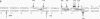Abstract
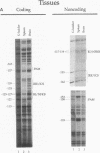
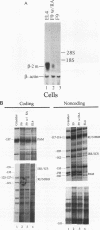
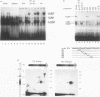
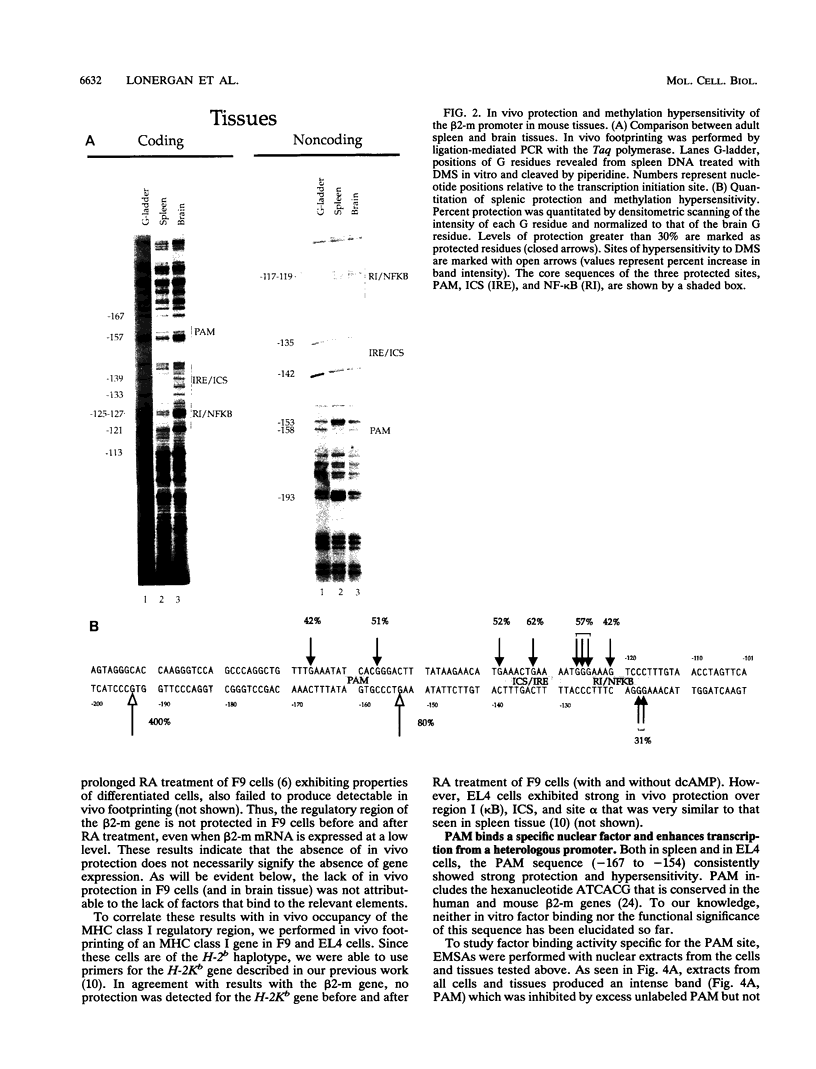
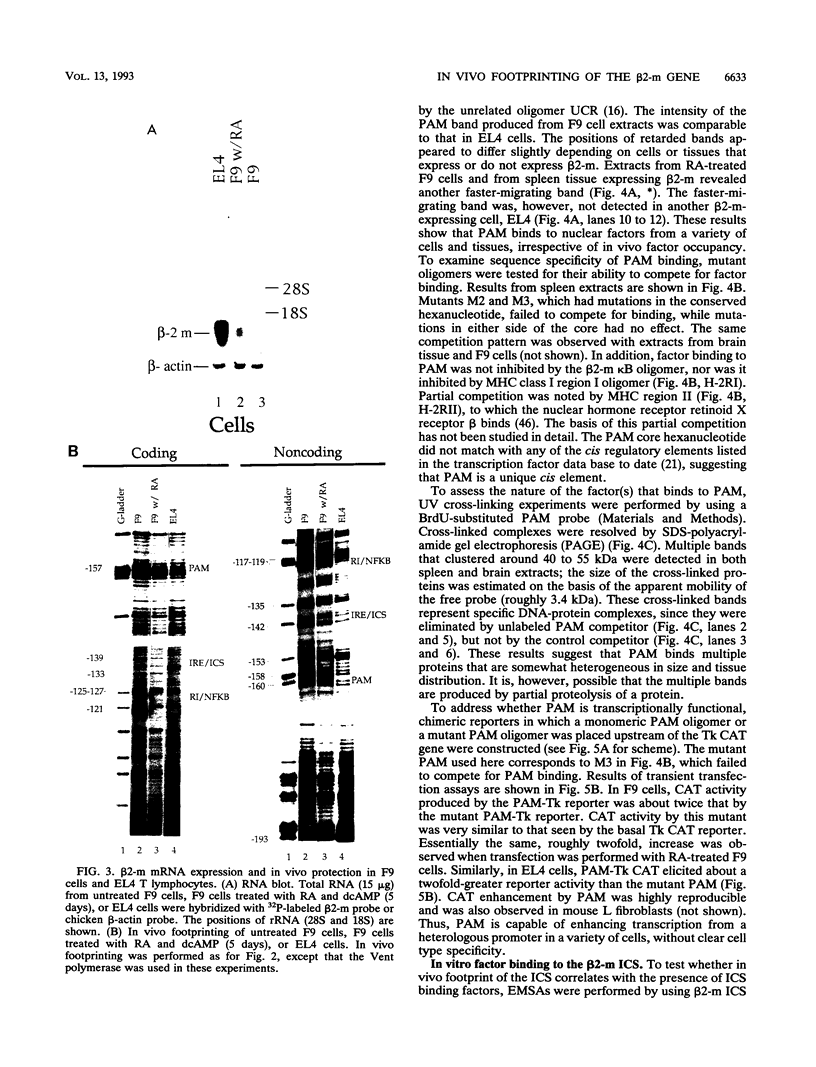
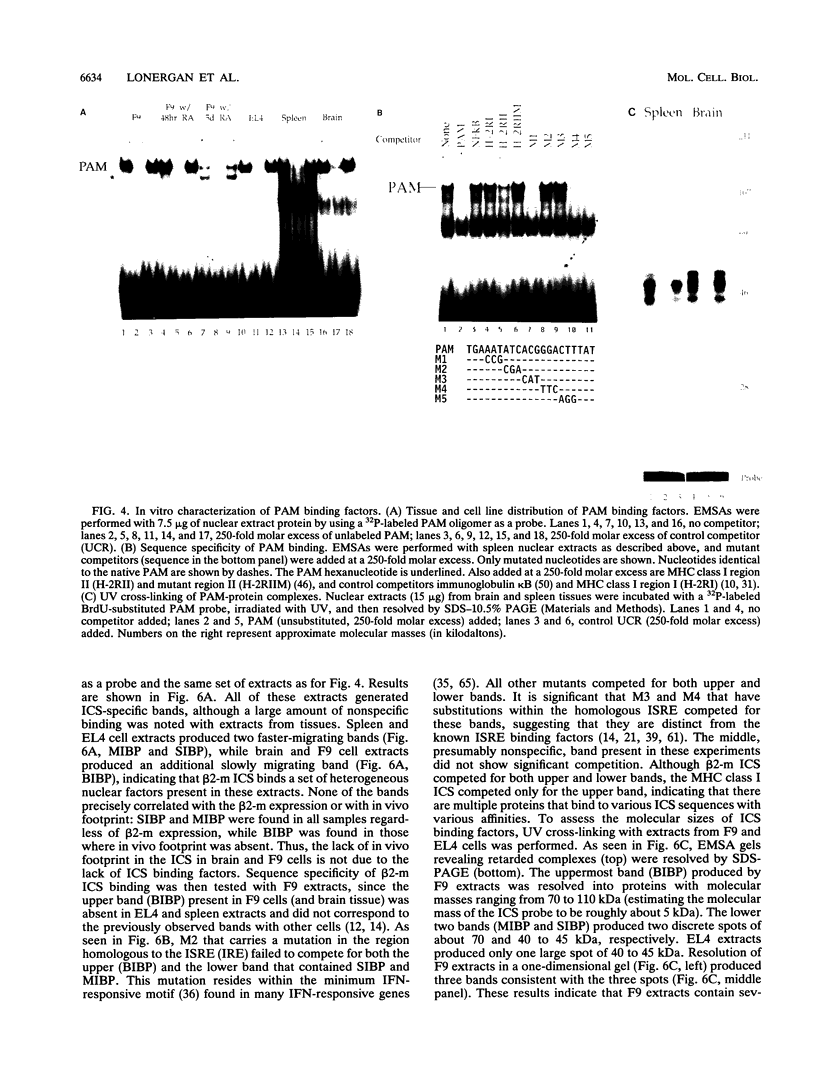
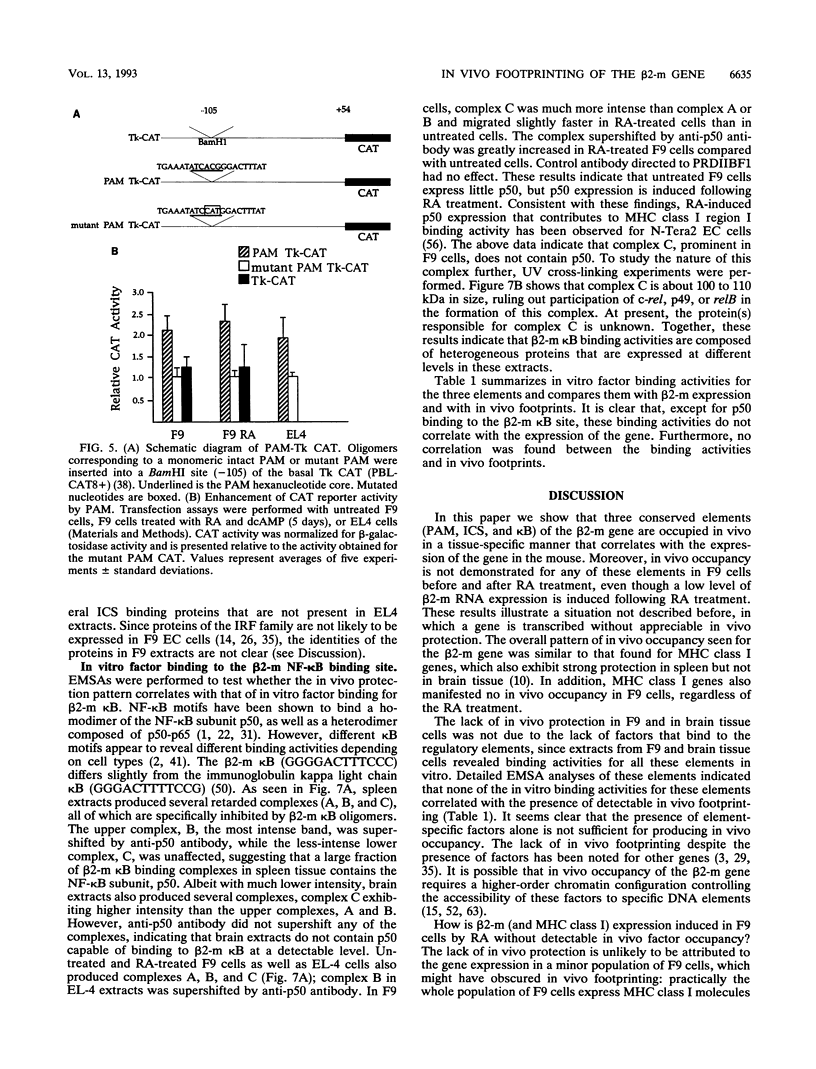
Expression of the beta 2-microglobulin (beta 2-m) and major histocompatibility complex (MHC) class I genes is coordinately regulated. By ligation-mediated polymerase chain reaction, we have analyzed in vivo factor binding to the promoter region of the murine beta 2-m gene. In adult spleen, in which beta 2-m is expressed, strong protection was found in three elements. Two of these elements, the beta 2-m NF-kappa B binding site and the interferon consensus sequence, are homologous to the regulatory elements of the MHC class I genes and were also found to be protected in spleen. A third protected element, PAM, identified in this work, is unique to the beta 2-m gene. None of the elements showed protection in brain tissue, in which neither the beta 2-m nor the MHC class I gene is expressed. In vivo footprinting was also performed with F9 embryonal carcinoma cells, in which expression of the beta 2-m and MHC class I genes is induced at a low level only upon stimulation with retinoic acid (RA). No in vivo protection was detected before and after RA treatment of F9 cells, indicating that RA induction of beta 2-m (and MHC class I) expression occurs without detectable in vivo factor occupancy, whereas EL4 T lymphocytes expressing beta 2-m at a high level exhibited strong protection similar to that in spleen. Despite the lack of in vivo occupancy, the nuclear factors specific for each of the three elements were present in brain tissue and F9 cells as well as in spleen tissue and EL4 cells. We show that PAM, an element identified by its in vivo protection, binds nuclear factors ranging from 40 to 50 kDa in size and is capable of enhancing transcription of a reporter in F9 and other cells. Taken together, these results indicate that in vivo factor occupancy for the beta 2-m and MHC class I promoters is coordinated and occurs through a mechanism other than mere expression of relevant factors.
