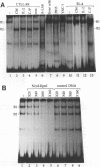
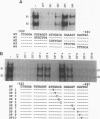
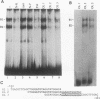
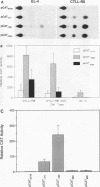
Abstract
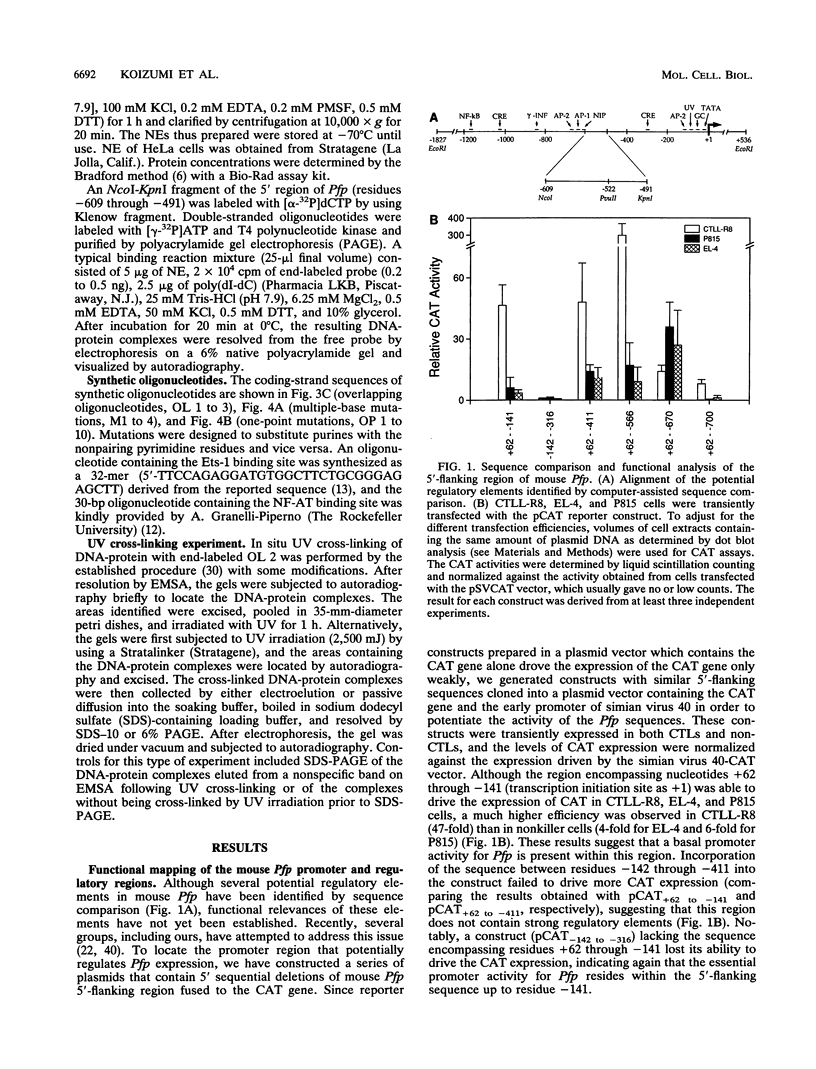
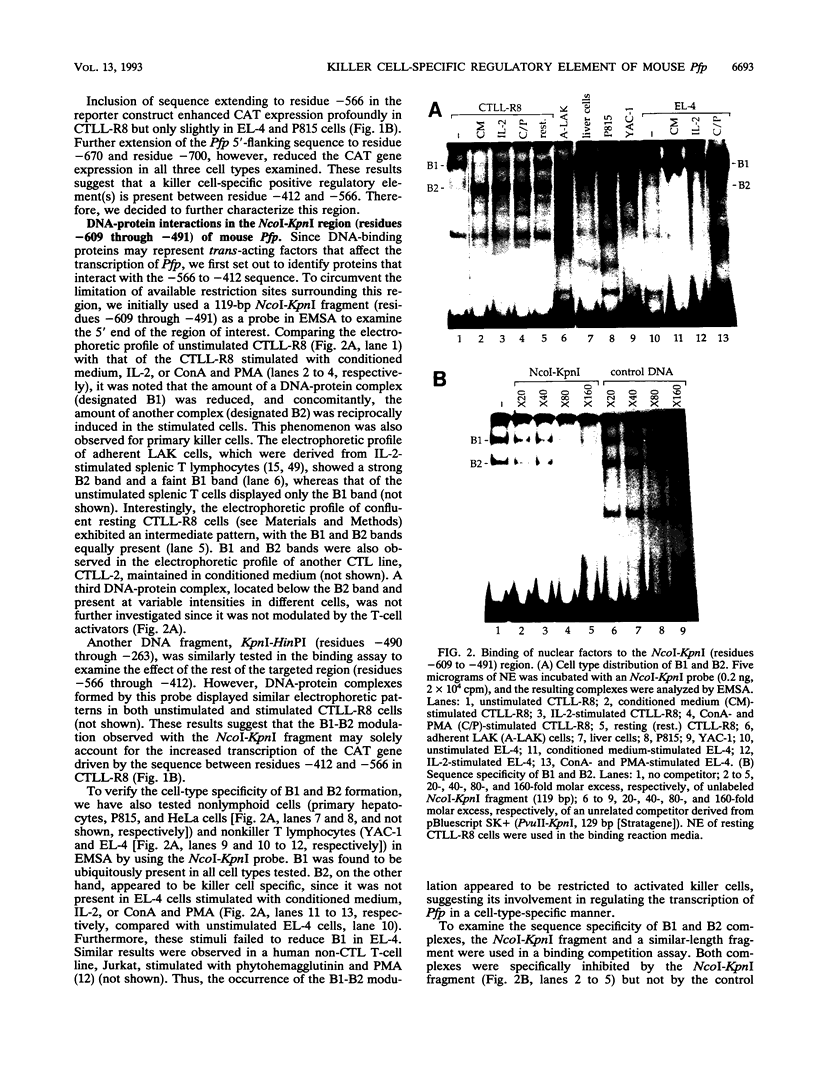
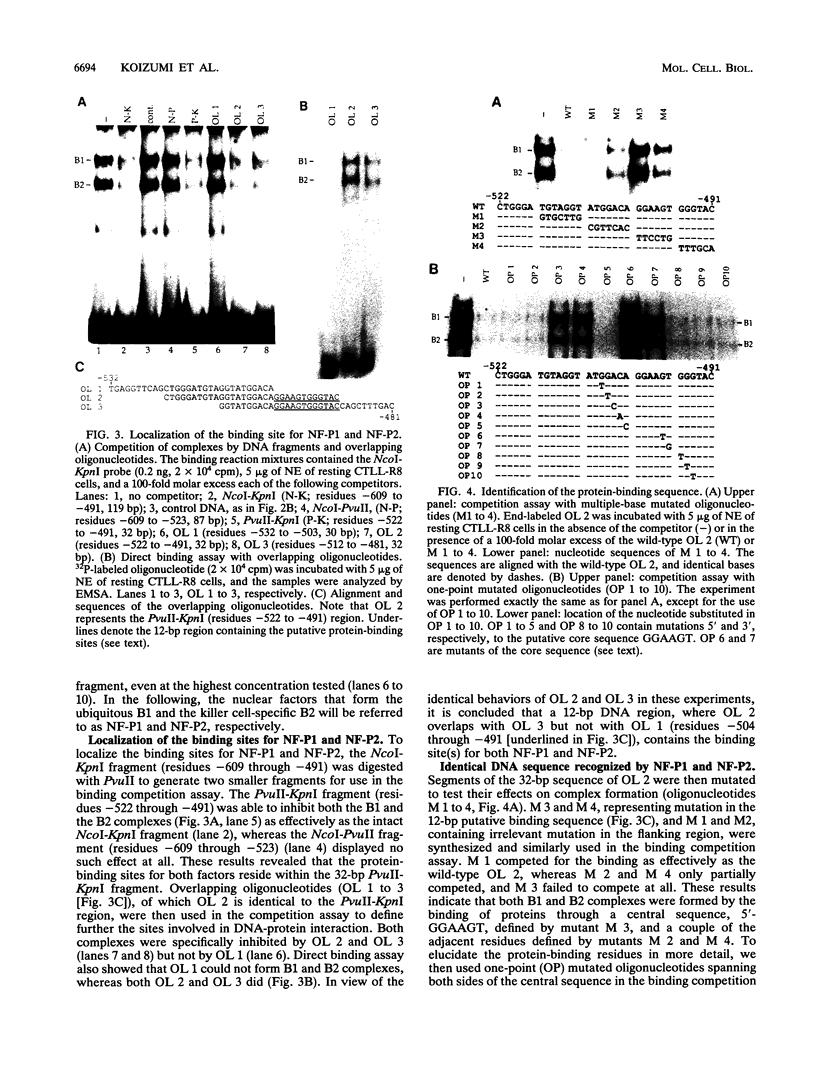
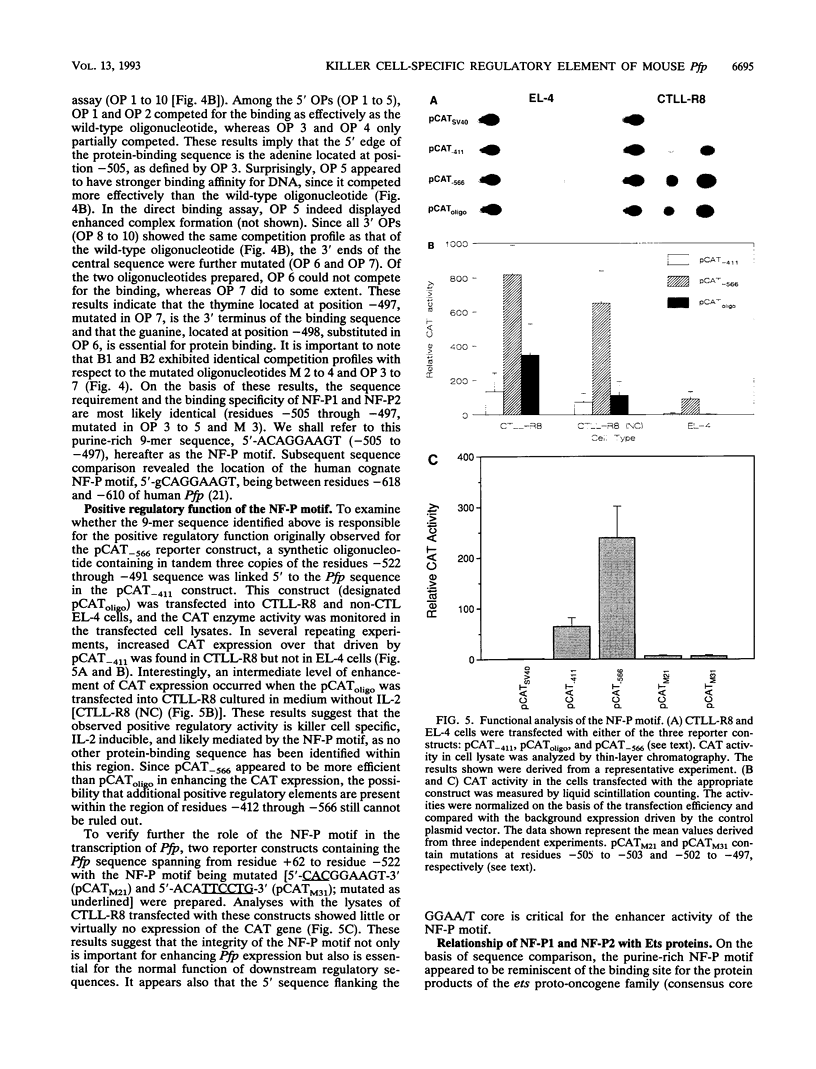
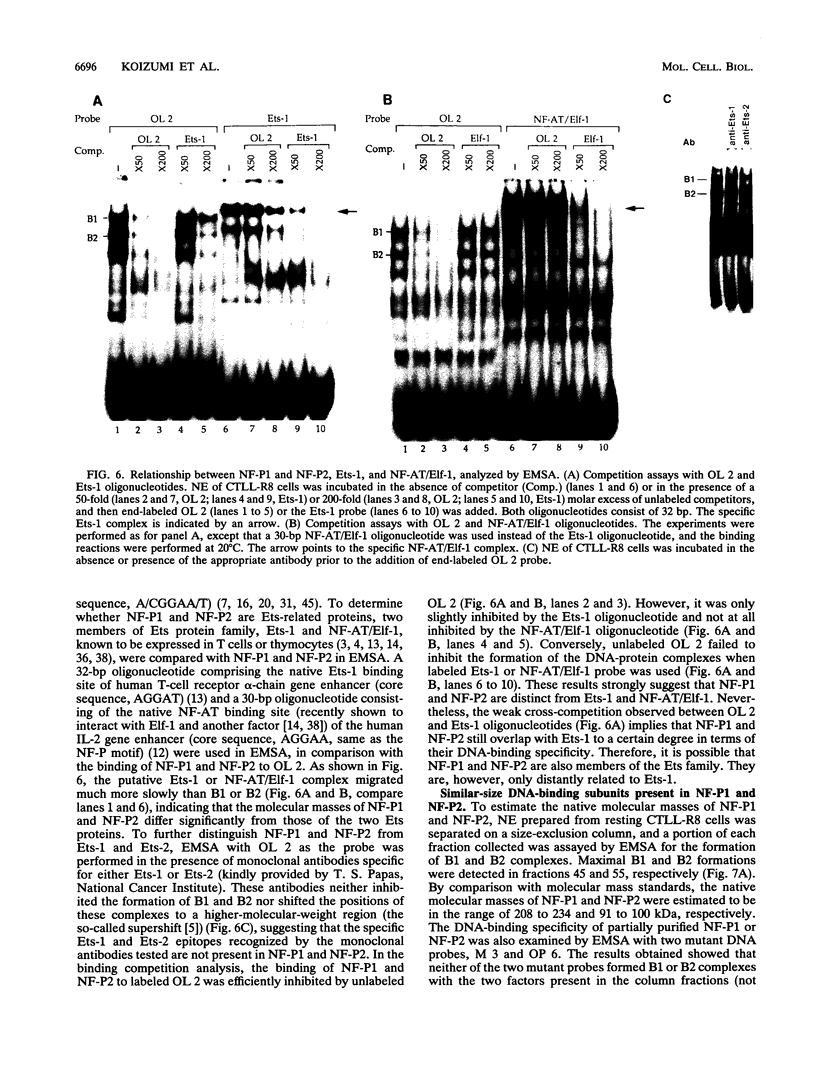
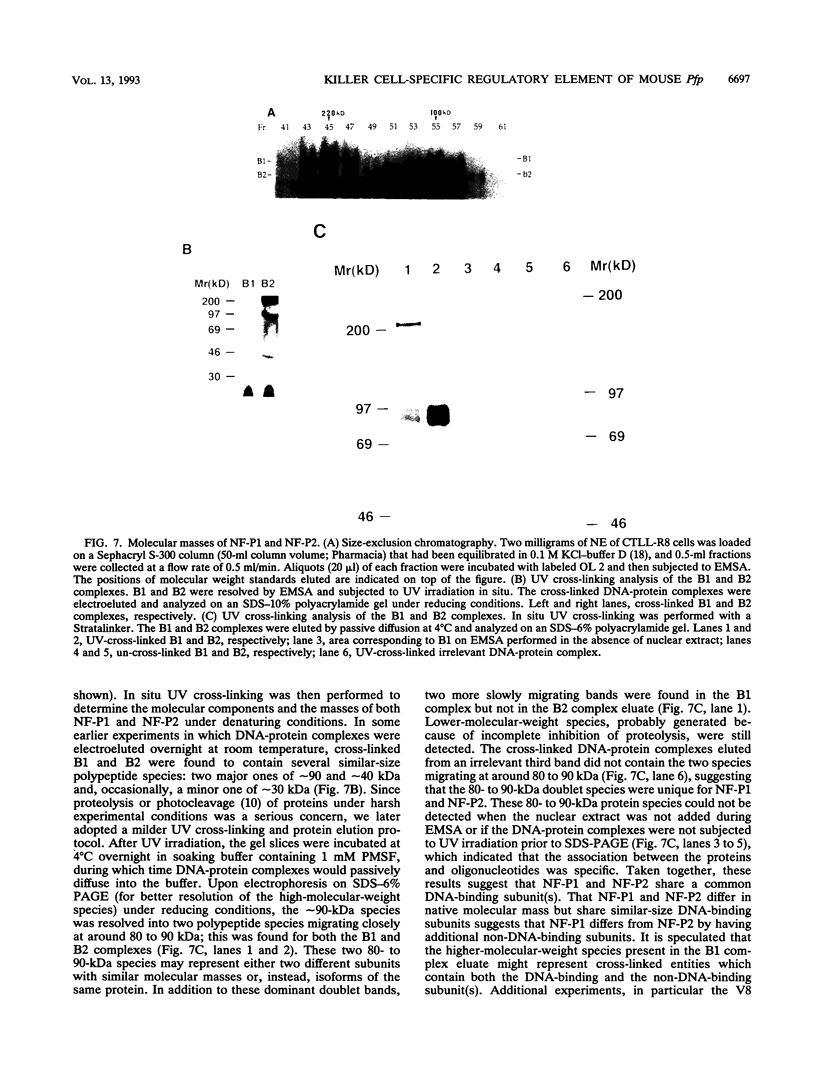
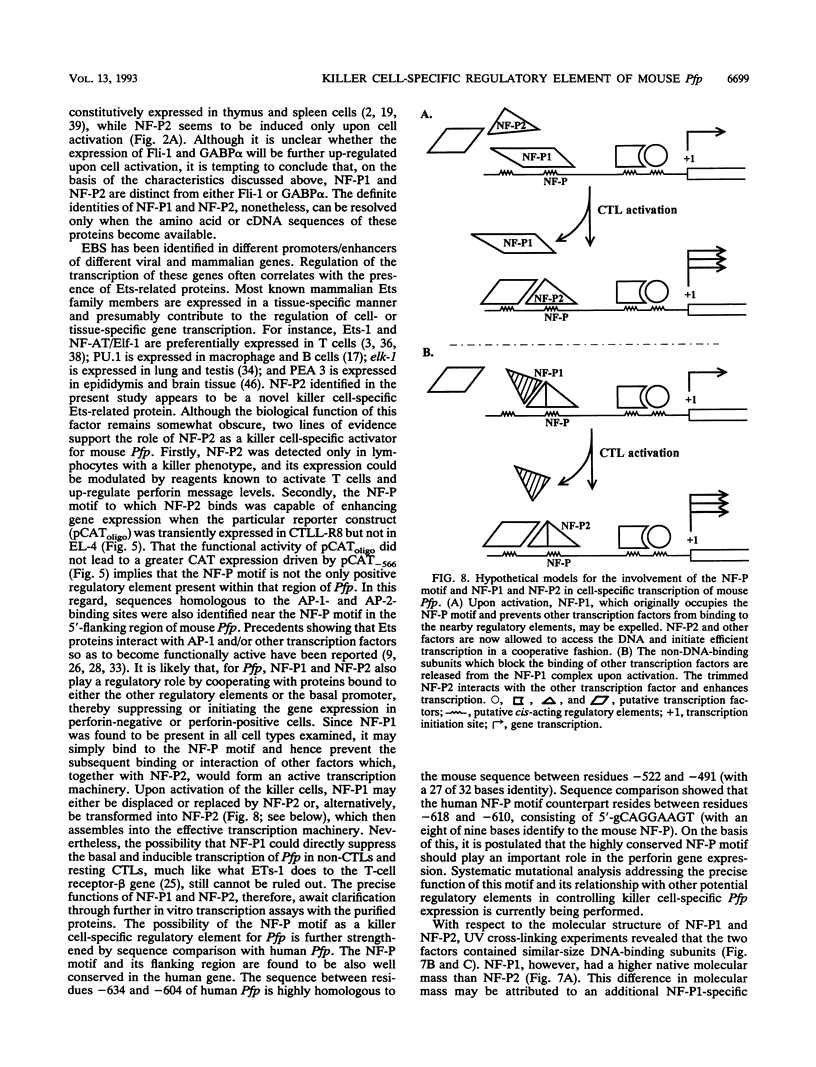
The gene encoding the cytolytic protein perforin is selectively expressed by activated killer lymphocytes. To understand the mechanisms underlying the cell-type-specific expression of this gene, we have characterized the regulatory functions and the DNA-protein interactions of the 5'-flanking region of the mouse perforin gene (Pfp). A region extending from residues +62 through -141, which possesses the essential promoter activity, and regions further upstream, which are able to either enhance or suppress gene expression, were identified. The region between residues -411 and -566 was chosen for further characterization, since it contains an enhancer-like activity. We have identified a 32-mer sequence (residues -491 to -522) which appeared to be capable of enhancing gene expression in a killer cell-specific manner. Within this segment, a 9-mer motif (5'-ACAGGAAGT-3', residues -505 to -497; designated NF-P motif), which is highly homologous to the Ets proto-oncoprotein-binding site, was found to interact with two proteins, NF-P1 and NF-P2. NF-P2 appears to be induced by reagents known to up-regulate the perforin message level and is present exclusively in killer cells. Electrophoretic mobility