Abstract
Objective:
Investigation of the association of excess adiposity with three different outcomes: all-cause mortality, coronary heart disease (CHD) mortality and incident CHD.
Design:
Cross-sectional surveys linked to hospital admissions and death records.
Subjects:
19 329 adults (aged 18–86 years) from a representative sample of the Scottish population.
Measurements:
Gender-stratified Cox proportional hazards models were used to estimate hazard ratios (HRs) for all-cause mortality, CHD mortality and incident CHD. Separate models incorporating the anthropometric measurements body mass index (BMI), waist circumference (WC) or waist–hip ratio (WHR) were created adjusted for age, year of survey, smoking status and alcohol consumption.
Results:
For both genders, BMI-defined obesity (⩾30 kg m−2) was not associated with either an increased risk of all-cause mortality or CHD mortality. However, there was an increased risk of incident CHD among the obese men (hazard ratio (HR)=1.78; 95% confidence interval=1.37–2.31) and obese women (HR=1.93; 95% confidence interval=1.44–2.59). There was a similar pattern for WC with regard to the three outcomes; for incident CHD, the HR=1.70 (1.35–2.14) for men and 1.71 (1.28–2.29) for women in the highest WC category (men ⩾102 cm, women ⩾88 cm), synonymous with abdominal obesity. For men, the highest category of WHR (⩾1.0) was associated with an increased risk of all-cause mortality (1.29; 1.04–1.60) and incident CHD (1.55; 1.19–2.01). Among women with a high WHR (⩾0.85) there was an increased risk of all outcomes: all-cause mortality (1.56; 1.26–1.94), CHD mortality (2.49; 1.36–4.56) and incident CHD (1.76; 1.31–2.38).
Conclusions:
In this study excess adiposity was associated with an increased risk of incident CHD but not necessarily death. One possibility is that modern medical intervention has contributed to improved survival of first CHD events. The future health burden of increased obesity levels may manifest as an increase in the prevalence of individuals living with CHD and its consequences.
Keywords: body mass index, waist circumference, waist–hip ratio, mortality, coronary heart disease, Scotland
Introduction
The high prevalence of obesity in Scotland1 and other developed countries2 raises concerns over its health impact. The relationship between overall body fat proportion, as summarised by body mass index (BMI), and all-cause mortality has been established by many studies to be U-shaped, with the nadir within the normal range (18.5–25 kg m−2).3, 4 However, some studies using contemporary population samples have identified an apparent attenuation of this association.5, 6, 7, 8 In these analyses, overweight (25–30 kg m−2) has been identified to be borderline protective, or even protective, and obesity (>30 kg m−2) was only associated with an increased risk of mortality once BMI exceeded 35 kg m−2. Flegal et al.,6, 9 upon comparing cohorts recruited at different times, suggested that the influence of obesity on mortality, and cardiovascular disease (CVD) mortality, may have decreased over time. Similarly, on comparison of two Canadian studies, one recruited in the 1980s5 and the other in the 1990s,7 excess adiposity was found to have a reduced association with mortality in the later study.
It has been suggested that improvements in medical care, particularly in relation to CVD, may be one of the contributors to the diminished impact of obesity on mortality over time.6, 9 Advances in treatment account for at least half the recent decline in coronary heart disease (CHD) mortality.10, 11 In a recent study of the Scottish Health Survey (SHeS) Cohort,12 we identified obesity to be associated with an increased risk of mortality once BMI was >40 kg m−2. We hypothesised that if medical improvements are, in part, weakening the association between increased adiposity and mortality, then a stronger positive association would exist for incident CHD than for mortality. We therefore examined the association between BMI, as well as waist circumference (WC), and waist–hip ratio (WHR) (indices of abdominal adiposity), with the three outcomes of all-cause mortality, CHD mortality and incident CHD within this same Scottish population sample, the SHeS Cohort.
Materials and methods
Survey method and subjects
The SHeSs 1995, 1998 and 2003 are cross-sectional nationally representative surveys designed to provide information on the health and health-related behaviours of people living in private households in Scotland; details are described elsewhere.13, 14, 15, 16 Samples were selected using a multi-stage stratified clustered probability sampling design. The surveys were age limited in 1995 (16–64 years) and 1998 (2–74 years). Data were collected during two household visits: first by an interviewer (where BMI was determined), then by a nurse. Body weight was measured to the nearest 100 g using electronic scales; an estimate was requested from respondents exceeding the scales' upper limit of 130 kg. Height was measured to the nearest millimetre using a stadiometer. Waist and hip circumferences were measured during the nurse visit using a tape with an insertion buckle. Both were taken at least twice and recorded to the nearest millimetre to provide a mean. The waist was defined as the midpoint between the lower rib and the upper margin of the iliac crest. The hips were measured at the widest circumference around the buttocks below the iliac crest. Pregnant women and participants who were chair bound were excluded from all the above measurements.
Measures
Anthropometric
BMI was calculated as weight (kg) divided by the square of the height (m2). Established cut-off points for BMI were used: underweight (<18.5 kg m−2), desirable weight (18.5–<25 kg m−2), overweight (25–<30 kg m−2) and obese (⩾30 kg m−2).17 WC was defined in gender-specific low, reference, moderate and high (abdominal obesity) categories (for men: <79 cm, 79–<94 cm, 94–<102 cm and ⩾102 cm, and for women: <68 cm, 68–<80 cm, 80–<88 cm and ⩾88 cm, respectively).18, 19 WHR (WC divided by hip circumference) was categorised as low, reference, moderate and high (abdominal obesity) separately for men (<0.85, 0.85–<0.95, 0.95–<1 and ⩾1) and women (<0.7, 0.7–<0.8, 0.8–<0.85 and ⩾0.85). WC and WHR included cut-offs recommended by a WHO consultation; the upper limits of the second lowest categories (reference) have been associated with increased health risk.17
Covariates
Age was recorded as age at the time of interview. Smoking status was categorised as never smoker, ex-smoker, light smoker (0–<10 cigarettes per day), moderate smoker (10–<20 cigarettes per day) and heavy smoker (⩾20 cigarettes per day). Pipe and cigar smokers were included with light smokers. Alcohol consumption was defined as never or very occasional drinker, ex-drinker, 0–<7 units per week, 7–<14 units per week, 14–<21 units per week, 21–<28 units per week and ⩾28 units per week (1 unit=10 ml ethanol). For women the last two categories were merged owing to low numbers. Two socioeconomic covariates were used: individual Registrar General's occupational social class and 2001 Carstairs score quintiles, an area-based deprivation measure.20
Outcome measures
These surveys, with participants' consent, have been linked to a national database of deaths (till December 2008) and the Scottish Morbidity Records.16 The latter are a patient-based database of acute hospital discharges from 1981 to December 2008. Three outcomes were investigated: all-cause mortality, CHD mortality defined by international classification of disease codes (ICD-9 410-414 and ICD-10 I20-I25) and incident CHD (fatal and non-fatal events). The latter was defined as first hospital admission, or death, using the same ICD codes plus the surgical interventions coronary artery bypass grafting and percutaneous transluminal coronary angioplasty.