Full text
PDF


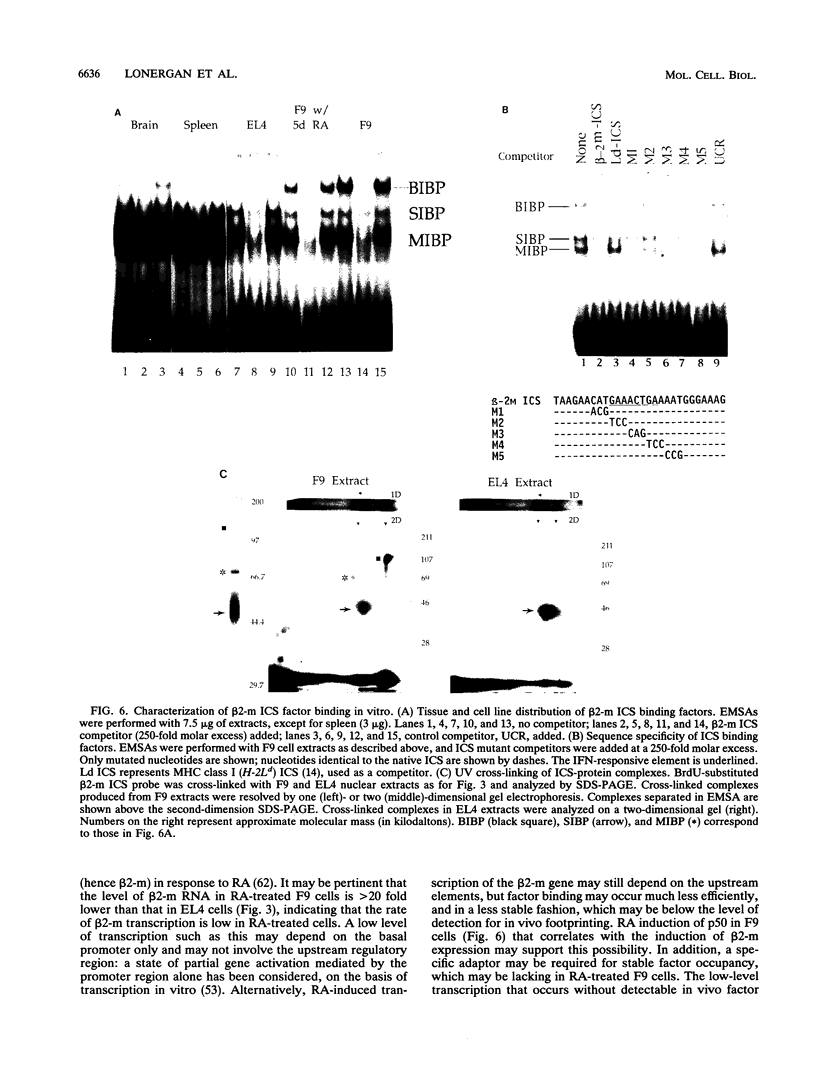
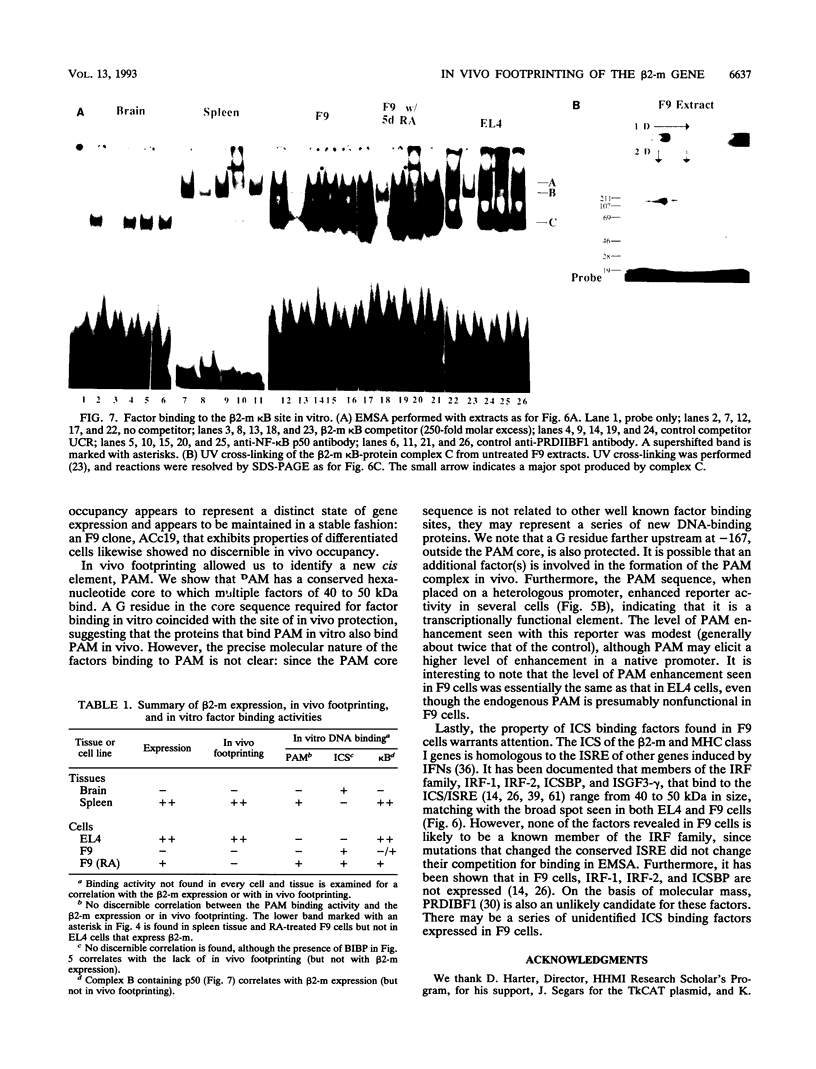


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Ballard D. W., Walker W. H., Doerre S., Sista P., Molitor J. A., Dixon E. P., Peffer N. J., Hannink M., Greene W. C. The v-rel oncogene encodes a kappa B enhancer binding protein that inhibits NF-kappa B function. Cell. 1990 Nov 16;63(4):803–814. doi: 10.1016/0092-8674(90)90146-6. [DOI] [PubMed] [Google Scholar]
- Becker P. B., Ruppert S., Schütz G. Genomic footprinting reveals cell type-specific DNA binding of ubiquitous factors. Cell. 1987 Nov 6;51(3):435–443. doi: 10.1016/0092-8674(87)90639-8. [DOI] [PubMed] [Google Scholar]
- Bours V., Villalobos J., Burd P. R., Kelly K., Siebenlist U. Cloning of a mitogen-inducible gene encoding a kappa B DNA-binding protein with homology to the rel oncogene and to cell-cycle motifs. Nature. 1990 Nov 1;348(6296):76–80. doi: 10.1038/348076a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. W., Nolan J. A., Conrad P. J., Vasavada H. A., Vasavada H. H., Ploegh H. L., Ganguly S., Janeway C. A., Jr, Weissman S. M. Tissue-specific and cell surface expression of human major histocompatibility complex class I heavy (HLA-B7) and light (beta 2-microglobulin) chain genes in transgenic mice. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7690–7694. doi: 10.1073/pnas.85.20.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Linnenbach A., Huebner K., Parnes J. R., Margulies D. H., Appella E., Seidman J. G. Control of expression of histocompatibility antigens (H-2) and beta 2-microglobulin in F9 teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5754–5758. doi: 10.1073/pnas.78.9.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel F., Morello D., Le Bail O., Chambon P., Cayre Y., Kourilsky P. Structure and expression of the mouse beta 2-microglobulin gene isolated from somatic and non-expressing teratocarcinoma cells. EMBO J. 1983;2(7):1061–1065. doi: 10.1002/j.1460-2075.1983.tb01546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargemont C., Dunon D., Deugnier M. A., Denoyelle M., Girault J. M., Lederer F., Lê K. H., Godeau F., Thiery J. P., Imhof B. A. Thymotaxin, a chemotactic protein, is identical to beta 2-microglobulin. Science. 1989 Nov 10;246(4931):803–806. doi: 10.1126/science.2683083. [DOI] [PubMed] [Google Scholar]
- David-Watine B., Israël A., Kourilsky P. The regulation and expression of MHC class I genes. Immunol Today. 1990 Aug;11(8):286–292. doi: 10.1016/0167-5699(90)90114-o. [DOI] [PubMed] [Google Scholar]
- Dey A., Thornton A. M., Lonergan M., Weissman S. M., Chamberlain J. W., Ozato K. Occupancy of upstream regulatory sites in vivo coincides with major histocompatibility complex class I gene expression in mouse tissues. Mol Cell Biol. 1992 Aug;12(8):3590–3599. doi: 10.1128/mcb.12.8.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew P. D., Lonergan M., Goldstein M. E., Lampson L. A., Ozato K., McFarlin D. E. Regulation of MHC class I and beta 2-microglobulin gene expression in human neuronal cells. Factor binding to conserved cis-acting regulatory sequences correlates with expression of the genes. J Immunol. 1993 Apr 15;150(8 Pt 1):3300–3310. [PubMed] [Google Scholar]
