Abstract
OBJECTIVES:
Our clinical experience suggested that elemental diets were associated with a reduction in aspiration pneumonia among bedridden patients with percutaneous endoscopic gastrostomy (PEG). We compared the effects of elemental and standard liquid diets on the risk of clinical aspiration pneumonia and gastric emptying in bedridden patients receiving PEG feedings.
METHODS:
Study 1: consecutive bedridden PEG patients received elemental diets or standard liquid diets in the same fashion. The frequency of defecation, diet aspirated from the trachea, and aspiration pneumonia during hospitalization were prospectively recorded. Study 2: a randomized, crossover trial using elemental or standard liquid diets containing 13C sodium acetate as a tracer given to bedridden PEG patients who had experienced aspiration pneumonia. 13C breath tests were performed to estimate gastric emptying.
RESULTS:
Study 1: 127 patients were enrolled, 60 with elemental and 67 with standard liquid diets. The diet was aspirated from the trachea in none (0%) with the elemental diet vs. 8 (11.9%) with standard liquid diets (P=0.0057); aspiration pneumonia developed none with the elemental diet vs. 5 (7.5%) with standard liquid diets (P=0.031) (number needed to treat 14, 95% confidence interval 7–85). Study 2: 19 patients were enrolled. The elemental diet was associated with a significant increase in the 10, 30 or 50% emptying (excretion) time (P<0.001) and increased the area under the curve (% dose/h) compared with the standard liquid diet (P<0.05).
CONCLUSIONS:
Elemental diets were associated with more rapid gastric empting and fewer episodes of aspiration than standard liquid diets in bedridden PEG patients. They may be preferred for bedridden PEG patients especially who have experienced aspiration pneumonia. Properly performed randomized-controlled trials are needed to prove this potential benefit.
INTRODUCTION
Percutaneous endoscopic gastrostomy (PEG) feeding is commonly used for bedridden elderly patients (1). However, tube feedings may be complicated by vomiting and with aspiration resulting in aspiration-induced pneumonia ((2,3,4)). The prevalence of aspiration pneumonia in such patients is reported to be in the range of 10–22% and is often caused by aspiration of refluxed stomach contents ((5,6,7)). It is unclear whether, or to what extent, these serious adverse effects of enteral feeding are related to changes in gastric emptying that may occur in bedridden PEG patients.
A number of methods have been proposed to reduce the risk of aspiration. Controlling the rate of delivery of the tube feeding with pump-assisted enteral feeding in long-term PEG patients has been reported to reduce the incidence of vomiting and regurgitation (8). Recent studies in Japan have also used semi-solid nutrients instead of liquid nutrients and have also reported a reduction in late complications of tube feedings, including regurgitation and diarrhea ((9,10)). High-viscosity liquid meals have been shown to accelerate gastric emptying in healthy subjects (11) whereas semi-solid nutrients were not associated with an improvement in gastric emptying in PEG patients (10).
Reducing the time the meal is in the stomach might also reduce aspiration risk. Factors that affect gastric emptying include the content and energy density of the meal (12). Based on our clinical impression that bedridden PEG patients who received elemental diets were at reduced risk of aspiration pneumonia, we hypothesized that elemental diets may be useful for the prevention of aspiration pneumonia possibly through more rapid gastric emptying than standard liquid diets. To test these hypotheses, we prospectively compared elemental diets with standard liquid diets on the frequency of aspirating the tube feedings from the trachea and the frequency of new aspiration pneumonias appearing during hospitalization. Then we performed a randomized, crossover trial to compare the effects of elemental diet and standard liquid diets on gastric emptying among bedridden PEG patients as well as normal controls.
METHODS
The study was done at the Showa Inan General Hospital. The Institutional Review Board of Showa Inan General Hospital approved the study protocol, and all subjects or their guardians gave written informed consent. The Study 2 was registered at Clinical Trials.gov (NCT No.01467765).