Sample
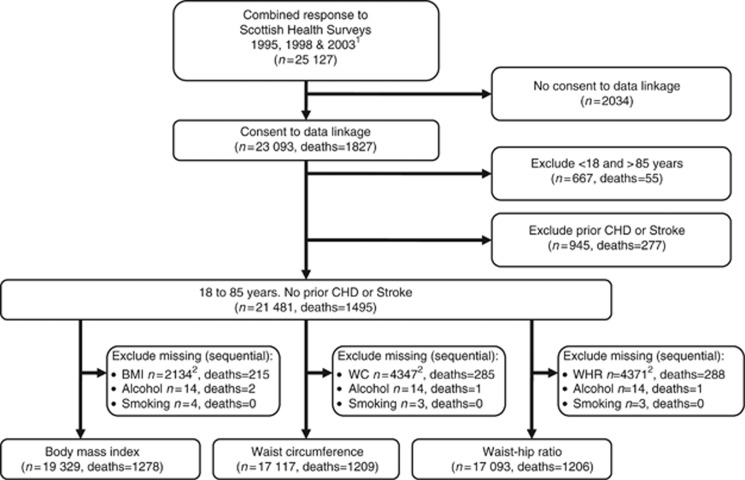
Response for the interview stage of the survey, where BMI was determined, declined from 81% in 1995 to 76% in 1998 and finally to 60% in 2003. The proportion of these progressing to the nurse interview, where WC and WHR were ascertained, also declined from 88% in 1995 to 67% in 2003. Individuals who had consented to data linkage (approximately 92%), were between 18 and 85 years of age, had no prior hospital admissions for CHD or stroke, and had complete data were included in analyses; Figure 1 summarises the combined sample derivation. Of the first event, CHD deaths in this sample, 36% had post mortems completed and 76% occurred without prior hospitalisation (numerator, CHD death with no admission; denominator, CHD death with no admission plus all deaths within 12 months of admission).
Figure 1.
Flow diagram of the derivation of the final combined sample size from the Scottish Health Survey Cohort for each anthropometric measure used in analyses. 1All respondents over 16 years of age 2includes 212 pregnant women.
Statistical analyses
For each of the anthropometric measures Cox proportional hazards models were used to estimate HRs for the three outcomes. Models were fitted within a multilevel framework to account for the data hierarchy of individuals within postcodes. The timescale was taken as time since interview (months) and censoring occurred at 31 December 2008. Models were adjusted for age (centred), smoking status, alcohol consumption and survey cohort. Additional adjustments were then made using either social class or area of deprivation. The second lowest category was used as referent for the anthropometric covariates; all other categorical covariates used the first category. A significant interaction between BMI and gender prompted stratification of all analyses by sex. Survival curves for each gender, and outcome, were generated using the Kaplan–Meier method with formal testing using the log-rank test. Adherence to the proportional hazards assumption was investigated by incorporation of time-dependent covariates in the models and by plotting smoothed Schoenfeld residuals against time; no violations of the assumption were identified. Sensitivity analyses excluding the first 3 or 5 years of follow-up were performed, as well as limiting analyses to individuals with complete data for the anthropometric measures. All statistical tests were two-tailed and statistical significance was taken as P<0.05. All the aforementioned analyses were performed using SAS v9.2 (SAS Institute Inc., Cary, NC, USA) and MLwiN v2.11(ref. 21) statistical software.
The anthropometric measures were also fitted as continuous variables using a fractional polynomial algorithm in fully adjusted, gender-stratified, single-level models.22 Command mfp in Stata/IC 10.1 (StataCorp LP., College Station, TX, USA) was used to fit the most appropriate first-degree, or second-degree, fractional polynomial to the data using power transformation(s) from, among others, the reciprocal, logarithm, square, cube and linear (no transformation). Age was simultaneously fitted using the same selection process.
Results
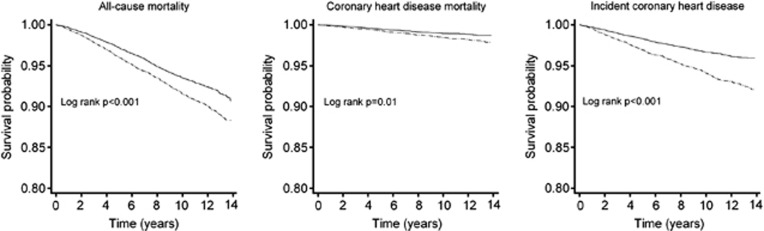
During a median follow-up of 10.1 years (extremes 6 days to 13.8 years; men=86 012 and women=112 804 person-years at risk) there were 757 deaths among men and 738 deaths among women (crude death rate of 8.8 for men and 6.5 for women per 1000 person-years) (Table 1). The crude CHD death rate was 1.5 and 1.0 per 1000 person-years and the crude incident CHD (fatal and non-fatal) rate was 6.1 and 3.2 per 1000 person-years for men and women, respectively. The risk of death, CHD death and incident CHD was greater for men than for women (Figure 2). Baseline data stratified by gender are presented in Table 1.
Table 1. Baseline characteristics for the Scottish Health Surveys Cohort sample stratified by gender.