shift assay and UV cross-linking experiments revealed that NF-P1 and NF-P2 may possess common DNA-binding subunits. However, the larger native molecular mass of NF-P1 suggests that NF-P1 contains an additional non-DNA-binding subunit(s). In view of the homology between the NF-P motif and other Ets proto-oncoprotein-binding sites, it is postulated that NF-P1 and NF-P2 belong to the Ets protein family. Results obtained from the binding competition assay, nevertheless, suggest that NF-P1 and NF-P2 are related to but distinct from Ets proteins, e.g., Ets-1, Ets-2, and NF-AT/Elf-1, known to be expressed in T cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abken H., Reifenrath B. A procedure to standardize CAT reporter gene assay. Nucleic Acids Res. 1992 Jul 11;20(13):3527–3527. doi: 10.1093/nar/20.13.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David Y., Giddens E. B., Letwin K., Bernstein A. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 1991 Jun;5(6):908–918. doi: 10.1101/gad.5.6.908. [DOI] [PubMed] [Google Scholar]
- Bhat N. K., Fisher R. J., Fujiwara S., Ascione R., Papas T. S. Temporal and tissue-specific expression of mouse ets genes. Proc Natl Acad Sci U S A. 1987 May;84(10):3161–3165. doi: 10.1073/pnas.84.10.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat N. K., Komschlies K. L., Fujiwara S., Fisher R. J., Mathieson B. J., Gregorio T. A., Young H. A., Kasik J. W., Ozato K., Papas T. S. Expression of ets genes in mouse thymocyte subsets and T cells. J Immunol. 1989 Jan 15;142(2):672–678. [PubMed] [Google Scholar]