Subjects
PEG patients
Study 1: In Japan, gastrostomy tube placement is performed using endoscopy and PEG patients remain in a semi-recumbent position for an hour after enteral nutrition administration. Consecutive bedridden PEG patients who were hospitalized with aspiration pneumonia, urinary tract infection, or biliary tact infection were prospectively enrolled from January 2010 to December 2011. These patients were admitted for fever, which is the most common reason for admission of PEG patients to our hospital.
Study 2: Consecutive bedridden PEG patients with recent aspiration pneumonias were entered from November 2011 to April 2012. Exclusion criteria included regular use of benzodiazepines or opioids, any clinical evidence of acute infection, a history of abdominal surgery, and an American Society of Anesthesiologists physical status of class IV or V.
Healthy subjects
Results with PEG patients were compared with six male healthy subjects in Study 2. Healthy subjects were studied in the morning, after fasting overnight. The exclusion criteria were neither previous abdominal surgery nor regular medication use.
Study design
Study 1
The selection of whether to start elemental or standard liquid diet used depended on which gastroenterologist first saw the patient (AH, elemental diet; MK, standard liquid diet). Elemental and standard liquid diets were administered at the same speed using a gravity-controlled method. The feeding time was always adjusted for 60–90 min by the nurses, regardless of the diet. The frequency of episode of diet being aspirated from the trachea, new aspiration pneumonias, and defecations/day during hospitalization were prospectively recorded. The nurses always record the characteristics of contents aspirated from the trachea for all patients in our gastroenterology ward. When PEG patients developed respiratory symptoms, chest X-rays were obtained. Aspiration pneumonia was defined as development of a consistent chest X-ray and requirement of treatment in addition to stopping PEG feeding. Significant feeding-induced diarrhea was defined as more than five defecations per day for 1 week without evidence of acute intestinal infection. The presence of any of these study features was scored throughout the hospitalization. For any feature (e.g., aspiration pneumonia), patients could only be counted once (the initial episode).
Study 2
PEG patients: This was a randomized, crossover trial. Two types of liquid test meals (200 kcal/200 ml of either the elemental (predigested) diet or the standard liquid diet) were labeled with 100 mg [13C]sodium acetate (Cambridge Isotope Laboratories, Cambridge, MA, USA) and administered in the first 15 min of the examination at the same speed using a gravity-controlled method using the PEG. For each, the feeding time was adjusted to exactly 15 min. All examinations were performed after an overnight fast. Both examinations with a 3-day washout interval between studies were conducted for all patients. Randomization was done using a table of random numbers. Subjects were then allocated using sealed opaque envelopes containing the randomization. The schedule of examinations remained blinded to the analyst (RS) until all the analyses were completed.
Healthy subjects: Both examinations with a 3-day washout interval between studies were conducted for all healthy subjects. The subjects were asked to orally consume (i.e., drink) the liquid test meals (300 kcal/300 ml of the elemental diet or standard liquid diet) labeled with 100 mg [13C]sodium acetate within 15 min and were then instructed to remain in the sitting position for 4 h.
Measurement of gastric emptying
During the 4 h after administration or ingestion of the test meal, a 13C breath test was performed ((11,13,14)) on breath samples obtained using an exclusive nasal cannula connected to an infrared spectrometer (Breath ID System; Exalenz Bioscience, Modiin, Israel) that analyzes the ratio of 13CO2 to 12CO2 in each breath sample. This system is mechanically identical to Microstream capnography for monitoring end-tidal CO2 and collects each subject's breath automatically under continuous suction, with virtually no subject cooperation ((15,16)).
Breath samples were stored in intermediate cells with built-in capnography to regulate carbon dioxide concentrations, which were subsequently passed on continuously and in sequence to an analysis chamber. The total number of breath samples ranged from 60 to 70 per subject. Ratio data obtained from breath tests are expressed as the rate of 13CO2 recovery (ratio of 13CO2 to 12CO2) per hour of each initially administered 13C substrate (% dose/h). In plots of % dose/h values, the slope of the resulting curve corresponds to the velocity of gastric emptying between data time points. Values in % dose/h were determined by calculating the ratio of 13CO2 to 12CO2 in the breath samples. Benefits of stable-isotope techniques that use 13C-labeled test meals for the breath test include ease of performance, non-invasiveness, and high sensitivity for the quantitative assessment of gastric emptying ((17,18)). This method allowed continuous and almost real-time measurement of the 12CO2/13CO2 ratio.