| Men | Women | |
|---|---|---|
| Sample size | 9320 | 12 161 |
| All-cause deaths, n (%) | 757 (8.1) | 738 (6.1) |
| Coronary heart disease deaths, n (%) | 132 (1.4) | 112 (0.9) |
| Incident CHD, n (%) | 511 (5.5) | 362 (3.0) |
| Person-years of follow-up | ||
| Mortality | 86 012 | 112 804 |
| Incident CHD | 84 155 | 111 464 |
| Death rate/1000 person-yearsa | 8.8 | 6.5 |
| Coronary heart disease death rate/1000 person-yearsa | 1.5 | 1.0 |
| Incident CHD event rate/1000 person-yearsa | 6.1 | 3.2 |
| Mean age (years) (s.d.) | 44.6 (15.0) | 45.1 (15.5) |
| Survey, n (%) | ||
| 1995 | 3108 (33.3) | 3914 (32.2) |
| 1998 | 3331 (35.7) | 4429 (36.4) |
| 2003 | 2881 (30.9) | 3818 (31.4) |
| BMI (kg m−2), n (%) | ||
| <18.5 (underweight) | 84 (0.9) | 201 (1.7) |
| 18.5–<25 (desirable weight) | 2983 (32.0) | 4633 (38.1) |
| 25–<30 (overweight) | 3711 (39.8) | 3511 (28.9) |
| ⩾30 (obese) | 1760 (18.9) | 2464 (20.3) |
| Missing | 782 (8.4) | 1352 (11.1) |
| WC (cm), n (%) | ||
| M <79, W <68 (low) | 719 (7.7) | 978 (8.0) |
| M 79–<94, W 68–<80 (reference) | 3477 (37.3) | 3528 (29.0) |
| M 94–<102, W 80–<88 (moderate) | 1743 (18.7) | 2163 (17.8) |
| M ⩾102, W ⩾88 (high) | 1677 (18.0) | 2849 (23.4) |
| Missing | 1704 (18.3) | 2643 (21.7) |
| WHR, n (%) | ||
| M <0.8, W <0.65 (low) | 1325 (14.2) | 421 (3.5) |
| M 0.8–<0.9, W 0.65–<0.75 (reference) | 4193 (45.0) | 4339 (35.7) |
| M 0.9–<1, W 0.75–<0.85 (moderate) | 1374 (14.7) | 2347 (19.3) |
| M ⩾1, W ⩾0.85 (high) | 717 (7.7) | 2394 (19.7) |
| Missing | 1711 (18.4) | 2660 (21.9) |
| Smoking status, n (%) | ||
| Never smoker | 3686 (39.5) | 5431 (44.7) |
| Ex-smoker | 2203 (23.6) | 2609 (21.5) |
| Light smoker | 871 (9.3) | 860 (7.1) |
| Moderate smoker | 1149 (12.3) | 1744 (14.3) |
| Heavy smoker | 1407 (15.1) | 1508 (12.4) |
| Missing | 4 (0) | 9 (0.1) |
| Alcohol consumption, n (%) | ||
| Never/occasional drinker | 297 (3.2) | 908 (7.5) |
| Ex-drinker | 346 (3.7) | 560 (4.6) |
| 0–<7 units/week | 2645 (28.4) | 6763 (55.6) |
| 7–<14 units/week | 1608 (17.3) | 2252 (18.5) |
| 14–<21 units/week | 1423 (15.3) | 971 (8.0) |
| 21–<28 units/week | 988 (10.6) | 335 (2.8) |
| ⩾ 28 units/week | 2004 (21.5) | 361 (3.0) |
| Missing | 9 (0.1) | 11 (0.1) |
| Individual social class, n (%) | ||
| I/II—professional/managerial | 2952 (31.7) | 3295 (27.1) |
| III—skilled non-manual | 944 (10.1) | 3807 (31.3) |
| III—skilled manual | 3131 (33.6) | 1058 (8.7) |
| IV—partly skilled | 1470 (15.8) | 2189 (18.0) |
| V—unskilled | 467 (5.0) | 1098 (9.0) |
| Other | 356 (3.8) | 714 (5.9) |
| Carstairs quintile, n (%) | ||
| 1—Least deprived | 1742 (18.7) | 2175 (17.9) |
| 2 | 2057 (22.1) | 2616 (21.5) |
| 3 | 2060 (22.1) | 2691 (22.1) |
| 4 | 1673 (18.0) | 2226 (18.3) |
| 5—Most deprived | 1768 (19.0) | 2433 (20.0) |
| Missing | 20 (0.2) | 20 (0.2) |
Abbreviations: BMI, body mass index; CHD, coronary heart disease, M, men; W, women; WC, waist circumference; WHR, waist-hip ratio.
Age limit was 18–85 years and no prior hospital admission for CHD or stroke. The missing anthropometric data included pregnant women (n=212), who were excluded from all analyses.
Crude incidence rates.
Figure 2.
Kaplan–Meier survival curves stratified by gender for the outcomes of all-cause mortality, CHD mortality and incident CHD in the Scottish Health Survey Cohort. Sample was age restricted to 18–85 years of age and excluded prior hospital admission for CHD or stroke. Men, dashed lines; Women, solid lines.
The anthropometric measures had the highest proportion of missing data, which increased from 1995 to 2003 and was greatest for the WC and WHR measures (Table 1, Figure 1 and Supplementary Table 1). The proportion of women in each survey was greater than men; in the combined sample 56.6% were women. The proportion classed as obese by BMI increased from 16.7% in 1995 to 22.1% in 2003. There was a similar increasing trend for the size of the highest WC and WHR categories. For the complete case samples used in the main analyses, on the basis of BMI (⩾30 kg m−2), 20.6% of men and 22.8% of women were obese (Tables 2 and 3). On the basis of WC (men⩾102 cm, women⩾88 cm) 22.0% of men and 29.9% of women had abdominal obesity. WHR (men⩾1.0, women⩾0.85) classified 9.4% of men and 25.2% of women as having abdominal obesity.
Table 2. Multilevel, multivariate HR and 95% confidence intervals for all-cause mortality, CHD mortality and incident CHD, by anthropometric measure, for men in the Scottish Health Survey Cohort.
