months post-vaccination indicated no long-term adverse events.
Figure 1. Analysis of liver enzymes and immune responses following yellow fever vaccination of a kidney transplant patient (KTX).
The KTX patient was immunized with YFV–17D in preparation for international travel, despite contraindications due to ongoing immunosuppressive therapy. A Blood chemistries are shown in relation to the time of vaccination and IVIG administration. B YFV–specific neutralizing antibody titers and T-cell frequencies were determined for the KTX patient as well as eight healthy controls (median age=28 yrs, range=26–36 yrs, 63% female). Neutralizing titers were determined using a standard PRNT50 measurement (6). Virus–specific CD4+ and CD8+ T cell frequencies are based on T cells capable of producing both INF–γ and TNF–α after direct ex vivo stimulation with live purified YFV using previously described flow cytometry and intracellular cytokine staining methods (7).
Retrospective testing was performed on patient blood samples and on the IVIG lot administered to the KTX patient. The IVIG lot yielded a YFV-specific 50% plaque reduction neutralizing test (PRNT50) value of 320, demonstrating the presence of YFV-specific neutralizing antibodies similar to ten other lots of IVIG sampled across four vendors (geometric mean PRNT50 = 226, range = 80–320). Blood samples were drawn from the patient at 20, 47, and 246 days after vaccination and analyzed for YFV-specific neutralizing antibody as well as CD4+ and CD8+ T cell responses (Figure 1B). For comparison, the immune responses of eight healthy adults at 30 days post-YFV-17D vaccination were examined. The patient seroconverted by 20 days post-vaccination with antibody titers within the lower range of the controls. In contrast to neutralizing antibody levels, the patient’s virus-specific CD4+ and CD8+ T cell responses remained below detection (i.e., equivalent to naïve controls) at all time points examined.
Discussion
YFV-17D is formally contraindicated for immunosuppressed patients due to increased risk of serious adverse events (4). In this report, we found that although the KTX subject had depressed cellular immunity, he was able to mount a YFV-specific neutralizing antibody response following vaccination.
Time to onset of YEL-AVD symptoms typically range from 4–8 days post-vaccination (4) and a prior report described a YEL-AVD case who had presented with fever and AST = 111 U/mL and ALT = 72 U/mL prior to rapid development of multiple organ failure ending in death on hospital day 4 (5). There is no approved therapy for YEL-AVD, however, IVIG is often administered to KTX patients following high-risk exposure to other viruses such as measles or varicella zoster virus and since IVIG was expected to contain YFV-specific neutralizing antibodies (4), the clinical team considered it prudent to treat the patient with IVIG prior to onset of severe viscerotropic disease since the therapeutic effect would be greatest if administered at the earliest stages of disease. While it is unknown if IVIG prevented viral dissemination or viscerotropic disease, it did not appear to inhibit successful YFV vaccination as judged by seroconversion. These results suggest that IVIG could be administered as a precaution against potential YFV-AVD or other YFV vaccine-associated adverse events.
Acknowledgements
This project was funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, R44 AI079898 (to MKS and IJA), R01 AI098723 (to MKS) and Oregon National Primate Research Center grant, 8P51 OD011092-53 (to MKS). The authors thank D.J. Norman, L. Strassfeld, M.R. Arnesen, and A. Mittalhenkle for insightful discussion.
Abbreviations
- IVIG
intravenous immunoglobulin
- KTX
kidney transplant
- YFV
yellow fever virus
- YEL-AVD
yellow fever associated viscerotropic disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
MKS, IJA, EH, EAP, MWL, and JMH, performed laboratory studies and/or data analysis associated with measuring antiviral immune responses, and KAM and DH participated in care of the patient. All authors participated in writing or reviewing of the case report.
Conflict of Interest Statement
The authors were not paid to write this article by a pharmaceutical company or other agency. OHSU, IJA, EH, EAP, and MKS have a financial interest in Najít Technologies, Inc., a company that may have a commercial interest in the results of this research and technology. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU. All other authors declare no financial conflicts of interest.
References
- 1.Azevedo LS, Lasmar EP, Contieri FL, et al. Yellow fever vaccination in organ transplanted patients: is it safe? A multicenter study. Transpl Infect Dis. 2012;14(3):237. doi: 10.1111/j.1399-3062.2011.00686.x. [DOI] [PubMed] [Google Scholar]
- 2.Gowda R, Cartwright K, Bremner JA, Green ST. Yellow fever vaccine: a successful vaccination of an immunocompromised patient. Eur J Haematol. 2004;72(4):299. doi: 10.1111/j.1600-0609.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 3.Yax JA, Farnon EC, Cary Engleberg N. Successful immunization of an allogeneic bone marrow transplant recipient with live, attenuated yellow Fever vaccine. J Travel Med. 2009;16(5):365. doi: 10.1111/j.1708-8305.2009.00336.x. [DOI] [PubMed] [Google Scholar]
- 4.Monath TP, Cetron MS, Teuwen DE. Yellow fever vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Saunders/Elsevier; 2008. p. 959. [Google Scholar]
- 5.Gerasimon G, Lowry K. Rare case of fatal yellow fever vaccine-associated viscerotropic disease. South Med J. 2005;98(6):653. doi: 10.1097/01.SMJ.0000157537.11806.DC. [DOI] [PubMed] [Google Scholar]
- 6.Amanna IJ, Raue HP, Slifka MK. Development of a new hydrogen peroxide-based vaccine platform. Nat Med. 2012;18(6):974. doi: 10.1038/nm.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammarlund E, Lewis MW, Hansen SG, et al. Duration of antiviral immunity after smallpox vaccination. Nature Medicine. 2003;9(9):1131. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]