Test meals
A commercially available predigested or elemental diet, Elental (1 kcal/ml, Ajinomoto Pharmaceutical, Tokyo, Japan) was used consisting of 16.5% protein (provided as amino acids), 79.5% carbohydrates (provided as dextrin (mean molecular weight 900)), 0.64% fat, and 3.36% vitamins and minerals dissolved in water to yield 1 kcal/ml. Ensure liquid (1 kcal/ml, Abbott Japan, Tokyo, Japan), consisting of 18% protein, 20% fat, and 62% carbohydrates, was used as the standard liquid diet.
Data analysis
Gastric emptying was evaluated by using three parameters: T10%, T30%, T50%: 10, 30, and 50% the gastric emptying (excretion) time, (i.e., the time at which 10, 30, and 50% of the total input of 13C is excreted (19). Although the T50% and scintigraphic half-emptying time are not identical, there is a linear correlation between T50% in the 13C breath test and scintigraphic half-emptying times ((18,19)). Total excretion of 13CO2 during 3 h was calculated by measuring the area under the % dose/h curve.
Statistical analysis
Data are presented as mean±s.d. Statistical tests to compare the measured results for the two groups were as follows: the χ2 test, with Yates' correction for continuity where appropriate, was used for comparison of categorical data. Fisher's exact test was used when the numbers were small. For parametric data, the Student's t-test was used when two means were compared. The number needed to treat and 95% confidence interval were calculated using SAS 9.2 (SAS Institute, Tokyo, Japan).
For Study 2, we estimated (from preliminary data) that % dose/h per subject in the standard liquid group would be 12 after 1 h in PEG patients. For a 25% increase in % dose/h in the elemental diet group, a total of at least 19 subjects would be required (α level 0.05, β level 0.2). The effects of the elemental diet on t1/2ex and total excretion of 13CO2 during 3 h were compared, with serving sequence and subject as variables, in an analysis of variance. A repeated-measures two-factor analysis of variance with interactions was used to analyze treatment and time effects in 19 PEG patients, and then a Bonferroni correction was applied to comparisons of two groups. Differences were considered significant if the P-value was <0.05. Statistical analysis was performed by using Prism 5 software (GraphPad Software, San Diego, CA).
RESULTS
Study 1: A total of 128 patients (average age of 80 years and 60 men) were enrolled: 60 were assigned to the elemental diet group and 67 to the standard liquid diet group. There were no significant differences between the two groups with respect to clinical baseline parameters, including age, gender, the cause of hospitalization, indications for PEG, amount and duration of PEG feeding, periods when the incidence of complications was observed, concurrent medical conditions, or proton pump inhibitor use (Table 1). As these patients became the bedridden, gastrostomy-fed state owing to cerebrovascular accident or central nervous system disorders, there were no significant differences between the two groups with regard to prior medical conditions and illness severity.
Table 1. Baseline characteristics of 127 PEG patients who were enrolled in Study 1.
| Group | Elemental diet (N=60) | Standard liquid diet (N=67) | P value |
|---|---|---|---|
| Gender (male/female) | 33/27 | 27/40 | 0.098 |
| Age (years)a | 79±8 | 81±7 | 0.88 |
| Indication for PEG: CVA/CNSD | 48/12 | 57/10 | 0.45 |
| Causes of hospitalization | |||
| Pneumonia | 43 | 47 | 0.95 |
| BTI | 12 | 15 | |
| UTI | 5 | 5 | |
| Amount of PEG feeding (ml)a | 900±0 | 1040±100 | 0.76 |
| Duration of PEG feeding (months)a | 21±4 | 17±4 | 0.66 |
| Period when the incidence of complications was observed (months)a | |||
| 11±7 | 12±9 | 0.74 | |
| Concurrent medical conditions | |||
| Chronic kidney disease | 3 | 4 | 0.81 |
| Chronic liver disease | 5 | 6 | 0.90 |
| COPD | 3 | 2 | 0.56 |
| Proton pump inhibitor use | 8 | 10 | 0.80 |
BTI, biliary tract infection; CNSD, central nervous system disorders; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; PEG, percutaneous endoscopic gastrostomy; UTI, urinary tract infection.