| Number at risk n (%) |
All-cause mortality |
CHD mortality |
Incident CHD |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events | Unadjusted | Adjusted | Events | Unadjusted | Adjusted | Events | Unadjusted | Adjusted | ||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| BMI (kg m−2) | ||||||||||
| <18.5 (underweight) | 84 (1.0) | 19 | 3.13 (1.96–5.01) | 2.53 (1.58–4.06) | 3 | 3.09 (0.94–10.1) | 2.37 (0.71–7.88) | 7 | 2.54 (1.17–5.48) | 2.02 (0.93–4.38) |
| 18.5–<25 (desirable weight) | 2977 (34.9) | 246 | 1 | 1 | 37 | 1 | 1 | 108 | 1 | 1 |
| 25–<30 (overweight) | 3711 (43.5) | 241 | 0.56 (0.47–0.67) | 0.64 (0.54–0.77) | 47 | 0.72 (0.47–1.11) | 0.86 (0.55–1.33) | 222 | 1.25 (0.99–1.57) | 1.48 (1.17–1.88) |
| ⩾30 (obese) | 1760 (20.6) | 148 | 0.70 (0.57–0.86) | 0.82 (0.67–1.01) | 30 | 0.94 (0.58–1.52) | 1.13 (0.69–1.86) | 128 | 1.47 (1.13–1.90) | 1.78 (1.37–2.31) |
| WC (cm) | ||||||||||
| <79 (low) | 714 (9.4) | 57 | 2.25 (1.69–3.01) | 1.89 (1.41–2.53) | 9 | 2.40 (1.15–4.98) | 1.92 (0.92–4.01) | 18 | 1.00 (0.61–1.63) | 0.84 (0.51–1.37) |
| 79–<94 (reference) | 3476 (45.7) | 243 | 1 | 1 | 37 | 1 | 1 | 150 | 1 | 1 |
| 94–<102 (moderate) | 1742 (22.9) | 140 | 0.86 (0.7–1.06) | 0.90 (0.73–1.11) | 27 | 1.07 (0.65–1.76) | 1.17 (0.71–1.94) | 113 | 1.20 (0.94–1.53) | 1.31 (1.02–1.68) |
| ⩾102 (high) | 1677 (22.0) | 189 | 1.06 (0.88–1.29) | 1.10 (0.90–1.33) | 39 | 1.44 (0.91–2.26) | 1.54 (0.98–2.44) | 155 | 1.58 (1.26–1.98) | 1.70 (1.35–2.14) |
| WHR | ||||||||||
| <0.65 (low) | 1320 (17.4) | 53 | 1.11 (0.83–1.49) | 1.04 (0.77–1.39) | 7 | 0.94 (0.43–2.09) | 0.83 (0.37–1.85) | 25 | 0.69 (0.46–1.05) | 0.64 (0.42–0.97) |
| 0.8–<0.9 (reference) | 4191 (55.1) | 303 | 1 | 1 | 49 | 1 | 1 | 203 | 1 | 1 |
| 0.9–<1 (moderate) | 1374 (18.1) | 154 | 1.01 (0.83–1.23) | 0.97 (0.80–1.18) | 36 | 1.42 (0.92–2.19) | 1.44 (0.93–2.23) | 128 | 1.38 (1.10–1.72) | 1.38 (1.10–1.72) |
| ⩾1 (high) | 717 (9.4) | 119 | 1.42 (1.15–1.76) | 1.29 (1.04–1.60) | 20 | 1.43 (0.85–2.41) | 1.36 (0.80–2.31) | 80 | 1.58 (1.22–2.05) | 1.55 (1.19–2.01) |
Abbreviations: 95% CI, 95% confidence interval; BMI, body mass index; CHD, coronary heart disease; HR, hazard ratios; WC, waist circumference; WHR, waist–hip ratio.
Estimates presented as unadjusted and adjusted for age, smoking status, alcohol consumption and year of survey.
Table 3. Multilevel, multivariate HR and 95% confidence intervals for all-cause mortality, CHD mortality and incident CHD, by anthropometric measure, for women in the Scottish Health Survey Cohort.
| Number at risk n (%) | All-cause mortality | CHD mortality | Incident CHD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events | Unadjusted | Adjusted | Events | Unadjusted | Adjusted | Events | Unadjusted | Adjusted | ||
| |
|
|
HR (95% CI) |
HR (95% CI) |
|
HR (95% CI) |
HR (95% CI) |
|
HR (95% CI) |
HR (95% CI) |
| BMI (kg m−2) | ||||||||||
| <18.5 (underweight) | 200 (1.9) | 28 | 3.52 (2.36–5.25) | 2.69 (1.80–4.03) | 2 | 1.81 (0.43–7.61) | 1.26 (0.30–5.34) | 4 | 1.26 (0.46–3.47) | 0.92 (0.33–2.51) |
| 18.5–<25 (desirable weight) | 4625 (42.8) | 216 | 1 | 1 | 27 | 1 | 1 | 80 | 1 | 1 |
| 25–<30 (overweight) | 3509 (32.5) | 223 | 0.89 (0.74–1.08) | 0.98 (0.81–1.18) | 35 | 1.06 (0.64–1.75) | 1.22 (0.73–2.03) | 128 | 1.49 (1.12–1.98) | 1.61 (1.21–2.14) |
| ⩾30 (obese) | 2463 (22.8) | 157 | 0.84 (0.68–1.04) | 0.93 (0.75–1.15) | 31 | 1.28 (0.76–2.15) | 1.51 (0.90–2.56) | 112 | 1.80 (1.35–2.42) | 1.93 (1.44–2.59) |
| WC (cm) | ||||||||||
| <79 (low) | 977 (10.3) | 47 | 1.68 (1.21–2.34) | 1.57 (1.13–2.19) | 4 | 1.22 (0.41–3.57) | 1.02 (0.35–2.99) | 11 | 0.77 (0.41–1.45) | 0.71 (0.37–1.34) |
| 79–<94 (reference) | 3522 (37.0) | 156 | 1 | 1 | 20 | 1 | 1 | 71 | 1 | 1 |
| 94–<102 (moderate) | 2162 (22.7) | 134 | 1.01 (0.8–1.27) | 1.05 (0.83–1.33) | 17 | 0.94 (0.49–1.79) | 1.01 (0.53–1.93) | 71 | 1.28 (0.92–1.78) | 1.33 (0.95–1.85) |
| ⩾102 (high) | 2847 (29.9) | 243 | 1.16 (0.95–1.43) | 1.20 (0.97–1.47) | 44 | 1.49 (0.87–2.55) | 1.64 (0.95–2.81) | 142 | 1.71 (1.28–2.30) | 1.71 (1.28–2.29) |
| WHR | ||||||||||
| <0.65 (low) | 420 (4.4) | 14 | 1.48 (0.85–2.56) | 1.72 (0.99–2.98) | 1 | 1.15 (0.15–8.74) | 1.31 (0.17–9.95) | 4 | 0.72 (0.26–1.97) | 0.79 (0.29–2.17) |
| 0.8–<0.9 (reference) | 4334 (45.7) | 147 | 1 | 1 | 15 | 1 | 1 | 77 | 1 | 1 |
| 0.9–<1 (moderate) | 2345 (24.7) | 176 | 1.54 (1.23–1.92) | 1.41 (1.13–1.76) | 26 | 2.07 (1.09–3.92) | 1.91 (1.01–3.63) | 89 | 1.59 (1.17–2.17) | 1.50 (1.10–2.05) |
| ⩾1 (high) | 2392 (25.2) | 240 | 1.77 (1.43–2.18) | 1.56 (1.26–1.94) | 43 | 2.71 (1.49–4.94) | 2.49 (1.36–4.56) | 125 | 1.95 (1.45–2.62) | 1.76 (1.31–2.38) |
Abbreviations: 95% CI, 95% confidence interval; BMI, body mass index; CHD, coronary heart disease; HR, hazard ratios; WC, waist circumference; WHR, waist–hip ratio.
Estimates presented as unadjusted and adjusted for age, smoking status, alcohol consumption and year of survey.
For both genders there was no increase in risk of all-cause death associated with BMI-defined obesity (men adjusted HR=0.82; 95% confidence interval=0.67–1.01 and women 0.93; 0.75–1.15) (Tables 2 and 3). A similar finding was determined for CHD death except that the estimates were >1, although still non-significant. Similarly, overweight, based on BMI, was not associated with altered risk, except for all-cause mortality in men, where it was protective (0.64; 0.54–0.77). In contrast, the risk of an incident CHD event was increased for men and women for both overweight and obesity, as defined by BMI. The highest risk was among the obese: for men the adjusted HR=1.78 (1.37–2.31) and for women the adjusted HR=1.93 (1.44–2.59). For all-cause mortality there was an increased risk of mortality associated with the underweight for both genders.
