Abstract
Between 20% and 50% of cardiovascular patients treated with clopidogrel, an anti‐P2Y12 drug, display high on‐treatment platelet reactivity (HTPR) and are not adequately protected from major adverse cardiovascular events (MACE). Despite a minor influence of the CYP2C19*2 genetic variant on the pharmacodynamic response to clopidogrel (5% to 12%) and a limited or absent value for predicting stent thrombosis and MACE, this latter polymorphism is currently considered an important candidate to tailor anti‐P2Y12 therapy during percutaneous coronary intervention. Seven studies have examined the value of CYP2C19*2 for predicting HTPR in comparison to a specific pharmacodynamic assay (VASP assay). Overall, the summarized sensitivity of the CYP2C19*2 genotype for predicting HTPR was 37.6% (95% CI: 32.2 to 43.3%), yielding a negative likelihood ratio of only 0.77 (95% CI: 0.68 to 0.86) which confirms its limited value as a routine clinical aid. A tailored anti‐P2Y12 treatment strategy restricted to CYP2C19*2 carriers may be of some help, but this restrictive approach leaves out noncarriers with HTPR. As for platelet function testing, there is currently no convincing data to support that using CYP2C19*2 genotyping as a tailored anti‐P2Y12 treatment would be an effective strategy and there is no urgency for CYP2C19 genotyping in clinical practice. Strategies incorporating genotyping, phenotyping, and clinical data in a stratified and sequential approach may be more promising.
Keywords: pharmacogenetics, platelet, thienopyridine
Introduction
Clopidogrel exerts its antithrombotic effect through irreversible inhibition of the platelet receptor for adenosine diphosphate (ADP) P2Y12. Between about 20% and 50% of patients treated with clopidogrel display high on‐treatment platelet reactivity (HTPR)1 and are not adequately protected from MACE. In the era of personalized medicine, effective strategies are needed to identify these patients and thus to tailor their antiplatelet treatment.
As HTPR on clopidogrel seems to be strongly heritable (h2=0.73),2 genotyping could theoretically help to identify patients at risk. Clopidogrel is a prodrug that needs to be metabolized to its active metabolite by cytochrome P450 (CYP) isoforms in the liver. Various loss‐ and gain‐of‐function genotypes of CYP isoforms are known to affect the response to clopidogrel. In particular, CYP2C19 loss‐of‐function variant *2 (rs4244285) has been linked both to a poor pharmacodynamic response to clopidogrel and to an increased risk of recurrent cardiovascular events, best evidenced in patients treated with percutaneous coronary interventions (PCI) and for the outcome of stent thrombosis.3–4 However, recent metanalyses have challenged this link between CYP2C19*2 and MACE.5–7 The reported association between loss‐of‐function alleles and poor cardiovascular outcomes was found to suffer from bias due to small‐study effects,6–7 with no risk increase being found in a pooled analysis of studies involving more than 500 patients.8 These inconsistencies in the observed relation between CYP2C19*2 and MACE are likely explained by the fact that CYP2C19*2 has only a minor influence (5% to 12%) on the pharmacodynamic response to clopidogrel.2,9–11
The capacity of CYP2C19*1/*2 genotyping to predict HTPR has been examined in several studies using various platelet function tests, including VASP assay, which is highly specific for P2Y12 receptor inhibition.12 In a PubMed search conducted on October 25, 2012 using the terms “clopidogrel,” “vasodilator‐stimulated phosphoprotein,” and “cytochrome,” we identified 22 studies, 7 of which provided substantive data on the association between CYP2C19 genotypes and HTPR.10,13–18 As shown in the Figure1, the summarized sensitivity19 of the CYP2C19*2 genotype for predicting HTPR was 37.6% (95% CI: 32.2 to 43.3%), yielding a summarized negative predictive value of only 52.3% (95% CI: 44.7% to 59.7%) and a negative likelihood ratio of only 0.77 (95% CI: 0.68 to 0.86). Thus, CYP2C19 genotyping would contribute little to excluding the risk of HTPR or MACE. Routine CYP2C19*1/*2 genotyping of all clopidogrel‐treated patients would fail to solve the problem of high on‐treatment platelet reactivity. HTPR in clopidogrel‐treated patients is indeed dependent on various other factors such as high body weight or high body mass index, clopidogrel absorption, drug‐drug interaction, underlying diseases such as diabetes, renal failure, old age, and the presence of an acute coronary syndrome.20–22 However, after exclusion of all identifiable genetic and non‐genetic factors, a large proportion of the variation in clopidogrel pharmacokinetics and pharmacodynamics remains unexplained at present.23
Figure 1.