Values are number of patients except for age, the amount, the duration of PEG feeding, and the period when the incidence of complications was observed.
Each value shows mean±s.d.
The number of patients who had diet aspirated from the trachea or who developed new aspiration pneumonias in the elemental group was significantly less than in the standard liquid diet group (0 vs. 8, P=0.0057; 0 vs. 5, P=0.031) (Table 2). The number of patients in whom diarrhea occurred in the elemental group was also less than that in the standard liquid diet group, although the difference was only of borderline significance (0 vs. 4; P=0.054). Number needed to treat of elemental diet for aspiration pneumonia and diarrhea was 14 (95% confidence interval 7–85) and 17 (95% confidence interval 9–333), respectively. In the standard liquid diet group, the ratios of patients with prior pneumonia/all patients with any of these features developed during hospitalization were high (4/8, 4/5, and 3/4) (Table 2). Although all patients promptly recovered associated with the administration of 2 g/day of ceftriaxone sodium for 4–7 days after admission, additional hospitalizations were required for nine patients with aspiration pneumonia or newly developed diarrhea in the standard liquid diet group.
Table 2. Clinical outcomes of 127 PEG patients who were enrolled in Study 1.
| Variable | Elemental diet (N=60) | Standard liquid diet (N=67) | P value | NNT (95% CI) |
|---|---|---|---|---|
| No. of patients with nutrients aspirated form the trachea (no. of patients with prior pneumonia ) | ||||
| 0 | 8 (4) | 0.0057 | ||
| No. of patients with aspiration pneumonia newly developed (no. of patients with prior pneumonia) | ||||
| 0 | 5 (4) | 0.031 | 14 (7–85) | |
| No. of patients with diarrhea (no. of patients with prior pneumonia ) | ||||
| 0 | 4 (3) | 0.054 | 17 (9–333) |
CI, confidence interval; NNT, number needed to treat; PEG, percutaneous endoscopic gastrostomy.
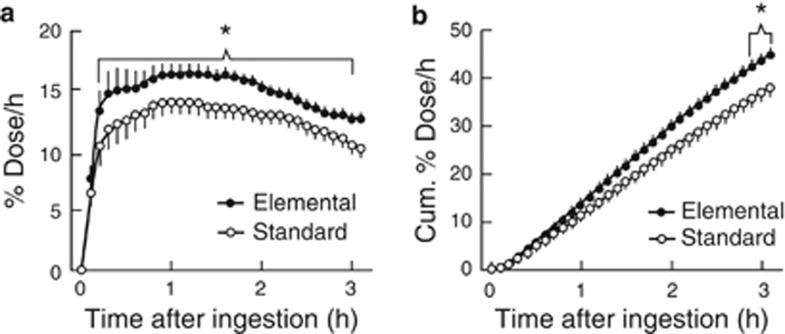
Study 2: Clinical features of the 19 PEG patients who were enrolled in Study 2 are shown in Table 3. The % dose/h curve showed a steep rise after the intake of elemental diet while the curve rose more slowly to its peak and then declined steadily (Figure 1). With bedridden PEG patients, the elemental diet not only resulted in a significant increase in the mathematically simulated 10, 30, or 50% emptying (excretion) time (P<0.001), but also increased the area under the curve (% dose/h) significantly as compared with standard liquid diet (P<0.05) (Table 4, Figure 1). The healthy subjects were younger than the patients (average age of 48 years, range 44–52). In the healthy subjects, there was no significant effect in either the mathematically simulated 10, 30, or 50% emptying (excretion) time or the area under the curve (% dose/h) in gastric emptying of the orally ingested meals (Table 5).
