Abstract
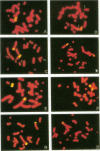
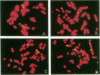
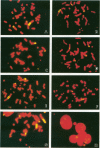
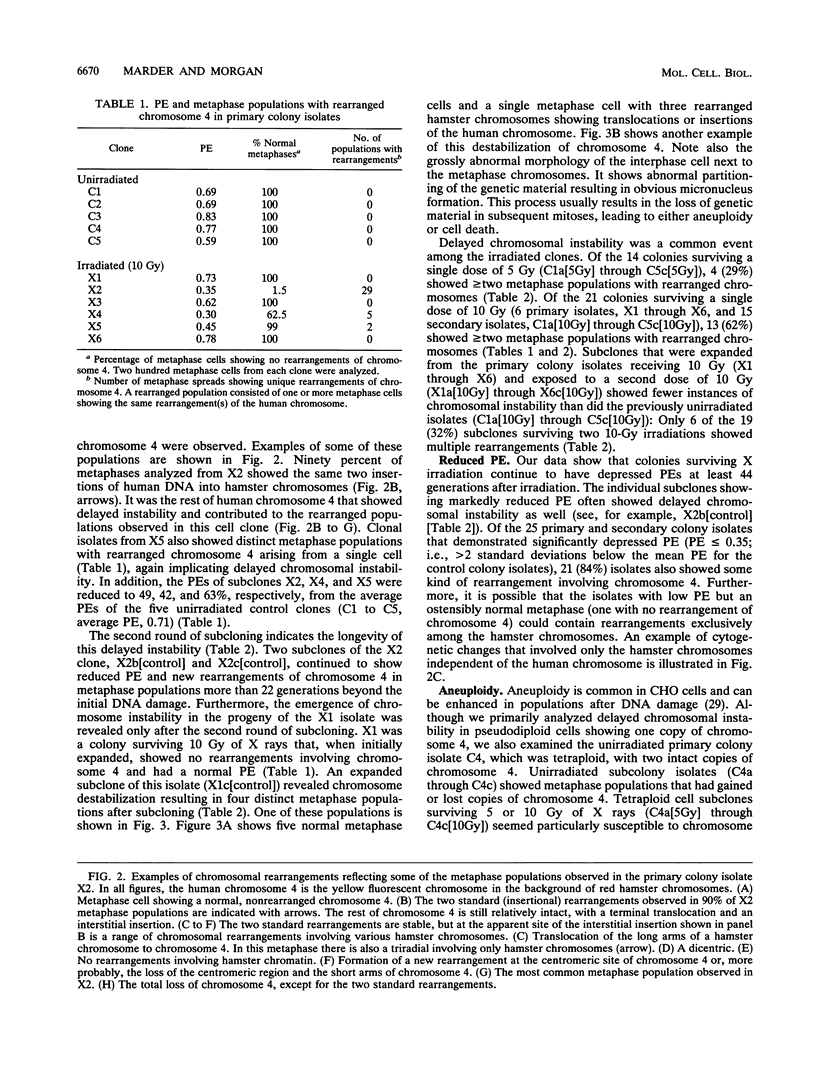
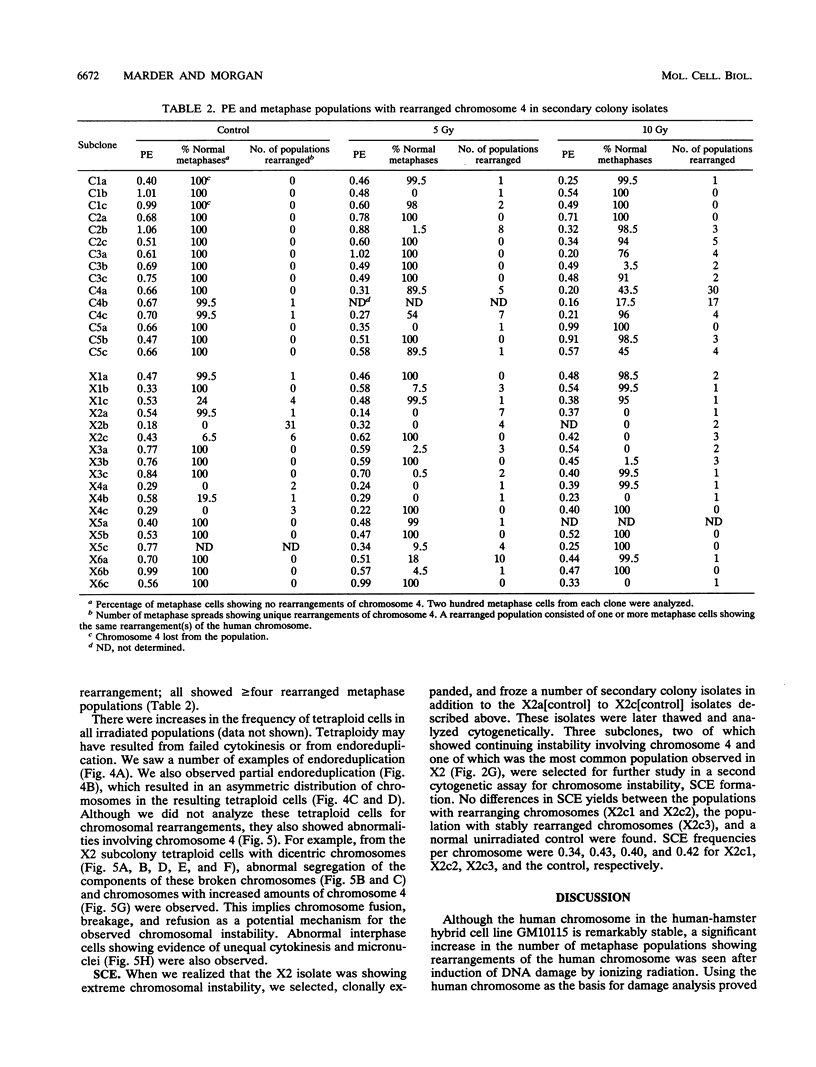
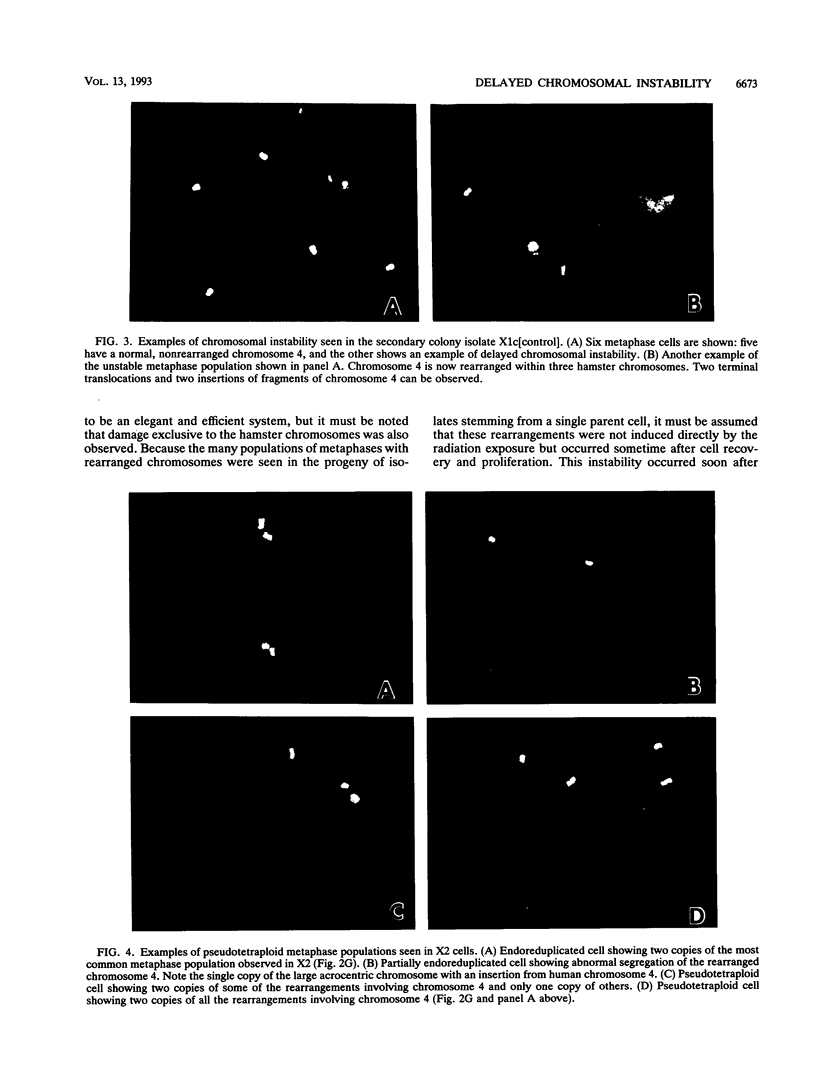
DNA damage induced by ionizing radiation can result in gene mutation, gene amplification, chromosome rearrangements, cellular transformation, and cell death. Although many of these changes may be induced directly by the radiation, there is accumulating evidence for delayed genomic instability following X-ray exposure. We have investigated this phenomenon by studying delayed chromosomal instability in a hamster-human hybrid cell line by means of fluorescence in situ hybridization. We examined populations of metaphase cells several generations after expanding single-cell colonies that had survived 5 or 10 Gy of X rays. Delayed chromosomal instability, manifested as multiple rearrangements of human chromosome 4 in a background of hamster chromosomes, was observed in 29% of colonies surviving 5 Gy and in 62% of colonies surviving 10 Gy. A correlation of delayed chromosomal instability with delayed reproductive cell death, manifested as reduced plating efficiency in surviving clones, suggests a role for chromosome rearrangements in cytotoxicity. There were small differences in chromosome destabilization and plating efficiencies between cells irradiated with 5 or 10 Gy of X rays after a previous exposure to 10 Gy and cells irradiated only once. Cell clones showing delayed chromosomal instability had normal frequencies of sister chromatid exchange formation, indicating that at this cytogenetic endpoint the chromosomal instability was not apparent. The types of chromosomal rearrangements observed suggest that chromosome fusion, followed by bridge breakage and refusion, contributes to the observed delayed chromosomal instability.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender M. A., Awa A. A., Brooks A. L., Evans H. J., Groer P. G., Littlefield L. G., Pereira C., Preston R. J., Wachholz B. W. Current status of cytogenetic procedures to detect and quantify previous exposures to radiation. Mutat Res. 1988 Sep;196(2):103–159. doi: 10.1016/0165-1110(88)90017-6. [DOI] [PubMed] [Google Scholar]
- Boothman D. A., Bouvard I., Hughes E. N. Identification and characterization of X-ray-induced proteins in human cells. Cancer Res. 1989 Jun 1;49(11):2871–2878. [PubMed] [Google Scholar]