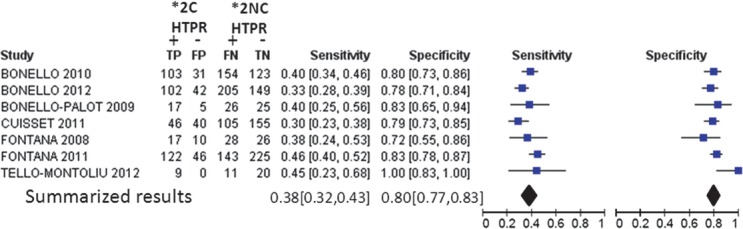
Sensitivity and specificity of the 2C19*1/*2 polymorphism for detecting high on‐treatment platelet reactivity (HTPR), as based on the vasodilator‐stimulated phosphoprotein (VASP) assay performed in clopidogrel‐treated patients. Patients are classified as either 2C19*2 carriers (*2C), corresponding to carriers of 1 or 2 *2 alleles, or 2C19*2 noncarriers (*2NC), corresponding to *1 homozygotes. The global sensitivity and specificity are depicted as a black diamonds. TP indicates true positives; FP, false positives; FN, false negatives; TN, true negatives.
In recent months, physicians have been targeted by aggressive marketing from the manufacturer of the Spartan RX CYP2C19 device (Spartan Biosciences), designed for rapid identification of CYP2C19*2 carriers. This device was recently tested in the Reassessment of Antiplatelet Therapy Using an Individualized Strategy Based on Genetic Evaluation (RAPID GENE) study,24 which addressed the issue of tailored treatment in CYP2C19*2 carriers only, using the newer thienopyrine drug prasugrel, whose bioactivation is not significantly affected by CYP genotypes.25 The working hypothesis of the study was confirmed as none of the 23 CYP2C19*2 carriers allocated to prasugrel had HTPR after 7 days of treatment, compared to 7 of the 23 CYP2C19*2 carriers allocated to standard clopidogrel treatment (P=0.009). However, this strategy failed to identify 18 patients with HTPR (9.6% [95% CI, 5.8 to 14.8] of the total population of 187 patients) who were not CYP2C19*2 carriers. Furthermore, this false‐negative rate of 9.6% is probably underestimated. Indeed, such patients were even more numerous in the Escalating Clopidogrel by Involving a Genetic Strategy‐ Thrombolysis In Myocardial Infarction 56 (ELEVATE‐TIMI56) study26 (23% [95% CI, 17 to 29] of 335 enrolled PCI patients) and in the Antiplatelet Drug Resistances and Ischemic Events (ADRIE) study10 (39% [95% CI, 34 to 44] of 538 enrolled stable cardiovascular outpatients). Thus, a strategy tailoring anti‐P2Y12 therapy to CYP2C19*2 carrier status would ignore the 10% to 39% of clopidogrel‐treated patients who have HTPR not associated with CYP2C19*2, leaving them exposed to a 4‐ to 8‐fold higher risk of recurrent ischemic events, including death from stent thrombosis.27–28 Conversely, using a global phenotype‐based strategy with a low VerifyNow P2Y12 cut‐off (208 P2Y12 reaction units [PRU]), only 10/2930 patients in the Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel (TRIGGER‐PCI) study still had HTPR (0.3%, 95% CI [0.2 to 0.6]).29 Altogether, 2C19*2 genotyping is technically reliable, can now be rapidly performed, and provides an unambiguous and permanent categorization for an individual patient, but it lacks sufficient predictive capability to be used on its own. The alternate platelet reactivity approach also has its own set of limitations, including the absence of a standardized technique and universal cut‐offs, and the variability of the phenotype over time. When compared with CYP genotyping head‐to‐head, platelet function testing emerges as a better, albeit imperfect predictor of MACE.30–31 In a large nonrandomized prospective study, patients with HTPR remained at an increased risk of MACE despite a higher maintenance dose of clopidogrel or ticlopidine.32 Some prospective studies suggested that tailored anti‐P2Y12 treatment is associated with a lower risk of stent thrombosis,33–35 but this was not confirmed in larger randomized clinical trials.36–37,29 Ongoing studies of strategies incorporating genotyping, phenotyping, and clinical data in a stratified and sequential approach may give more favorable results. Alternatively, the use of new P2Y12 inhibitors such as prasugrel and the nonthienopyridine drug ticagrelor in all patients might largely overcome the problem of HTPR without the need for testing. Pending the results of additional controlled studies, we consider that “personalized” antiplatelet treatment based on CYP2C19 genotyping has no valid place in clinical practice yet, and that there is currently no urgency for CYP2C19 genotyping.