- Drezen J. M., Babinet C., Morello D. Transcriptional control of MHC class I and beta 2-microglobulin genes in vivo. J Immunol. 1993 Apr 1;150(7):2805–2813. [PubMed] [Google Scholar]
- Driggers P. H., Ennist D. L., Gleason S. L., Mak W. H., Marks M. S., Levi B. Z., Flanagan J. R., Appella E., Ozato K. An interferon gamma-regulated protein that binds the interferon-inducible enhancer element of major histocompatibility complex class I genes. Proc Natl Acad Sci U S A. 1990 May;87(10):3743–3747. doi: 10.1073/pnas.87.10.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Felsenfeld G., Reitman M. Control of globin gene transcription. Annu Rev Cell Biol. 1990;6:95–124. doi: 10.1146/annurev.cb.06.110190.000523. [DOI] [PubMed] [Google Scholar]
- Flanagan J. R., Becker K. G., Ennist D. L., Gleason S. L., Driggers P. H., Levi B. Z., Appella E., Ozato K. Cloning of a negative transcription factor that binds to the upstream conserved region of Moloney murine leukemia virus. Mol Cell Biol. 1992 Jan;12(1):38–44. doi: 10.1128/mcb.12.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Stark G. R. alpha-Interferon-induced transcription of HLA and metallothionein genes containing homologous upstream sequences. Nature. 1985 Apr 18;314(6012):637–639. doi: 10.1038/314637a0. [DOI] [PubMed] [Google Scholar]
- Fujita T., Miyamoto M., Kimura Y., Hammer J., Taniguchi T. Involvement of a cis-element that binds an H2TF-1/NF kappa B like factor(s) in the virus-induced interferon-beta gene expression. Nucleic Acids Res. 1989 May 11;17(9):3335–3346. doi: 10.1093/nar/17.9.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity P. A., Wold B. J. Effects of different DNA polymerases in ligation-mediated PCR: enhanced genomic sequencing and in vivo footprinting. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1021–1025. doi: 10.1073/pnas.89.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D. A relational database of transcription factors. Nucleic Acids Res. 1990 Apr 11;18(7):1749–1756. doi: 10.1093/nar/18.7.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Güssow D., Rein R., Ginjaar I., Hochstenbach F., Seemann G., Kottman A., Ploegh H. L. The human beta 2-microglobulin gene. Primary structure and definition of the transcriptional unit. J Immunol. 1987 Nov 1;139(9):3132–3138. [PubMed] [Google Scholar]
- Harada H., Fujita T., Miyamoto M., Kimura Y., Maruyama M., Furia A., Miyata T., Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989 Aug 25;58(4):729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- Harada H., Willison K., Sakakibara J., Miyamoto M., Fujita T., Taniguchi T. Absence of the type I IFN system in EC cells: transcriptional activator (IRF-1) and repressor (IRF-2) genes are developmentally regulated. Cell. 1990 Oct 19;63(2):303–312. doi: 10.1016/0092-8674(90)90163-9. [DOI] [PubMed] [Google Scholar]