Among men the adjusted HR estimates associated with WC-defined abdominal obesity increased from 1.10 (0.90–1.33) for all-cause mortality, to 1.54 (0.98–2.44) for CHD mortality and finally to 1.70 (1.35–2.14) for incident CHD (Table 2). This pattern was repeated for women with HRs: respectively, 1.20 (0.97–1.47), 1.64 (0.95–2.81) and 1.71 (1.28–2.29) (Table 3). For both genders there were no increased risks associated with moderate WC, whereas a low WC was associated with a significant increased risk for all-cause mortality only. Men with a high WHR had an increased risk of all-cause mortality and there was an increased risk of incident CHD for men who were classed as having a moderate WHR (1.38; 1.10–1.72) or high WHR (1.55; 1.19–2.01) (Table 2). In contrast, women with moderate or high WHR had an increased risk of suffering from all the three outcomes. For those with a WHR denoting abdominal obesity the adjusted HRs were 1.56 (1.26–1.94), 2.49 (1.36–4.56) and 1.76 (1.31–2.38) for all-cause mortality, CHD mortality and incident CHD, respectively (Table 3).
Adjustment for social class or deprivation had minimal impact on the estimates for the anthropometric measures but did identify inverse socioeconomic gradients for both genders and all outcomes (data available on request). Sensitivity analyses excluding early follow-up or restricting the sample to complete cases for anthropometric measures had minimal impact on estimates (data available on request).
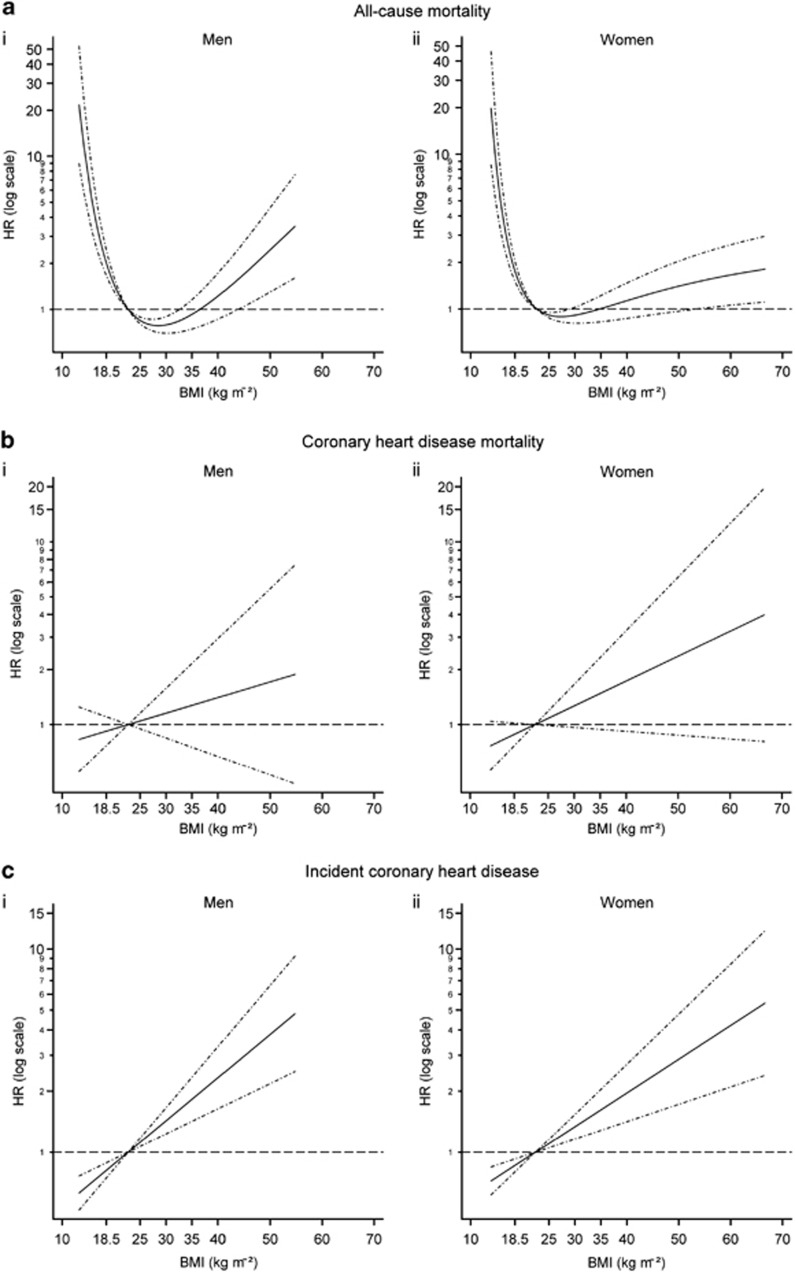
The fractional polynomial algorithm selected a U-shape (two-term fractional polynomials) to best describe the association between all-cause mortality and either continuous BMI or WC, for both genders (Figure 3 and Supplementary Figure 1). For all the other outcomes and for all models involving WHR, a linear relationship was identified (Figure 3 and Supplementary Figures 2–6). A linear relationship for age was deemed most appropriate in all models.
Figure 3.
Functional form of the association of BMI with the relative hazard of (a) all-cause mortality, (b) CHD mortality, (c) incident CHD for (i) men and (ii) women, estimated in a Cox proportional hazards model adjusted for age, smoking, alcohol consumption and survey year. For (a) functions were fitted using two-term fractional polynomial functions with powers: (i) men (−0.5, −0.5) and (ii) women (−2, −2). For (b) and (c) linear functions were selected. Functions standardised such that HR=1 at the mean of the BMI reference category (18.5–25 kg m−2): (i) men=22.72 kg m−2 and (ii) women=22.45 kg m−2. Dot–dash lines indicate 95% confidence interval.
Discussion
In the SHeS cohort, a nationally representative sample, the risk of incident CHD was higher for individuals with greater adiposity, irrespective of the anthropometric measure utilised. There appeared to be a positive gradient in the strength of association as the outcome changed from all-cause mortality to CHD mortality to incident CHD. This would imply that excess adiposity was associated with a significant increase in the risk of a CHD event occurring, but not of that event being fatal. Avoidance of fatality during, and following, an event could be related to not only its severity, a secular decline in which has been observed by some studies,23, 24 but also timely use of evidence-based medical interventions that have been developed over recent decades.