Disclosures
Dr Fontana has received a research grant from Evolva and honoraria from Bayer and CSL Behring. Dr Cattaneo has received research grants and advisory board honoraria from AstraZeneca, Eli Lilly, Daiichi Sankyo, Evolva. Drs Combescure and Reny have no conflicts of interest to declare.
References
- 1.Mallouk N, Labruyere C, Reny JL, Chapelle C, Piot M, Fontana P, Gris JC, Delavenne X, Mismetti P, Laporte S. Prevalence of poor biological response to clopidogrel: a systematic review. Thromb Haemost. 2012; 107:494-506 [DOI] [PubMed] [Google Scholar]
- 2.Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009; 302:849-857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, Horne BD, Hulot JS, Kastrati A, Montalescot G, Neumann FJ, Shen L, Sibbing D, Steg PG, Trenk D, Wiviott SD, Sabatine MS. Reduced‐function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta‐analysis. JAMA. 2010; 304:1821-1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hulot JS, Collet JP, Silvain J, Pena A, Bellemain‐Appaix A, Barthelemy O, Cayla G, Beygui F, Montalescot G. Cardiovascular risk in clopidogrel‐treated patients according to cytochrome P450 2C19*2 loss‐of‐function allele or proton pump inhibitor coadministration: a systematic meta‐analysis. J Am Coll Cardiol. 2010; 56:134-143 [DOI] [PubMed] [Google Scholar]
- 5.Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta‐analysis. JAMA. 2011; 306:2704-2714 [DOI] [PubMed] [Google Scholar]
- 6.Bauer T, Bouman HJ, van Werkum JW, Ford NF, ten Berg JM, Taubert D. Impact of CYP2C19 variant genotypes on clinical efficacy of antiplatelet treatment with clopidogrel: systematic review and meta‐analysis. BMJ. 2011; 343:d4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zabalza M, Subirana I, Sala J, Lluis‐Ganella C, Lucas G, Tomas M, Masia R, Marrugat J, Brugada R, Elosua R. Meta‐analyses of the association between cytochrome CYP2C19 loss‐ and gain‐of‐function polymorphisms and cardiovascular outcomes in patients with coronary artery disease treated with clopidogrel. Heart. 2012; 98:100-108 [DOI] [PubMed] [Google Scholar]
- 8.Cattaneo M. Response variability to clopidogrel: is tailored treatment, based on laboratory testing, the right solution? J Thromb Haemost. 2012; 10:327-336 [DOI] [PubMed] [Google Scholar]
- 9.Hochholzer W, Trenk D, Fromm MF, Valina CM, Stratz C, Bestehorn HP, Buttner HJ, Neumann FJ. Impact of cytochrome P450 2C19 loss‐of‐function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J Am Coll Cardiol. 2010; 55:2427-2434 [DOI] [PubMed] [Google Scholar]
- 10.Fontana P, James R, Barazer I, Berdague P, Schved JF, Rebsamen M, Vuilleumier N, Reny JL. Relationship between paraoxonase‐1 activity, its Q192R genetic variant and clopidogrel responsiveness in the ADRIE study. J Thromb Haemost. 2011; 9:1664-1666 [DOI] [PubMed] [Google Scholar]
- 11.Bouman HJ, Harmsze AM, van Werkum JW, Breet NJ, Bergmeijer TO, Ten Cate H, Hackeng CM, Deneer VH, Ten Berg JM. Variability in on‐treatment platelet reactivity explained by CYP2C19*2 genotype is modest in clopidogrel pretreated patients undergoing coronary stenting. Heart. 2011; 97:1239-1244 [DOI] [PubMed] [Google Scholar]
- 12.Cattaneo M. Resistance to antiplatelet drugs: molecular mechanisms and laboratory detection. J Thromb Haemost. 2007; 5suppl 1:230-237 [DOI] [PubMed] [Google Scholar]
- 13.Bonello L, Armero S, Ait Mokhtar O, Mancini J, Aldebert P, Saut N, Bonello N, Barragan P, Arques S, Giacomoni MP, Bonello‐Burignat C, Bartholomei MN, Dignat‐George F, Camoin‐Jau L, Paganelli F. Clopidogrel loading dose adjustment according to platelet reactivity monitoring in patients carrying the 2C19*2 loss of function polymorphism. J Am Coll Cardiol. 2010; 56:1630-1636 [DOI] [PubMed] [Google Scholar]