- Israël A., Kimura A., Kieran M., Yano O., Kanellopoulos J., Le Bail O., Kourilsky P. A common positive trans-acting factor binds to enhancer sequences in the promoters of mouse H-2 and beta 2-microglobulin genes. Proc Natl Acad Sci U S A. 1987 May;84(9):2653–2657. doi: 10.1073/pnas.84.9.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L., Jeannotte L., Bikoff E. K., Robertson E. J. Analysis of beta 2-microglobulin gene expression in the developing mouse embryo and placenta. J Immunol. 1990 Nov 15;145(10):3474–3482. [PubMed] [Google Scholar]
- Kara C. J., Glimcher L. H. In vivo footprinting of MHC class II genes: bare promoters in the bare lymphocyte syndrome. Science. 1991 May 3;252(5006):709–712. doi: 10.1126/science.1902592. [DOI] [PubMed] [Google Scholar]
- Keller A. D., Maniatis T. Identification and characterization of a novel repressor of beta-interferon gene expression. Genes Dev. 1991 May;5(5):868–879. doi: 10.1101/gad.5.5.868. [DOI] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Kimura A., Israël A., Le Bail O., Kourilsky P. Detailed analysis of the mouse H-2Kb promoter: enhancer-like sequences and their role in the regulation of class I gene expression. Cell. 1986 Jan 31;44(2):261–272. doi: 10.1016/0092-8674(86)90760-9. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Marrack P., Kappler J. W., Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990 Jun 8;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Fisher C. A., Whelan J. P. Striking paucity of HLA-A, B, C and beta 2-microglobulin on human neuroblastoma cell lines. J Immunol. 1983 May;130(5):2471–2478. [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Darnell J. E., Jr Cytoplasmic activation of ISGF3, the positive regulator of interferon-alpha-stimulated transcription, reconstituted in vitro. Genes Dev. 1989 Sep;3(9):1362–1371. doi: 10.1101/gad.3.9.1362. [DOI] [PubMed] [Google Scholar]
- MacDonald N. J., Kuhl D., Maguire D., Näf D., Gallant P., Goswamy A., Hug H., Büeler H., Chaturvedi M., de la Fuente J. Different pathways mediate virus inducibility of the human IFN-alpha 1 and IFN-beta genes. Cell. 1990 Mar 9;60(5):767–779. doi: 10.1016/0092-8674(90)90091-r. [DOI] [PubMed] [Google Scholar]
- MacGregor G. R., Caskey C. T. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989 Mar 25;17(6):2365–2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez E., Givel F., Wahli W. The estrogen-responsive element as an inducible enhancer: DNA sequence requirements and conversion to a glucocorticoid-responsive element. EMBO J. 1987 Dec 1;6(12):3719–3727. doi: 10.1002/j.1460-2075.1987.tb02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M., Fujita T., Kimura Y., Maruyama M., Harada H., Sudo Y., Miyata T., Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988 Sep 9;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- Miyazaki J., Appella E., Ozato K. Negative regulation of the major histocompatibility class I gene in undifferentiated embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9537–9541. doi: 10.1073/pnas.83.24.