- Bhat N. K., Papas T. S. Characterization and uses of monoclonal antibody derived against DNA binding domain of the ets family of genes. Hybridoma. 1992 Jun;11(3):277–294. doi: 10.1089/hyb.1992.11.277. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown T. A., McKnight S. L. Specificities of protein-protein and protein-DNA interaction of GABP alpha and two newly defined ets-related proteins. Genes Dev. 1992 Dec;6(12B):2502–2512. doi: 10.1101/gad.6.12b.2502. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek H., Tantravahi R. V., Rao V. N., Reddy E. S., Reddy E. P. Myb and Ets proteins cooperate in transcriptional activation of the mim-1 promoter. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1291–1295. doi: 10.1073/pnas.89.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I. R., Lee-Eiford A., Mocz G., Phillipson C. A., Tang W. J., Gibbons B. H. Photosensitized cleavage of dynein heavy chains. Cleavage at the "V1 site" by irradiation at 365 nm in the presence of ATP and vanadate. J Biol Chem. 1987 Feb 25;262(6):2780–2786. [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., McHugh P. Characterization of a protein that regulates the DNA-binding activity of NF-AT, the nuclear factor of activated T cells. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11431–11434. doi: 10.1073/pnas.88.24.11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho I. C., Bhat N. K., Gottschalk L. R., Lindsten T., Thompson C. B., Papas T. S., Leiden J. M. Sequence-specific binding of human Ets-1 to the T cell receptor alpha gene enhancer. Science. 1990 Nov 9;250(4982):814–818. doi: 10.1126/science.2237431. [DOI] [PubMed] [Google Scholar]