A similar positive relationship between BMI, WC or WHR and incident CHD has been identified by many studies.25, 26, 27, 28 It is likely that the contribution of obesity to CHD risk is mediated by the major CVD risk factors of hypertension, dyslipidaemia and diabetes. Overweight and obesity represent a clustering of these risk factors and are associated with an increased risk of developing these conditions.4, 29 A substantial component of the increased risk of CHD associated with excess adiposity is accounted for by such risk factors.25, 28 There may be an independent effect of obesity on the risk of CHD.25, 30
Although there was a tendency for an increased risk of CHD mortality associated with excess adiposity according to all three measures, in contrast to incident CHD, it was only significant according to WHR among women. Like incident CHD there was evidence that there was a linear relationship between each of the measures and CHD. Obesity has been associated with increased risk for fatal CHD events by other studies,4, 30 whereas for intermediate categories, such as BMI overweight, the increase has been shown to be more marginal.9, 31 The findings for all-cause mortality of a U-shaped relationship with BMI and WC are similar to that found in our previous study, albeit this time stratified by gender.12 Another study that incorporated the SHeS cohort also found a decrease in the mortality risk associated with overweight as defined by BMI.8 Others have found there to be a non-significant decrease, or increase, in the association with overweight.6, 7, 31 Some research contradicts this, having identified that overweight, and also obesity, is associated with an increased risk of all-cause mortality.4
Part of the lack of association between overweight according to BMI and mortality is undoubtedly a consequence of its inability to distinguish between fat mass and lean mass. It has limited accuracy in the intermediate range (overweight) where there is a greater heterogeneity in body fat proportion and this misclassifies many individuals, particularly men.32 This might explain the comparatively lower risk for men associated with the overweight categories for all outcomes seen in this study. The inability of BMI to differentiate a lack of lean mass from a lack of fat mass33, 34 may partly influence the association of underweight with the increased all-cause mortality seen here and elsewhere.6, 7 The increased association between underweight and all-cause mortality in part reflects its principal association with non-cancer non-CVD mortality, in particular respiratory disease.9, 4, 12
WC and WHR summarise overall fat proportion as well as fat distribution; in particular they are proxies for visceral fat accumulation. It is this that may be particularly deleterious in relation to CVD via its role, along with dyslipidaemia, systemic inflammation and insulin resistance, in metabolic syndrome.35 In this study the three measures were qualitatively similar in their association with incident CHD for both genders. WHR, although identifying increased risk for all-cause mortality for men in the highest category, was particularly effective in demonstrating increased risk for both moderate and high-categorised women for both mortality outcomes. Women in the high category for WHR had an almost 150% increased risk of CHD mortality relative to the normal category. A stronger association between abdominal obesity and incident CHD for women, as seen in this study, has previously been identified.25, 26 The evidence as to the best anthropometric measure in relation to mortality risk or CVD risk is conflicting; many studies have found equivalence,3, 27, 28 others have found either WC29 or WHR36 to be superior. It was not our intention to compare the different measures formally and care should be taken as the thresholds denoting excess adiposity used here are not necessarily comparable. It would seem prudent to consider more than one measure in epidemiological studies. A further investigation of this data set could involve use of WC or WHR in combination, or in mutual adjustment, with BMI, as this may better capture the health risks of increased adiposity.3
Despite adjusting our analyses for possible confounders there is the potential for residual confounding to remain. As we were interested in the impact of excess adiposity, we did not adjust for the known intermediary CVD risk factors such as cholesterol and blood pressure. Similarly, we did not adjust for physical activity, as this will, in combination with diet, determine adiposity, although it may have a separate cardio-protective effect. In previous analyses, using this data set, we did not identify any major change in the estimates for all-cause mortality following exclusion of smokers, individuals over 64 years, early follow-up or pre-existing disease.12 Neither did sensitivity analyses using age as the timescale, survey weights, multiple imputation for missing data (including non-responders to the nurse) or limiting analyses to those registered with general practitioners within Scotland. In this study exclusion of early follow-up to account for the potential effect of occult disease had minimal impact, as seen in other studies.4, 8
This study has many strengths, not least because it was conducted on a contemporary, nationally representative sample using anthropometric variables obtained by standardised measurement. However, there are limitations, not least the likelihood of measurement or responder bias. There is the potential for the misclassification of the cause of death or reason for hospital admission; an assessment comparing the clinical coding in Scottish Morbidity Records data to full medical records has determined it to be 94% accurate for CHD, with 99% completeness, for the period 2004–2006.37 As these were cross-sectional surveys no information was available on past or future changes in variables. For excess adiposity the length of time it has been endured, age at which it occurred and any subsequent weight loss may all be important in its relationship with mortality.38, 39, 40, 41 There will be bias as a result of non-response to the original interview and subsequent attrition because of non-consent to the nurse visit and/or data linkage. The declining response proportion across the surveys raises questions about their representativeness, especially the 2003 SHeS. Assessment of non-response was not possible using these SHeS data; in 2003 response was modestly skewed towards those in less-deprived areas.42 Univariate analyses identified that non-responders to the SHeS nurse interview, when compared with responders, were more likely to be younger, smokers and of lower socioeconomic status.43 Evidence is sparse as to how non-participants differ from participants in health surveys conducted in Britain; some less contemporary studies indicate that they may be younger, of lower education or socioeconomic status, lead unhealthier lifestyles and have higher mortality.44, 45 This is similar to more contemporary studies from other countries.46, 47, 48
There is some evidence that overweight and obese individuals with a BMI of <35 kg m−2 may have a better chance of surviving a CHD event than those of a normal weight.49 This may be influenced by the finding that first CHD events may occur at a younger age in obese patients than those of normal weight.50, 51 Clinicians have been demonstrated to pursue more aggressive treatments, despite no difference in disease severity, in patients with higher BMI50, 52 and in younger patients.53 Obese patients with CHD have higher risk factor levels such as hypertension and hypercholesterolaemia that can be targeted by secondary prevention. The inability of BMI to differentiate body composition could also contribute to this obesity paraadox.54
Although medical treatment accounts for at least half of the recent decline in CHD mortality, the remainder can be attributed to population CVD-risk factor reductions.10, 11 Obese people in contemporary populations have a much lower prevalence of major CVD risk factors (except diabetes) than 40 years ago, although they still have the highest levels.55, 56 A reduction in the risk factors that mediate the association of obesity with CHD could attenuate the mortality–adiposity relationship and the severity of CHD events.
This study suggests that excess adiposity is associated with an increased risk of incident CHD but not necessarily death. Modern medical interventions may have contributed to improved survival of first CHD events. Suffering a non-fatal CHD event adversely affects an individual's quality of life57 and increases their risk of further CVD incidents,58 with life expectancy halved after a diagnosis of acute myocardial infarction.59 The future health burden of increased population obesity levels may manifest as an increase in the prevalence of individuals living with CHD and its consequences. Public health policies to reduce obesity, such as Scotland's route map towards healthy weight,60 and other related CVD-risk factors, such as hypertension and dyslipidaemia,61 are essential if the Scottish population is to be spared heavy financial and health burdens as a consequence of CHD, now and in the future.