- Boothman D. A., Wang M., Lee S. W. Induction of tissue-type plasminogen activator by ionizing radiation in human malignant melanoma cells. Cancer Res. 1991 Oct 15;51(20):5587–5595. [PubMed] [Google Scholar]
- Brown W. R., MacKinnon P. J., Villasanté A., Spurr N., Buckle V. J., Dobson M. J. Structure and polymorphism of human telomere-associated DNA. Cell. 1990 Oct 5;63(1):119–132. doi: 10.1016/0092-8674(90)90293-n. [DOI] [PubMed] [Google Scholar]
- Carrano A. V., Minkler J., Piluso D. On the fate of stable chromosomal aberrations. Mutat Res. 1975 Oct;30(1):153–156. [PubMed] [Google Scholar]
- Chang W. P., Little J. B. Delayed reproductive death as a dominant phenotype in cell clones surviving X-irradiation. Carcinogenesis. 1992 Jun;13(6):923–928. doi: 10.1093/carcin/13.6.923. [DOI] [PubMed] [Google Scholar]
- Chang W. P., Little J. B. Delayed reproductive death in X-irradiated Chinese hamster ovary cells. Int J Radiat Biol. 1991 Sep;60(3):483–496. doi: 10.1080/09553009114552331. [DOI] [PubMed] [Google Scholar]
- Chang W. P., Little J. B. Evidence that DNA double-strand breaks initiate the phenotype of delayed reproductive death in Chinese hamster ovary cells. Radiat Res. 1992 Jul;131(1):53–59. [PubMed] [Google Scholar]
- Chang W. P., Little J. B. Persistently elevated frequency of spontaneous mutations in progeny of CHO clones surviving X-irradiation: association with delayed reproductive death phenotype. Mutat Res. 1992 Nov 16;270(2):191–199. doi: 10.1016/0027-5107(92)90130-t. [DOI] [PubMed] [Google Scholar]
- Countryman P. I., Heddle J. A. The production of micronuclei from chromosome aberrations in irradiated cultures of human lymphocytes. Mutat Res. 1976 Dec;41(2-3):321–332. doi: 10.1016/0027-5107(76)90105-6. [DOI] [PubMed] [Google Scholar]
- ELKIND M. M., SUTTON H. X-ray damage and recovery in mammalian cells in culture. Nature. 1959 Oct 24;184:1293–1295. doi: 10.1038/1841293a0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Nebert D. W., Hollander M. C., Luethy J. D., Papathanasiou M., Fargnoli J., Holbrook N. J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989 Oct;9(10):4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgojo L., Little J. B. Expression of lethal mutations in progeny of irradiated mammalian cells. Int J Radiat Biol. 1989 Apr;55(4):619–630. doi: 10.1080/09553008914550661. [DOI] [PubMed] [Google Scholar]