- 14.Bonello L, Camoin‐Jau L, Mancini J, Bessereau J, Grosdidier C, Alessi MC, Ostorero M, Dignat‐George F, Paganelli F. Factors associated with the failure of clopidogrel dose‐adjustment according to platelet reactivity monitoring to optimize P2Y12‐ADP receptor blockade. Thromb Res. 2012; 130:70-74 [DOI] [PubMed] [Google Scholar]
- 15.Bonello‐Palot N, Armero S, Paganelli F, Mancini J, De Labriolle A, Bonello C, Levy N, Maillard L, Barragan P, Dignat‐George F, Camoin‐Jau L, Bonello L. Relation of body mass index to high on‐treatment platelet reactivity and of failed clopidogrel dose adjustment according to platelet reactivity monitoring in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2009; 104:1511-1515 [DOI] [PubMed] [Google Scholar]
- 16.Cuisset T, Quilici J, Cohen W, Fourcade L, Saut N, Pankert M, Gaborit B, Carrieri P, Morange PE, Bonnet JL, Alessi MC. Usefulness of high clopidogrel maintenance dose according to CYP2C19 genotypes in clopidogrel low responders undergoing coronary stenting for non ST elevation acute coronary syndrome. Am J Cardiol. 2011; 108:760-765 [DOI] [PubMed] [Google Scholar]
- 17.Fontana P, Senouf D, Mach F. Biological effect of increased maintenance dose of clopidogrel in cardiovascular outpatients and influence of the cytochrome P450 2C19*2 allele on clopidogrel responsiveness. Thromb Res. 2008; 121:463-468 [DOI] [PubMed] [Google Scholar]
- 18.Tello‐Montoliu A, Jover E, Marin F, Bernal A, Lozano ML, Sanchez‐Vega B, Pastor FJ, Hurtado JA, Valdes M, Vicente V, Rivera J. Influence of CYP2C19 polymorphisms in platelet reactivity and prognosis in an unselected population of non ST elevation acute coronary syndrome. Rev Esp Cardiol. 2012; 65:219-226 [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986; 7:177-188 [DOI] [PubMed] [Google Scholar]
- 20.Gilard M, Arnaud B, Cornily JC, Le Gal G, Lacut K, Le Calvez G, Mansourati J, Mottier D, Abgrall JF, Boschat J. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double‐blind OCLA (Omeprazole Clopidogrel Aspirin) study. J Am Coll Cardiol. 2008; 51:256-260 [DOI] [PubMed] [Google Scholar]
- 21.Geisler T, Grass D, Bigalke B, Stellos K, Drosch T, Dietz K, Herdeg C, Gawaz M. The residual platelet aggregation after deployment of intracoronary stent (PREDICT) score. J Thromb Haemost. 2008; 6:54-61 [DOI] [PubMed] [Google Scholar]
- 22.Fontana P, Berdague P, Castelli C, Nolli S, Barazer I, Fabbro‐Peray P, Schved JF, Bounameaux H, Mach F, de Moerloose P, Reny JL. Clinical predictors of dual aspirin and clopidogrel poor responsiveness in stable cardiovascular patients from the ADRIE study. J Thromb Haemost. 2010; 8:2614-2623 [DOI] [PubMed] [Google Scholar]
- 23.Frelinger AL, III, Bhatt DL, Lee RD, Mulford DJ, Wu J, Nudurupati S, Nigam A, Lampa M, Brooks JK, Barnard MR, Michelson AD. Clopidogrel pharmacokinetics and pharmacodynamics vary widely despite exclusion or control of polymorphisms (CYP2C19, ABCB1, PON1), noncompliance, diet, smoking, co‐medications (including proton pump inhibitors), and pre‐existent variability in platelet function. J Am Coll Cardiol. 2013; 61:872-879 [DOI] [PubMed] [Google Scholar]
- 24.Roberts JD, Wells GA, Le May MR, Labinaz M, Glover C, Froeschl M, Dick A, Marquis JF, O'Brien E, Goncalves S, Druce I, Stewart A, Gollob MH, So DY. Point‐of‐care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof‐of‐concept trial. Lancet. 2012; 379:1705-1711 [DOI] [PubMed] [Google Scholar]
- 25.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias WL, Braunwald E, Sabatine MS. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009; 119:2553-2560 [DOI] [PubMed] [Google Scholar]