Table 3. Clinical features of bedridden PEG patients who were enrolled in Study 2.
| Group | (N=19) |
|---|---|
| Gender (male/female) | 11/8 |
| Age (years)a | 79.4±3.9 |
| Body height (cm)a | 153.4±5.8 |
| Body weight (kg)a | 47.8±5.1 |
| Indication for PEG: CVA/CNSD | 12/7 |
| Amount of PEG feeding (ml)a | 900±0 |
| Duration of PEG feeding (months)a | 16±5 |
| Frequency of aspiration pneumonia experienced beforea | 1.6 vs. 0.7 |
| Concurrent medical conditions | |
| Diabetes mellitus | 4 |
| Chronic liver disease | 3 |
| Proton pump inhibitor use | 5 |
CNSD, central nervous system disorders; CVA, cerebrovascular accident; PEG, percutaneous endoscopic gastrostomy.
Values are number of patients except for age, body height, body weight, the amount and the duration of PEG feeding.
Each value shows mean±s.d.
Figure 1.
Effects of the elemental diet or standard liquid diet on gastric emptying in gastrostomy patients. (a) Curves of 13CO2 excretion (% dose/h) after administration of test nutrients. (b) Cumulative excretion of 13CO2 (Cum. % dose/h) constructed from a. All values are mean+s.d. *There were significant differences between elemental diet and standard liquid diet group (N=19, P<0.05).
Table 4. Comparison of elemental diet and standard liquid diet on gastric emptying in bedridden PEG patients.
| Group | Elemental diet (N=19) | Standard liquid diet (N=19) | P value |
|---|---|---|---|
| T10% | 0.82±0.24 | 1.00±0.37 | 0.0064 |
| T30% | 2.06±0.42 | 2.53±0.76 | 0.0030 |
| T50% | 3.57±0.62 | 4.93±1.69 | 0.0017 |
PEG, percutaneous endoscopic gastrostomy.
All values (h) are mean±s.d.
T10%, T30%, T50%: 10, 30, and 50% the gastric excretion (emptying) time, i.e., the time at which 10, 30, and 50% of the total input of 13C is excreted.
Table 5. Comparison of elemental diet and standard liquid diet on gastric emptying in healthy subjects.
| Group | Elemental diet (N=6) | Standard liquid diet (N=6) | P value |
|---|---|---|---|
| T10% | 0.82±0.09 | 0.90±0.20 | 0.40 |
| T30% | 2.14±0.44 | 2.31±0.67 | 0.61 |
| T50% | 4.41±1.37 | 5.30±2.41 | 0.41 |
All values (h) are mean±s.d.
T10%, T30%, T50%: 10, 30, and 50% the gastric excretion (emptying) time, i.e., the time at which 10, 30, and 50% of the total input of 13C is excreted.
No complications, including diarrhea and aspiration pneumonia, which required any treatments occurred in either group during the study period.
DISCUSSION
We demonstrated that use of an elemental diet was associated with a reduced incidence of aspiration pneumonia compared with standard liquid diets among bedridden patients receiving PEG feeding despite the fact that the two diets were administered identically, especially among those who had previously experienced aspiration pneumonia.
Study 2 tested our hypothesis that in bedridden PEG patients elemental diet was emptied from the stomach more rapidly than standard liquid diet. We showed that the elemental diet empted significantly faster than the standard liquid diet among bedridden PEG patients administered by PEG. From the % dose/h curves that we obtained in PEG patients (Figure 1), the elemental diet initially entered the duodenum more rapidly from the stomach. In addition, the elemental diet appeared to be was less likely to induce diarrhea than the standard liquid diet (Table 2).