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor J. A., Walker W. H., Doerre S., Ballard D. W., Greene W. C. NF-kappa B: a family of inducible and differentially expressed enhancer-binding proteins in human T cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10028–10032. doi: 10.1073/pnas.87.24.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello D., Daniel F., Baldacci P., Cayre Y., Gachelin G., Kourilsky P. Absence of significant H-2 and beta 2-microglobulin mRNA expression by mouse embryonal carcinoma cells. Nature. 1982 Mar 18;296(5854):260–262. doi: 10.1038/296260a0. [DOI] [PubMed] [Google Scholar]
- Morello D., Duprey P., Israel A., Babinet C. Asynchronous regulation of mouse H-2D and beta-2 microglobulin RNA transcripts. Immunogenetics. 1985;22(5):441–452. doi: 10.1007/BF00418090. [DOI] [PubMed] [Google Scholar]
- Mueller P. R., Salser S. J., Wold B. Constitutive and metal-inducible protein:DNA interactions at the mouse metallothionein I promoter examined by in vivo and in vitro footprinting. Genes Dev. 1988 Apr;2(4):412–427. doi: 10.1101/gad.2.4.412. [DOI] [PubMed] [Google Scholar]
- Mueller P. R., Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989 Nov 10;246(4931):780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- Nagata T., Segars J. H., Levi B. Z., Ozato K. Retinoic acid-dependent transactivation of major histocompatibility complex class I promoters by the nuclear hormone receptor H-2RIIBP in undifferentiated embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):937–941. doi: 10.1073/pnas.89.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Wan Y. J., Orrison B. M. Mouse major histocompatibility class I gene expression begins at midsomite stage and is inducible in earlier-stage embryos by interferon. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2427–2431. doi: 10.1073/pnas.82.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Eldridge J. D. alpha-Cardiac actin is the major sarcomeric isoform expressed in embryonic avian skeletal muscle. Science. 1984 Jun 29;224(4656):1436–1438. doi: 10.1126/science.6729461. [DOI] [PubMed] [Google Scholar]
- Picard D., Schaffner W. A lymphocyte-specific enhancer in the mouse immunoglobulin kappa gene. Nature. 1984 Jan 5;307(5946):80–82. doi: 10.1038/307080a0. [DOI] [PubMed] [Google Scholar]
- Reik A., Schütz G., Stewart A. F. Glucocorticoids are required for establishment and maintenance of an alteration in chromatin structure: induction leads to a reversible disruption of nucleosomes over an enhancer. EMBO J. 1991 Sep;10(9):2569–2576. doi: 10.1002/j.1460-2075.1991.tb07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard-Foy H., Hager G. L. Sequence-specific positioning of nucleosomes over the steroid-inducible MMTV promoter. EMBO J. 1987 Aug;6(8):2321–2328. doi: 10.1002/j.1460-2075.1987.tb02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder R. G. The complexities of eukaryotic transcription initiation: regulation of preinitiation complex assembly. Trends Biochem Sci. 1991 Nov;16(11):402–408. doi: 10.1016/0968-0004(91)90164-q. [DOI] [PubMed] [Google Scholar]