- Hahn P., Morgan W. F., Painter R. B. The role of acentric chromosome fragments in gene amplification. Somat Cell Mol Genet. 1987 Nov;13(6):597–608. doi: 10.1007/BF01534480. [DOI] [PubMed] [Google Scholar]
- Hahn P., Nevaldine B., Morgan W. F. X-ray induction of methotrexate resistance due to dhfr gene amplification. Somat Cell Mol Genet. 1990 Sep;16(5):413–423. doi: 10.1007/BF01233191. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Allshire R. C. Human telomeres: fusion and interstitial sites. Trends Genet. 1989 Oct;5(10):326–331. doi: 10.1016/0168-9525(89)90137-6. [DOI] [PubMed] [Google Scholar]
- Holmberg K., Fält S., Johansson A., Lambert B. Clonal chromosome aberrations and genomic instability in X-irradiated human T-lymphocyte cultures. Mutat Res. 1993 Apr;286(2):321–330. doi: 10.1016/0027-5107(93)90197-n. [DOI] [PubMed] [Google Scholar]
- Honda T., Sadamori N., Itoh M., Kusumi O. Clonal fibroblastic cell lines established from a heavily exposed atomic bomb survivor. Mutat Res. 1993 Apr;291(2):125–133. doi: 10.1016/0165-1161(93)90151-o. [DOI] [PubMed] [Google Scholar]
- Iliakis G. The role of DNA double strand breaks in ionizing radiation-induced killing of eukaryotic cells. Bioessays. 1991 Dec;13(12):641–648. doi: 10.1002/bies.950131204. [DOI] [PubMed] [Google Scholar]
- Kadhim M. A., Macdonald D. A., Goodhead D. T., Lorimore S. A., Marsden S. J., Wright E. G. Transmission of chromosomal instability after plutonium alpha-particle irradiation. Nature. 1992 Feb 20;355(6362):738–740. doi: 10.1038/355738a0. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A., Latt S. A. Evolution of chromosomal regions containing transfected and amplified dihydrofolate reductase sequences. Mol Cell Biol. 1983 Apr;3(4):699–711. doi: 10.1128/mcb.3.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Martin S., Trask B., Hamlin J. L. Sister chromatid fusion initiates amplification of the dihydrofolate reductase gene in Chinese hamster cells. Genes Dev. 1993 Apr;7(4):605–620. doi: 10.1101/gad.7.4.605. [DOI] [PubMed] [Google Scholar]
- Martins M. B., Sabatier L., Ricoul M., Pinton A., Dutrillaux B. Specific chromosome instability induced by heavy ions: a step towards transformation of human fibroblasts? Mutat Res. 1993 Feb;285(2):229–237. doi: 10.1016/0027-5107(93)90111-r. [DOI] [PubMed] [Google Scholar]