Non-caloric water leaves the stomach quickly, whereas solutions of carbohydrates and proteins leave the stomach more slowly but at a similar speed; lipids clear most slowly (20). Fatty diets not only empty slowly but also have also been reported to be associated with gastroesophageal regurgitation (21). The standard liquid diet used contained 20% fat. In contrast, the elemental diet was low lipid (0.64 % fat) suggesting that differences in lipid content may be responsible for much of the differences seen in terms of gastric emptying and aspiration. However, it is unknown how low-residue diets, which are one of the characteristics of elemental diet, are related to gastric emptying and aspiration.
We used gravity feeding. Previous studies have suggested significant advantages to pump-assisted enteral nutrition over gravity-controlled enteral nutrition with regard to regurgitation, diarrhea, and aspiration-induced pneumonia rate, and a lower frequency of vomiting (8). Another approach to reduce aspiration is to use post-pyloric feeding, such as a jejunal extension tube through a gastrostomy, or a direct percutaneous endoscopic jejunostomy has also been used to decrease gastroesophageal regurgitation and prevent aspiration ((22,23)). Both pump-assisted enteral nutrition and post-pyloric feeding require pumps and are thus more elaborate to manage than gravity-controlled nutrition using PEG. In our study, gravity-controlled feeding of an elemental diet was superior to gravity-controlled standard diet tube feedings in terms of aspiration even among those who had suffered aspiration previously and had the additional advantage of not requiring a pump. The elemental diet (Elental) used is a high osmolality nutrient (1 kcal/ml equals 760 mOsm/l) and is typically given at <100 ml/h using a pump when used for treatment of patients with Crohn's disease in Japan. However, when the elemental diet was fed at the rate of 200–300 ml/h to PEG patients, diarrhea did not occur in any patient. In Study 2, 200 ml of elemental diet was administered exactly in 15 min to 19 PEG patients, and diarrhea was not produced in any. These results suggest that volumes of 300 ml are well tolerated when infused into the stomach. Future studies will be needed to identify the break point where the osmolality overwhelms the ability of the proximal intestine to compensate. We hypothesize that the fact that the elemental diet is low lipid and low residue may be responsible for its advantages in terms of aspiration and diet-associated diarrhea.
In Japan, semi-solid nutrients are commonly administered at a bolus fashion in PEG patients ((9,10)). Although a pump is not required, this method requires time and effort to prepare semi-solid meals. This study was part of our search for a simple method for feeding that does not require additional equipment or efforts but which is associated with a reduced rate of diarrhea, gastroesophageal regurgitation, and aspiration in patients requiring PEG feedings. Our results are consistent with the notion that elemental diet is associated with a reduction in aspiration and aspiration pneumonia in bedridden PEG patients and can be used without changing the regular schedule and approach for PEG feedings.
One potential disadvantage is that elemental diet is more expensive than standard liquid diets and the additional expense may not be covered by US or European health-care systems. In Japan, the cost of Elental (300 kcal, 447 JPY) is 2.4 times greater than that of Ensure liquid (300 kcal, 189 JPY). Cost effectiveness is suggested but a definitive answer in terms of whether the higher costs of predigested diet more than offset the expenses associated with aspiration pneumonia must await the results of prospective controlled trials. However, the present study suggests that based on the rapid recovery from aspiration pneumonia and reduction of aspiration pneumonia associated with the use of elemental diets in bedridden PEG patients, such studies are warranted especially among those who have suffered from aspiration pneumonia. Studies are also needed to investigate whether and when changing from an elemental diet back to a standard liquid diet after stabilization of patients' general condition would be a preferred approach as it would reduce the time high-cost elemental diet were needed.
The present studies have limitations. The first study was a non-randomized, non-blinded, pilot study without allocation concealment and there is therefore an increased risk of bias. Such trials may also tend to exaggerate intervention benefits. The randomized, non-blinded crossover study was more methodologically rigorous and although it supports the hypothesis, by itself, it cannot prove it. In the first trial, the effects were similar among the different groups of subjects including those who had experienced aspiration previously suggesting that they can be generalized. Study 2 also has the theoretically issue of “carry-over” between treatments, which could confound the estimates of the treatment effects. In practice, “carry-over” effects can be avoided with a sufficiently long “wash-out” period between treatments. However, in this type of patient a long wash-out period may result in changes the patients' condition. In this study, two different examinations were conducted within 3 days after an overnight fast.