- Jain J., McCaffrey P. G., Valge-Archer V. E., Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature. 1992 Apr 30;356(6372):801–804. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- Joag S. V., Liu C. C., Kwon B. S., Clark W. R., Young J. D. Expression of mRNAs for pore-forming protein and two serine esterases in murine primary and cloned effector lymphocytes. J Cell Biochem. 1990 May;43(1):81–88. doi: 10.1002/jcb.240430108. [DOI] [PubMed] [Google Scholar]
- Karim F. D., Urness L. D., Thummel C. S., Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A., Gunther C. V., Nye J. A. The ETS-domain: a new DNA-binding motif that recognizes a purine-rich core DNA sequence. Genes Dev. 1990 Sep;4(9):1451–1453. doi: 10.1101/gad.4.9.1451. [DOI] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- Koizumi H., Liu C. C., Zheng L. M., Joag S. V., Bayne N. K., Holoshitz J., Young J. D. Expression of perforin and serine esterases by human gamma/delta T cells. J Exp Med. 1991 Feb 1;173(2):499–502. doi: 10.1084/jem.173.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarco K., Thompson C. C., Byers B. P., Walton E. M., McKnight S. L. Identification of Ets- and notch-related subunits in GA binding protein. Science. 1991 Aug 16;253(5021):789–792. doi: 10.1126/science.1876836. [DOI] [PubMed] [Google Scholar]
- Leiden J. M. Transcriptional regulation during T-cell development: the alpha TCR gene as a molecular model. Immunol Today. 1992 Jan;13(1):22–30. doi: 10.1016/0167-5699(92)90200-q. [DOI] [PubMed] [Google Scholar]
- Lichtenheld M. G., Podack E. R. Structure and function of the murine perforin promoter and upstream region. Reciprocal gene activation or silencing in perforin positive and negative cells. J Immunol. 1992 Oct 15;149(8):2619–2626. [PubMed] [Google Scholar]