- McClintock B. The Stability of Broken Ends of Chromosomes in Zea Mays. Genetics. 1941 Mar;26(2):234–282. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonca M. S., Antoniono R. J., Redpath J. L. Delayed heritable damage and epigenetics in radiation-induced neoplastic transformation of human hybrid cells. Radiat Res. 1993 May;134(2):209–216. [PubMed] [Google Scholar]
- Minkler J. L., Piluso D., Gofman J. W., Tandy R. K. A long-term effect of radiation on chromosomes of cultured human fibroblasts. Mutat Res. 1971 Sep;13(1):67–75. doi: 10.1016/0027-5107(71)90126-6. [DOI] [PubMed] [Google Scholar]
- Morgan W. F., Schwartz J. L., Murnane J. P., Wolff S. Effect of 3-aminobenzamide on sister chromatid exchange frequency in X-irradiated cells. Radiat Res. 1983 Mar;93(3):567–571. [PubMed] [Google Scholar]
- Mothersill C., Seymour C. The influence of lethal mutations on the quantification of radiation transformation frequencies. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Apr;51(4):723–729. doi: 10.1080/09553008414552241. [DOI] [PubMed] [Google Scholar]
- Murnane J. P., Yu L. C. Acquisition of telomere repeat sequences by transfected DNA integrated at the site of a chromosome break. Mol Cell Biol. 1993 Feb;13(2):977–983. doi: 10.1128/mcb.13.2.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onfelt A., Ramel C. Some aspects on the organization of microfilaments and microtubules in relation to nondisjunction. Environ Health Perspect. 1979 Aug;31:45–52. doi: 10.1289/ehp.793145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampfer S., Streffer C. Increased chromosome aberration levels in cells from mouse fetuses after zygote X-irradiation. Int J Radiat Biol. 1989 Jan;55(1):85–92. doi: 10.1080/09553008914550091. [DOI] [PubMed] [Google Scholar]
- Perry P., Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974 Sep 13;251(5471):156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Ruiz J. C., Wahl G. M. Chromosomal destabilization during gene amplification. Mol Cell Biol. 1990 Jun;10(6):3056–3066. doi: 10.1128/mcb.10.6.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINCLAIR W. K. X-RAY-INDUCED HERITABLE DAMAGE (SMALL-COLONY FORMATION) IN CULTURED MAMMALIAN CELLS. Radiat Res. 1964 Apr;21:584–611. [PubMed] [Google Scholar]
- Sabatier L., Dutrillaux B., Martin M. B. Chromosomal instability. Nature. 1992 Jun 18;357(6379):548–548. doi: 10.1038/357548a0. [DOI] [PubMed] [Google Scholar]