In conclusion, elemental diet tube feedings were associated with more rapid gastric empting and fewer episodes of aspiration than standard liquid tube feedings among bedridden PEG patients, and suggest that low-lipid-containing elemental diets may be a good alternative for tube feeding, especially for those who have previously experienced aspiration pneumonia. Future studies about evaluation pump vs. gravity feeding, costs, and whether and when it is best to switch from predigested diet to standard tube feeding are now needed. In addition, further studies with proper design are needed to prove that elemental diets truly reduce the risk for aspiration pneumonia in bedridden, gastrostomy-fed patients.
STUDY HIGHLIGHTS
Guarantor of the article: Akira Horiuchi, MD.
Specific author contributions: Acquisition of data: Akira Horiuchi and Masashi Kajiyama. Analysis and interpretation of data: Study 1, Akira Horiuchi and Masashi Kajiyama. Study 2, Ryosei Sakai and Manabu Suzuki. Drafting of the manuscript: Akira Horiuchi. Critical revision of the manuscript: Akira Horiuchi, Yoshiko Nakayama, Ryosei Sakai, Manabu Suzuki, Masashi Kajiyama and Naoki Tanaka.
Financial support : Study 2 was funded in part by Ajinomoto, Japan, which supplied the elemental diets and provided the technology for the gastric emptying test and its analysis.
Potential competing interests: Ryosei Sakai and Manabu Suzuki are employed in research positions by the Ajinomoto. We thank David Y. Graham, MD, for his editorial advice.
References
- Moller P, Lindberg CG, Zilling T. Gastrostomy by various techniques: evaluation of indications, outcome, and complications. Scand J Gastroenterol. 1999;34:1050–1054. doi: 10.1080/003655299750025174. [DOI] [PubMed] [Google Scholar]
- Sartori S, Trevisani L, Tassinari D, et al. Prevention of aspiration pneumonia during long-term feeding by percutaneous endoscopic gastrostomy: might cisapride play any role? an open pilot study. Support Care Cancer. 1994;2:188–190. doi: 10.1007/BF00417479. [DOI] [PubMed] [Google Scholar]
- Hull MA, Rawlings J, Murray FE, et al. Audit of outcome of long-term enteral nutrition by percutaneous endoscopic gastrostomy. Lancet. 1993;341:869–872. doi: 10.1016/0140-6736(93)93072-9. [DOI] [PubMed] [Google Scholar]
- Senft M, Fietkau R, Iro H, Sailer D, Sauer R. The influence of supportive nutritional therapy via percutaneous endoscopically guided gastrostomy on the quality of life of cancer patients. Support Care Cancer. 1993;1:272–275. doi: 10.1007/BF00366049. [DOI] [PubMed] [Google Scholar]
- Ballan KK, Vinjamuri S, Maltby P, et al. Gastroesophageal reflux in patients fed by percutaneous endoscopic gastrostomy (PEG): detection by a simple scintigraphic method. Am J Gastroenterol. 1998;93:946–949. doi: 10.1111/j.1572-0241.1998.00284.x. [DOI] [PubMed] [Google Scholar]
- James A, Kapur K, Hawthorne AB. Long-term outcome of percutaneous endoscopic gastrostomy feeding in patients with dysphasic stroke. Age Ageing. 1998;27:671–676. doi: 10.1093/ageing/27.6.671. [DOI] [PubMed] [Google Scholar]
- Finocchiaro C, Galetti R, Ferrari A, et al. Percutaneous endoscopic gastrostomy: a long-term follow-up. Nutrition. 1997;13:520–523. doi: 10.1016/s0899-9007(97)00030-0. [DOI] [PubMed] [Google Scholar]