- Lichtenheld M. G., Podack E. R. Structure of the human perforin gene. A simple gene organization with interesting potential regulatory sequences. J Immunol. 1989 Dec 15;143(12):4267–4274. [PubMed] [Google Scholar]
- Liu C. C., Joag S. V., Kwon B. S., Young J. D. Induction of perforin and serine esterases in a murine cytotoxic T lymphocyte clone. J Immunol. 1990 Feb 15;144(4):1196–1201. [PubMed] [Google Scholar]
- Liu C. C., Rafii S., Granelli-Piperno A., Trapani J. A., Young J. D. Perforin and serine esterase gene expression in stimulated human T cells. Kinetics, mitogen requirements, and effects of cyclosporin A. J Exp Med. 1989 Dec 1;170(6):2105–2118. doi: 10.1084/jem.170.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod K., Leprince D., Stehelin D. The ets gene family. Trends Biochem Sci. 1992 Jul;17(7):251–256. doi: 10.1016/0968-0004(92)90404-w. [DOI] [PubMed] [Google Scholar]
- Majérus M. A., Bibollet-Ruche F., Telliez J. B., Wasylyk B., Bailleul B. Serum, AP-1 and Ets-1 stimulate the human ets-1 promoter. Nucleic Acids Res. 1992 Jun 11;20(11):2699–2703. doi: 10.1093/nar/20.11.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Mavrothalassitis G. J., Papas T. S. Positive and negative factors regulate the transcription of the ETS2 gene via an oncogene-responsive-like unit within the ETS2 promoter region. Cell Growth Differ. 1991 May;2(5):215–224. [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Molitor J. A., Walker W. H., Doerre S., Ballard D. W., Greene W. C. NF-kappa B: a family of inducible and differentially expressed enhancer-binding proteins in human T cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10028–10032. doi: 10.1073/pnas.87.24.