- 26.Mega JL, Hochholzer W, Frelinger AL, III, Kluk MJ, Angiolillo DJ, Kereiakes DJ, Isserman S, Rogers WJ, Ruff CT, Contant C, Pencina MJ, Scirica BM, Longtine JA, Michelson AD, Sabatine MS. Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA. 2011; 306:2221-2228 [DOI] [PubMed] [Google Scholar]
- 27.Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Jukema JW, Huisman MV. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta‐analysis. Am Heart J. 2007; 154:221-231 [DOI] [PubMed] [Google Scholar]
- 28.Combescure C, Fontana P, Mallouk N, Berdague P, Labruyere C, Barazer I, Gris JC, Laporte S, Fabbro‐Peray P, Reny JL. Clinical implications of clopidogrel non‐response in cardiovascular patients: a systematic review and meta‐analysis. J Thromb Haemost. 2010; 8:923-933 [DOI] [PubMed] [Google Scholar]
- 29.Trenk D, Stone GW, Gawaz M, Kastrati A, Angiolillo DJ, Muller U, Richardt G, Jakubowski JA, Neumann FJ. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug‐eluting stents: results of the TRIGGER‐PCI (Testing Platelet Reactivity in Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J Am Coll Cardiol. 2012; 59:2159-2164 [DOI] [PubMed] [Google Scholar]
- 30.Campo G, Parrinello G, Ferraresi P, Lunghi B, Tebaldi M, Miccoli M, Marchesini J, Bernardi F, Ferrari R, Valgimigli M. Prospective evaluation of on‐clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention relationship with gene polymorphisms and clinical outcome. J Am Coll Cardiol. 2011; 57:2474-2483 [DOI] [PubMed] [Google Scholar]
- 31.Trenk D, Hochholzer W, Fromm MF, Chialda LE, Pahl A, Valina CM, Stratz C, Schmiebusch P, Bestehorn HP, Buttner HJ, Neumann FJ. Cytochrome P450 2C19 681G>A polymorphism and high on‐clopidogrel platelet reactivity associated with adverse 1‐year clinical outcome of elective percutaneous coronary intervention with drug‐eluting or bare‐metal stents. J Am Coll Cardiol. 2008; 51:1925-1934 [DOI] [PubMed] [Google Scholar]
- 32.Parodi G, Marcucci R, Valenti R, Gori AM, Migliorini A, Giusti B, Buonamici P, Gensini GF, Abbate R, Antoniucci D. High residual platelet reactivity after clopidogrel loading and long‐term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011; 306:1215-1223 [DOI] [PubMed] [Google Scholar]
- 33.Bonello L, Camoin‐Jau L, Armero S, Com O, Arques S, Burignat‐Bonello C, Giacomoni MP, Bonello R, Collet F, Rossi P, Barragan P, Dignat‐George F, Paganelli F. Tailored clopidogrel loading dose according to platelet reactivity monitoring to prevent acute and subacute stent thrombosis. Am J Cardiol. 2009; 103:5-10 [DOI] [PubMed] [Google Scholar]
- 34.Bonello L, Camoin‐Jau L, Arques S, Boyer C, Panagides D, Wittenberg O, Simeoni MC, Barragan P, Dignat‐George F, Paganelli F. Adjusted clopidogrel loading doses according to vasodilator‐stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol. 2008; 51:1404-1411 [DOI] [PubMed] [Google Scholar]
- 35.Siller‐Matula JM, Francesconi M, Dechant C, Jilma B, Maurer G, Delle‐Karth G, Gouya G, Ruzicka K, Podczeck‐Schweighofer A, Christ G. Personalized antiplatelet treatment after percutaneous coronary intervention: the MADONNA study. Int J Cardiol. 2012 [DOI] [PubMed] [Google Scholar]
- 36.Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, Puri S, Robbins M, Garratt KN, Bertrand OF, Stillablower ME, Aragon JR, Kandzari DE, Stinis CT, Lee MS, Manoukian SV, Cannon CP, Schork NJ, Topol EJ. Standard‐ vs high‐dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011; 305:1097-1105 [DOI] [PubMed] [Google Scholar]
- 37.Collet JP, Cuisset T, Range G, Cayla G, Elhadad S, Pouillot C, Henry P, Motreff P, Carrie D, Boueri Z, Belle L, Van Belle E, Rousseau H, Aubry P, Monsegu J, Sabouret P, O'Connor SA, Abtan J, Kerneis M, Saint‐Etienne C, Barthelemy O, Beygui F, Silvain J, Vicaut E, Montalescot G. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012; 367:2100-2109 [DOI] [PubMed] [Google Scholar]