- Shang E, Geiger N, Sturm JW, et al. Pump-assisted enteral nutrition can prevent aspiration in bedridden percutaneous endoscopic gastrostomy patients. JPEN. 2004;28:180–183. doi: 10.1177/0148607104028003180. [DOI] [PubMed] [Google Scholar]
- Kanie J, Suzuki Y, Akatsu H, et al. Prevention of late complications by half-solid enteral nutrients in percutaneous endoscopic gastrostomy tube feeding. Gerontology. 2004;50:417–419. doi: 10.1159/000080181. [DOI] [PubMed] [Google Scholar]
- Nishiwaki S, Araki H, Shirakami Y, et al. Inhibition of gastroesophageal reflux by semi-solid nutrients in patients with percutaneous endoscopic gastrostomy. JPEN. 2009;33:513–519. doi: 10.1177/0148607108327045. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y, Kusano M, Kawamura O, et al. High-viscosity liquid meal accelerates gastric emptying. Neurogastroenterol Motil. 2007;19:879–886. doi: 10.1111/j.1365-2982.2007.00972.x. [DOI] [PubMed] [Google Scholar]
- Hays NP, Roberts SB. The anorexia of aging in humans. Physiol Behav. 2006;88:257–266. doi: 10.1016/j.physbeh.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Ghoos YF, Maes BD, Geypens BJ, et al. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology. 1993;104:1640–1647. doi: 10.1016/0016-5085(93)90640-x. [DOI] [PubMed] [Google Scholar]
- Zai H, Kusano M, Hosaka H, et al. Monosodium L-glutamate added to a high-energy, high-protein liquid diet promotes gastric emptying. Am J Clin Nutr. 2009;89:431–435. doi: 10.3945/ajcn.2008.26180. [DOI] [PubMed] [Google Scholar]
- Agus MS, Alexander JL, Mantell PA. Continuous non-invasive end-tidal CO2 monitoring in pediatric inpatients with diabetic ketoacidosis. Pediatr Diabetes. 2006;7:196–200. doi: 10.1111/j.1399-5448.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- Singh S, Allen WD, Jr, Venkataraman ST, et al. Utility of a novel quantitative handheld microstream capnometer during transport of critically ill children. Am J Emerg Med. 2006;24:302–307. doi: 10.1016/j.ajem.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Braden B, Adams S, Duan LP, et al. The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology. 1995;108:1048–1055. doi: 10.1016/0016-5085(95)90202-3. [DOI] [PubMed] [Google Scholar]
- Mossi S, Meyer-Wyss B, Beglinger C, et al. Gastric emptying of liquid meals measured noninvasively in humans with [13C]acetate breath test. Dig Dis Sci. 1994;39:107S–109S. doi: 10.1007/BF02300386. [DOI] [PubMed] [Google Scholar]
- Chapman MJ, Besanko LK, Burgstad CM, et al. Gastric emptying of a liquid nutrient meal in the critically ill: relationship between scintigraphic and carbon breath test measurement. Gut. 2011;60:1336–1343. doi: 10.1136/gut.2010.227934. [DOI] [PubMed] [Google Scholar]
- Stacher G, Bergmann H, Gaupmann G, et al. Fat preload delays gastric emptying: reversal by cisapride. Br J Clin Pharmacol. 1990;30:839–845. doi: 10.1111/j.1365-2125.1990.tb05449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB, Satia JA, Rabeneck L. Dietary intake and the risk of gastro-oesophageal reflux disease: a cross sectional study in volunteers. Gut. 2005;54:11–17. doi: 10.1136/gut.2004.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsky JL, Aszodi A. Percutaneous endoscopic jejunostomy. Am J Gastroenterol. 1984;79:113–116. [PubMed] [Google Scholar]
- Shike M, Wallach C, Lacier H. Direct percutaneous endoscopic jejunostomies. Gastrointest Endosc. 1991;37:62–65. doi: 10.1016/s0016-5107(91)70625-1. [DOI] [PubMed] [Google Scholar]