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye J. A., Petersen J. M., Gunther C. V., Jonsen M. D., Graves B. J. Interaction of murine ets-1 with GGA-binding sites establishes the ETS domain as a new DNA-binding motif. Genes Dev. 1992 Jun;6(6):975–990. doi: 10.1101/gad.6.6.975. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Hengartner H., Lichtenheld M. G. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- Pongubala J. M., Nagulapalli S., Klemsz M. J., McKercher S. R., Maki R. A., Atchison M. L. PU.1 recruits a second nuclear factor to a site important for immunoglobulin kappa 3' enhancer activity. Mol Cell Biol. 1992 Jan;12(1):368–378. doi: 10.1128/mcb.12.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. N., Huebner K., Isobe M., ar-Rushdi A., Croce C. M., Reddy E. S. elk, tissue-specific ets-related genes on chromosomes X and 14 near translocation breakpoints. Science. 1989 Apr 7;244(4900):66–70. doi: 10.1126/science.2539641. [DOI] [PubMed] [Google Scholar]
- Scheidereit C., Cromlish J. A., Gerster T., Kawakami K., Balmaceda C. G., Currie R. A., Roeder R. G. A human lymphoid-specific transcription factor that activates immunoglobulin genes is a homoeobox protein. Nature. 1988 Dec 8;336(6199):551–557. doi: 10.1038/336551a0. [DOI] [PubMed] [Google Scholar]
- Shaw J. P., Utz P. J., Durand D. B., Toole J. J., Emmel E. A., Crabtree G. R. Identification of a putative regulator of early T cell activation genes. Science. 1988 Jul 8;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- Sommer L., Hagenbüchle O., Wellauer P. K., Strubin M. Nuclear targeting of the transcription factor PTF1 is mediated by a protein subunit that does not bind to the PTF1 cognate sequence. Cell. 1991 Nov 29;67(5):987–994. doi: 10.1016/0092-8674(91)90371-5. