Abstract
Objective
To test whether reduction in hostility increases autonomic regulation of the heart.
Methods
In this randomized controlled trial, participants were 158 healthy adults, 20–45 years of age, who were one SD above national norms on the Cook-Medley Hostility and the Spielberger Trait Anger Indices. Participants also were interviewed using the Interpersonal Hostility Assessment Technique (IHAT). They were randomly assigned to a 12-week cognitive behavior therapy (CBT) program for hostility reduction or a wait list control condition. The main outcome measure was cardiac autonomic modulation, measured as RR interval variability (RRV) derived from 24-ECG recordings.
Results
In a MANOVA assessing psychological outcomes of hostility, anger, and IHAT scores, there was a significant treatment effect with an average reduction across the three outcomes that was approximately .7 SD (ES=.685, se=.184, p<.001) greater for the intervention group than for the control group.
In contrast, the change in HR was −0.14 bpm (95%CI= −2.43, 2.14) in treatment participants and −1.36 bpm (95%CI= −3.28, 0.61) in wait list participants. HF RRV, an index of cardiac parasympathetic modulation, increased by 0.07 ln msec2 (95%CI= −0.10, 0.24) for participants in the treatment condition and decreased by 0.04 ln msec2 (95%CI= −0.18, 0.10) for participants in the wait list condition. These differences were not significant. The findings for other indices of RRV were similar.
Conclusions
Reduction of hostility and anger was not accompanied by increases in cardiac autonomic modulation. These findings raise questions about the status of disordered ANS regulation of the heart as a pathophysiological mechanism underlying the hostility – heart disease relationship and about whether hostility itself is a mechanism or merely a marker of elevated risk of heart disease.
Trial Registration
clinicaltrials.gov NCT00365196
Keywords: Parasympathetic nervous system, hostility, cognitive behavior therapy, randomized controlled trial
Two hundred years ago, the British surgeon Sir John Hunter complained, “My life is at the mercy of any scoundrel who chooses to annoy me,” not long before he died of a heart attack provoked by a violent argument (1). Since that time, the contribution of hostility and anger to the development of CAD has become relatively well established although evidence linking hostility and anger to poor outcomes in patients with existing disease is less firm.
In a prospective study of 409 male and 321 female initially healthy residents of Glostrup, Denmark, Barefoot et al. showed that an abbreviated version of the Cook-Medley hostility scale predicted acute myocardial infarction and total mortality, even after control of standard risk factors (2). Data from the Kuopio Ischemic Heart Disease Risk Factor Study demonstrated that men with high levels of hostility were at greater than twice the risk of all-cause mortality, cardiovascular mortality, and myocardial infarction relative to men with lower levels. Adjustment for biological and socioeconomic risk factors did not reduce this risk although adjustment for behavioral risk factors did (3). Data from the Multiple Risk Factor Intervention Trial revealed that high hostile men were more likely than their low hostile counterparts to die of cardiovascular disease (4)
Among patients, cynical hostility was associated with progression of carotid artery atherosclerosis even after adjustment for conventional risk factors (5). In a case-control study among post-menopausal women, hostile affect from the Cook-Medley scale was linearly associated with risk for MI (6). Potential for hostility, measured by the Type A structured interview, predicted restenosis after angioplasty (7).
In a systematic analysis, Suls and Bunde (8) concluded that the evidenced linking hostility and anger to heart disease in initially healthy subjects was reasonably solid, a conclusion consistent with the findings from two older meta-analyses (9, 10). In contrast, the evidence for an effect in patients with existing disease was weaker. Another recent meta-analysis concluded that hostility and anger were associated with heart disease in both healthy and patient populations (11).
The mechanisms linking hostility and anger to elevated risk of heart disease are unclear but one candidate is dysregulation of the autonomic nervous system. Abundant evidence demonstrates that reduced autonomic regulation of the heart, measured as RR interval variability (RRV), predicts the development of heart disease in initially healthy subjects in community studies (12, 13) as well as poorer survival in patients with myocardial infarction (14–16) or heart failure (17).
Evidence also links hostility and anger to autonomic dysfunction. HF RRV during the daytime was inversely related to hostility in younger subjects (18). In the laboratory, hostility was inversely related to HF RRV at rest (19) and in response to cognitive challenge (20) and carotid-cardiac vagal baroreflex testing (21). Among hostile type A men, administration of isoproterenol was associated with reduced parasympathetic antagonism compared to the response in less hostile type B men (22). In response to cold forehead stimulation, a vagomimetic stimulus, HR deceleration was smaller in high hostile compared to low hostile subjects (23) and in type A compared to type B subjects (24). These data suggest an inverse association between hostility and cardiac vagal modulation.