Acknowledgments
The Social and Public Health Sciences Unit is jointly funded by the Medical Research Council and the Chief Scientist Office of the Scottish Government Health Directorate. This research was funded by the Chief Scientist Office as part of the ‘Measuring health, variations in health and determinants of health' programme, MC_US_A540_0001. Scottish Health Surveys Cohort are freely available data and were created and are maintained by the Information Services Division of NHS Scotland.16
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on International Journal of Obesity website (http://www.nature.com/ijo)
Supplementary Material
References
- Bromley C, Bradshaw P, Given L.(eds). The Scottish Health Survey 2008 The Scottish Government: Edinburgh; 2009 , http://www.scotland.gov.uk/Publications/2009/09/28102003/0 (accessed 20 March 2012). [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischon T, Boeing H, Hoffman K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- Prospective Studies Collaboration. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmarzyk PT, Craig CL, Bouchard C. Underweight, overweight and obesity: relationships with mortality in the 13-year follow-up of the Canada fitness survey. J Clin Epidemiol. 2001;54:916–920. doi: 10.1016/s0895-4356(01)00356-0. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Graubard BJ, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- Orpana HM, Berthelot J-M, Kaplan MS, Feeny DH, McFarland B, Ross NA. BMI and mortality: results from a national longitudinal study of Canadian adults. Obesity. 2010;18:214–218. doi: 10.1038/oby.2009.191. [DOI] [PubMed] [Google Scholar]
- Czernichow S, Kengne AP, Stamatakis E, Hamer M, Batty GD. Body mass index, waist circumference and waist hip ratio: which is the better discriminator of cardiovascular disease mortality risk? Evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev. 2011;12:680–687. doi: 10.1111/j.1467-789X.2011.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Graubard BJ, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- Capewell S, Morrison CE, McMurray JJ. Contribution of modern cardiovascular treatment and risk factor changes to the decline in coronary heart disease mortality in Scotland between 1975 and 1994. Heart. 1999;81:380–386. doi: 10.1136/hrt.81.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal B, Critchley JA, Capewell S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation. 2004;109:1101–1107. doi: 10.1161/01.CIR.0000118498.35499.B2. [DOI] [PubMed] [Google Scholar]
- Hotchkiss JW, Leyland AH. The relationship between body size and mortality in the linked Scottish Health Surveys: cross-sectional surveys with follow-up. Int J Obes. 2011;35:838–851. doi: 10.1038/ijo.2010.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Erens B.(eds). The Scottish Health Survey 1995 The Stationery Office: Edinburgh; 1997 , http://www.archive.official-documents.co.uk/document/scottish/shealth/shhm.htm (accessed 20 Mar 2012). [Google Scholar]
- Shaw A, McMunn A, Field J.(eds). The Scottish Health Survey 1998 The Stationery Office: Edinburgh; 2000 , http://www.sehd.scot.nhs.uk/scottishhealthsurvey/sh8-00.html (accessed 20 March 2012). [Google Scholar]
- Bromley C, Sproston K, Shelton N.(eds). The Scottish Health Survey 2003 The Scottish Executive: Edinburgh; 2005 , http://www.scotland.gov.uk/Publications/2005/12/02160336/03367 (accessed 20 Mar 2012). [Google Scholar]
- Gray L, Batty GD, Craig P, Stewart C, Whyte B, Finlayson A, et al. Cohort profile: The Scottish Health Surveys Cohort: linkage of study participants to routinely collected records for mortality, hospital discharge, cancer and offspring birth characteristics in three nationwide studies. Int J Epidemiol. 2010;39:345–350. doi: 10.1093/ije/dyp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation Obesity: Preventing and Managing the Global Epidemic WHO Technical Report Series 894; 2000 , http://whqlibdoc.who.int/trs/WHO_TRS_894.pdf (accessed 20 March 2012). [PubMed] [Google Scholar]
- Lean MEJ, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. Br Med J. 1995;311:158–161. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JA, MacInnis RJ, Peeters A, Hopper JL, Giles GG, English DR. A comparison of adiposity measures as predictors of all-cause mortality: the Melbourne Mollaborative Cohort Study. Obesity. 2007;15:994–1003. doi: 10.1038/oby.2007.622. [DOI] [PubMed] [Google Scholar]
- McLoone P.Carstairs Scores for Scottish Postcode Sectors from the 2001 Census Medical Research Council Social & Public Health Sciences Unit: Glasgow; 2004 , http://www.sphsu.mrc.ac.uk/files/File/library/other%20reports/Carstairs_report.pdf (accessed 19 March 2012). [Google Scholar]
- Rasbash J, Browne WJ, Healy M, Cameron B. MLwiN Version 2.1. Centre for Multilevel Modelling, University of Bristol: Bristol; 2009. [Google Scholar]
- Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- Myerson M, Coady S, Taylor H, Rosamond WD, Goff DC. Declining Severity of Myocardial Infarction From 1987 to 2002: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2009;119:503–514. doi: 10.1161/CIRCULATIONAHA.107.693879. [DOI] [PubMed] [Google Scholar]
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- Rexrode KM, Buring JE, Manson JE. Abdominal and total adiposity and risk of coronary heart disease in men. Int J Obes. 2001;25:1047–1056. doi: 10.1038/sj.ijo.0801615. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Ebrahim S, Ben-Shlomo Y, Martin RM, Whincup PH, Yarnell JW, et al. Comparison of the associations of body mass index and measures of central adiposity and fat mass with coronary heart disease, diabetes, and all-cause mortality: a study using data from 4 UK cohorts. Am J Clin Nutr. 2010;91:547–556. doi: 10.3945/ajcn.2009.28757. [DOI] [PubMed] [Google Scholar]
- The emerging risk factors collaboration. Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DM, Lee J, Youlian Liao Y, Natarajan S. Independent effect and population impact of obesity on fatal coronary heart disease in adults. Prev Med. 2006;42:6–72. doi: 10.1016/j.ypmed.2005.09.011. [DOI] [PubMed] [Google Scholar]
- McGee DL. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. 2005;15:87–97. doi: 10.1016/j.annepidem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazon-Clavell ML, Korinek J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32:959–996. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DB, Zhu S, Plankey M, Faith MS, Heo M. Differential associations of body mass index and adiposity with all-cause mortality among men in the first and second National Health and Nutrition Examination Surveys (NHANES I and NHANES II) follow-up studies. Int J Obes. 2002;26:410–416. doi: 10.1038/sj.ijo.0801925. [DOI] [PubMed] [Google Scholar]