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Wang C. Y., Ho I. C., Bohjanen P. R., Petryniak B., June C. H., Miesfeldt S., Zhang L., Nabel G. J., Karpinski B. cis-acting sequences required for inducible interleukin-2 enhancer function bind a novel Ets-related protein, Elf-1. Mol Cell Biol. 1992 Mar;12(3):1043–1053. doi: 10.1128/mcb.12.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. C., Brown T. A., McKnight S. L. Convergence of Ets- and notch-related structural motifs in a heteromeric DNA binding complex. Science. 1991 Aug 16;253(5021):762–768. doi: 10.1126/science.1876833. [DOI] [PubMed] [Google Scholar]
- Trapani J. A., Dupont B. Novel putative promoter/enhancer sequences are shared by the mouse and human perforin (Pfp) genes. Tissue Antigens. 1990 Nov;36(5):228–234. doi: 10.1111/j.1399-0039.1990.tb01833.x. [DOI] [PubMed] [Google Scholar]
- Trapani J. A., Kwon B. S., Kozak C. A., Chintamaneni C., Young J. D., Dupont B. Genomic organization of the mouse pore-forming protein (perforin) gene and localization to chromosome 10. Similarities to and differences from C9. J Exp Med. 1990 Feb 1;171(2):545–557. doi: 10.1084/jem.171.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Nabholz M. Perforin-mediated target cell lysis by cytolytic T lymphocytes. Annu Rev Immunol. 1990;8:279–302. doi: 10.1146/annurev.iy.08.040190.001431. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Petryniak B., Ho I. C., Thompson C. B., Leiden J. M. Evolutionarily conserved Ets family members display distinct DNA binding specificities. J Exp Med. 1992 May 1;175(5):1391–1399. doi: 10.1084/jem.175.