- Seymour C. B., Mothersill C., Alper T. High yields of lethal mutations in somatic mammalian cells that survive ionizing radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Jul;50(1):167–179. doi: 10.1080/09553008614550541. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Stark M. B., Gorman P. A., Stark G. R. Fusions near telomeres occur very early in the amplification of CAD genes in Syrian hamster cells. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5427–5431. doi: 10.1073/pnas.89.12.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon E., Borrow J., Goddard A. D. Chromosome aberrations and cancer. Science. 1991 Nov 22;254(5035):1153–1160. doi: 10.1126/science.1957167. [DOI] [PubMed] [Google Scholar]
- Stamato T., Weinstein R., Peters B., Hu J., Doherty B., Giaccia A. Delayed mutation in Chinese hamster cells. Somat Cell Mol Genet. 1987 Jan;13(1):57–65. doi: 10.1007/BF02422299. [DOI] [PubMed] [Google Scholar]
- Toledo F., Le Roscouet D., Buttin G., Debatisse M. Co-amplified markers alternate in megabase long chromosomal inverted repeats and cluster independently in interphase nuclei at early steps of mammalian gene amplification. EMBO J. 1992 Jul;11(7):2665–2673. doi: 10.1002/j.1460-2075.1992.tb05332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. F. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- Ward J. F., Limoli C. L., Calabro-Jones P. M., Aguilera J. An examination of the repair saturation hypothesis for describing shouldered survival curves. Radiat Res. 1991 Jul;127(1):90–96. [PubMed] [Google Scholar]
- Ward J. F. The yield of DNA double-strand breaks produced intracellularly by ionizing radiation: a review. Int J Radiat Biol. 1990 Jun;57(6):1141–1150. doi: 10.1080/09553009014551251. [DOI] [PubMed] [Google Scholar]
- Weichselbaum R. R., Hallahan D. E., Sukhatme V., Dritschilo A., Sherman M. L., Kufe D. W. Biological consequences of gene regulation after ionizing radiation exposure. J Natl Cancer Inst. 1991 Apr 3;83(7):480–484. doi: 10.1093/jnci/83.7.480. [DOI] [PubMed] [Google Scholar]
- Windle B., Draper B. W., Yin Y. X., O'Gorman S., Wahl G. M. A central role for chromosome breakage in gene amplification, deletion formation, and amplicon integration. Genes Dev. 1991 Feb;5(2):160–174. doi: 10.1101/gad.5.2.160. [DOI] [PubMed] [Google Scholar]