- Bigaard J, Frederiksen K, Tjonneland A, Thomsen BL, Overvad K, Heitmann BL, et al. Body fat and fat-free mass and all-cause mortality. Obes Res. 2004;12:1042–1049. doi: 10.1038/oby.2004.131. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- NHS National Services Scotland NHS hospital data quality. Towards better data from Scottish hospitals: An assessment of SMR01 and associated data 2004 - 2006 ISD Scotland publications: Edinburgh; 2007 , http://www.isdscotland.org/Health-Topics/Stroke/Background-and-Policy/SMR01%20Scotland%20Report%202007.pdf (accessed 19 March 2012). [Google Scholar]
- Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- Yan LL, Daviglus ML, Liu K, Stamler J, Wang R, Pirzada A, et al. Midlife body mass index and hospitalization and mortality in older age. JAMA. 2006;295:190–198. doi: 10.1001/jama.295.2.190. [DOI] [PubMed] [Google Scholar]
- Strandberg TE, Strandberg AY, Salomaa VV, Pitkala KH, Tilvis RS, Sirola J, et al. Explaining the obesity paradox: cardiovascular risk, weight change, and mortality during long-term follow-up in men. Eur Heart J. 2009;30:1720–1727. doi: 10.1093/eurheartj/ehp162. [DOI] [PubMed] [Google Scholar]
- Adbullah A, Wolfe R, Stoelwonder JU, de Courten M, Stevenson C, Walls HL, et al. The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int J Epidemiol. 2011;40:985–996. doi: 10.1093/ije/dyr018. [DOI] [PubMed] [Google Scholar]
- Lawder R, Harding O, Stockton D, Fischbacher C, Brewster DH, Chalmers J, et al. Is the Scottish population living dangerously? Prevalence of multiple risk factors: the Scottish Health Survey 2003. BMC Public Health. 2010;10:330. doi: 10.1186/1471-2458-10-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss JW, Davies CA, Gray L, Bromley C, Capewell S, Leyland AH. Trends in cardiovascular disease biomarkers and their socioeconomic patterning among adults in the Scottish population 1995 to 2009: cross-sectional surveys. BMJ Open 2012. 2:pii: e000771. doi: 10.1136/bmjopen-2011-000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M, Shaper AG, Cook DG. Non-participation and mortality in a prospective study of cardiovascular disease. J Epidemiol Community Health. 1987;41:295–299. doi: 10.1136/jech.41.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen E, Nutbeam D, Moore L. Demographic characteristics and health behaviours of consenters to medical examination. Results from the Welsh Heart Health Survey. J Epidemiol Community Health. 1992;46:455–459. doi: 10.1136/jech.46.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard AJ, Selmer R, Bjertness E, Thelle D. The Oslo Health Study: The impact of self-selection in a large population-based survey. Int J Equity Health. 2004;3:3. doi: 10.1186/1475-9276-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousilahti P, Salomaa V, Kuulasmaa K, Niemela M, Vartiainen E. Total and cause specific mortality among participants and non-participants of population based health surveys: a comprehensive follow up of 54 372 Finnish men and women. J Epidemiol Community Health. 2005;59:310–315. doi: 10.1136/jech.2004.024349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandhagen E, Berg C, Lissner L, Nunez L, Rosengren A, Toren K, et al. Selection bias in a population survey with registry linkage: potential effect on socioeconomic gradient in cardiovascular risk. Eur J Epidemiol. 2010;25:163–172. doi: 10.1007/s10654-010-9427-7. [DOI] [PubMed] [Google Scholar]
- Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- Eisenstein EL, McGuire DK, Bhapkar MV, Kristinsson A, Hochman JS, Kong DF, et al. Elevated body mass index and intermediate-term clinical outcomes after acute coronary syndrome. Am J Med. 2005;118:981–990. doi: 10.1016/j.amjmed.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Wells B, Gentry M, Ruiz-Arango A, Dias J, Landolfo CK. Relation between body mass index and clinical outcome in acute myocardial infarction. Am J Cardiol. 2006;98:474–477. doi: 10.1016/j.amjcard.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Steinberg BJ, Cannon CP, Hernandez AF, Pan W, Peterson ED, Fonarow GC. Medical therapies and invasive treatments for coronary artery disease by body mass: The ‘obesity paradox' in the get with the guidelines database. Am J Cardiol. 2007;100:1331–1335. doi: 10.1016/j.amjcard.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Barakat K, Wilkinson P, Deaner A, Fluck D, Ranjadayalan K, Timmis A. How should age affect management of acute myocardial infarction? A prospective cohort study. Lancet. 1999;353:955–959. doi: 10.1016/S0140-6736(98)07114-1. [DOI] [PubMed] [Google Scholar]
- Romero-Corral A, Somers VK, Sierra-Johnson J, Jensen MK, Thomas G, Squires RW, et al. Diagnostic performance of body mass index to detect obesity in patients with coronary artery disease. Eur Heart J. 2007;28:2087–2093. doi: 10.1093/eurheartj/ehm243. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- Rosengren A, Eriksson M, Hansson PO, Svardsudd K, Willhelmsen L, Johansson S, et al. Obesity and trends in cardiovascular risk factors over 40 years in Swedish men aged 50. J Intern Med. 2009;266:268–276. doi: 10.1111/j.1365-2796.2009.02116.x. [DOI] [PubMed] [Google Scholar]
- Schweikert B, Hunger M, Meisinger C, Konig H-H, Gapp O, Holle R. Quality of life several years after myocardial infarction: comparing the MONICA/KORA registry to the general population. Eur Heart J. 2009;30:436–443. doi: 10.1093/eurheartj/ehn509. [DOI] [PubMed] [Google Scholar]
- Tunstall-Pedoe H, Woodward M, Tavendale R. Comparison of the prediction by 27 different factors of coronary heart disease and death in men and women of the Scottish heart health study: cohort study. Br Med J. 1997;315:722–729. doi: 10.1136/bmj.315.7110.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capewell S, Livingston BM, Macintyre K, Chalmers J, Boyd J, Finlayson A, et al. Trends in case-fatality in 117 718 patients admitted with acute myocardial infarction in Scotland. Eur Heart J. 2000;21:1833–1840. doi: 10.1053/euhj.2000.2318. [DOI] [PubMed] [Google Scholar]
- The Scottish Government Preventing overweight and obesity in Scotland A route map towards healthy weight Scottish Government: Edinburgh; 2010 , http://www.scotland.gov.uk/Resource/Doc/302783/0094795.pdf (accessed 20 March 2012). [Google Scholar]
- National Institute for Health and Clinical Excellence NICE public health guidance 25. Prevention of cardiovascular disease at population level NICE: London; 2010 , http://www.nice.org.uk/nicemedia/live/13024/49273/49273.pdf (accessed 20 March 2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.