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., Ascione R., Papas T. S. Molecular analysis of the ets genes and their products. Crit Rev Oncog. 1990;1(4):409–436. [PubMed] [Google Scholar]
- Woods D. B., Ghysdael J., Owen M. J. Identification of nucleotide preferences in DNA sequences recognised specifically by c-Ets-1 protein. Nucleic Acids Res. 1992 Feb 25;20(4):699–704. doi: 10.1093/nar/20.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J. H., Cowie A., Lachance P., Hassell J. A. Molecular cloning and characterization of PEA3, a new member of the Ets oncogene family that is differentially expressed in mouse embryonic cells. Genes Dev. 1992 Mar;6(3):481–496. doi: 10.1101/gad.6.3.481. [DOI] [PubMed] [Google Scholar]
- Youn B. S., Liu C. C., Kim K. K., Young J. D., Kwon M. H., Kwon B. S. Structure of the mouse pore-forming protein (perforin) gene: analysis of transcription initiation site, 5' flanking sequence, and alternative splicing of 5' untranslated regions. J Exp Med. 1991 Apr 1;173(4):813–822. doi: 10.1084/jem.173.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D. Killing of target cells by lymphocytes: a mechanistic view. Physiol Rev. 1989 Jan;69(1):250–314. doi: 10.1152/physrev.1989.69.1.250. [DOI] [PubMed] [Google Scholar]
- Zychlinsky A., Karim M., Nonacs R., Young J. D. A homogeneous population of lymphokine-activated killer (LAK) cells is incapable of killing virus-, bacteria-, or parasite-infected macrophages. Cell Immunol. 1990 Jan;125(1):261–267. doi: 10.1016/0008-8749(90)90080-b. [DOI] [PubMed] [Google Scholar]