- Schindler C., Fu X. Y., Improta T., Aebersold R., Darnell J. E., Jr Proteins of transcription factor ISGF-3: one gene encodes the 91-and 84-kDa ISGF-3 proteins that are activated by interferon alpha. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7836–7839. doi: 10.1073/pnas.89.16.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segars J. H., Nagata T., Bours V., Medin J. A., Franzoso G., Blanco J. C., Drew P. D., Becker K. G., An J., Tang T. Retinoic acid induction of major histocompatibility complex class I genes in NTera-2 embryonal carcinoma cells involves induction of NF-kappa B (p50-p65) and retinoic acid receptor beta-retinoid X receptor beta heterodimers. Mol Cell Biol. 1993 Oct;13(10):6157–6169. doi: 10.1128/mcb.13.10.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinsson L., Peterson P. A. Beta 2-microglobulin induces intracellular transport of human class I transplantation antigen heavy chains in Xenopus laevis oocytes. J Cell Biol. 1984 Jul;99(1 Pt 1):226–232. doi: 10.1083/jcb.99.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman T., Rein A., Orrison B., Langloss J., Bratthauer G., Miyazaki J., Ozato K. Establishment of cell lines from somite stage mouse embryos and expression of major histocompatibility class I genes in these cells. J Immunol. 1988 Jun 15;140(12):4378–4387. [PubMed] [Google Scholar]
- Simister N. E., Mostov K. E. An Fc receptor structurally related to MHC class I antigens. Nature. 1989 Jan 12;337(6203):184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Veals S. A., Schindler C., Leonard D., Fu X. Y., Aebersold R., Darnell J. E., Jr, Levy D. E. Subunit of an alpha-interferon-responsive transcription factor is related to interferon regulatory factor and Myb families of DNA-binding proteins. Mol Cell Biol. 1992 Aug;12(8):3315–3324. doi: 10.1128/mcb.12.8.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y. J., Orrison B. M., Lieberman R., Lazarovici P., Ozato K. Induction of major histocompatibility class I antigens by interferons in undifferentiated F9 cells. J Cell Physiol. 1987 Feb;130(2):276–283. doi: 10.1002/jcp.1041300214. [DOI] [PubMed] [Google Scholar]
- Weih F., Nitsch D., Reik A., Schütz G., Becker P. B. Analysis of CpG methylation and genomic footprinting at the tyrosine aminotransferase gene: DNA methylation alone is not sufficient to prevent protein binding in vivo. EMBO J. 1991 Sep;10(9):2559–2567. doi: 10.1002/j.1460-2075.1991.tb07796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore L. A., Maniatis T. Postinduction repression of the beta-interferon gene is mediated through two positive regulatory domains. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7799–7803. doi: 10.1073/pnas.87.20.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. R. Transcriptional regulation of interferon-stimulated genes. Eur J Biochem. 1991 Aug 15;200(1):1–11. doi: 10.1111/j.1432-1033.1991.tb21041.x. [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]