Thus, considerable evidence supports associations among hostility and anger, heart disease, and autonomic nervous system dysfunction. Moreover, behavioral interventions that target hostility as part of more comprehensive stress management protocols have been shown to reduce risk factors and even disease endpoints (cf. Rozanski et al (25) for a review). Nonetheless, no randomized trials have examined whether reduction of hostility enhances cardiac autonomic regulation. The capacity of cognitive behavior therapy to successfully reduce hostility, on the other hand, is well established as several meta-analyses have determined (26, 27). In this paper, we report the results of a randomized controlled trial testing the hypothesis that a cognitively oriented hostility reduction program would improve autonomic regulation of the heart.
METHODS
Study Design
The study was a randomized controlled trial measuring the effects of cognitive behavior therapy for hostility, vs. a wait-list control, on 24-hour levels of RR interval variability (RRV). All subjects provided informed consent. The Institutional Review Board of Columbia University Medical Center approved this study.
Study Participants
We sought healthy young adults, 20–45 years of age, who were high in hostility. Subjects were recruited from the New York City metropolitan area by print and radio advertising. They received free treatment and were paid for testing sessions.
Subjects were eligible if they scored ≥ 26 on the 50 point Cook-Medley Hostility Scale (28) and ≥ 25 on the 30 point trait anger scale of the Spielberger State–Trait Anger Expression Inventory (STAXI) questionnaire (29). Exclusion criteria included current symptoms of affective disorder, psychosis, or substance abuse, current usage of psychoactive medication, and any medical condition that affected the autonomic nervous system or cardiovascular system.
Each eligible participant met individually with a clinician to determine the appropriateness of cognitive behavior therapy for the presenting anger problem. During this interview, the clinician asked about the participant’s reasons for volunteering for the study, probed for a history of violent behavior or problems with the law, and collected a brief psychiatric history to rule out serious clinical issues including mood, anxiety or personality disorders, and substance use/abuse. The clinician also reviewed the study protocol, including the possibility that the participant could be assigned to the wait list condition. Participants who were deemed to be in need of immediate treatment or for whom our CBT protocol was considered an inappropriate treatment were referred for treatment elsewhere.
Experimental Protocol
Subjects meeting enrollment criteria reported to the laboratory at 8:00 am for time-1 testing, after having eaten only a light breakfast and abstaining from caffeinated beverages. A Marquette 8500 ECG recorder was attached to two ECG leads (CM2 and CM5). After the recorder was attached, subjects were evaluated in a laboratory psychophysiology testing study. Data from this psychophysiology study will be reported elsewhere.
After completing the laboratory study, subjects resumed their regular daily activities. They removed the Holter recorder the following morning and returned it by prepaid mailer.
Once the ECG recording was returned, subjects were randomized to either active CBT treatment for hostility or to a wait-list control condition. After completion of 12 weekly sessions of treatment or the wait list, subjects returned for the time-2 testing session and 24-hour ECG monitoring. After this second session, wait-list subjects began treatment. Time-3 laboratory testing and 24-hour ECG recording was conducted as a six-month post-treatment follow-up for the treatment group and immediately after completing treatment for the wait-list group. Here we report results for the time-1 and time-2 testing sessions. Data collection staff were blind to treatment group assignment. Subjects received free treatment plus $75 for each testing session.
CBT Treatment of Hostility
The cognitive-behavioral treatment protocol involved 12 weekly individual therapy sessions, and included six categories of methods: (1) Psychoeduction, (2) Self-monitoring, (3) Cognitive Therapy, (4) Behavior Therapy (including social and communication skills training, and problem-solving training), (5) Relaxation and Visualization Exposure, and (6) In Vivo Exposure (i.e. contrived exposure to high risk situations combined with application of cognitive-behavioral skills). Protocol details have been described elsewhere (30).
Measurement of Outcome Variables
Psychological Effects of Treatment
Multiple measures of hostility were collected. The Cook-Medley and STAXI questionnaires and IHAT interview were administered at all data collection sessions. In addition to pre- and post-treatment assessments, CBT patients kept treatment diaries of anger events, and conducted daily self-ratings of anger levels throughout the 12-week treatment period. This diary was a standard cognitive-behavioral self-monitoring form on which patients describe any anger-producing episodes rated 3 or higher on a 0 to 10 scale. The second form, a daily self-rating form adapted from Borkovec and Costello (31), was completed by the patient just before going to bed at night. On this form, the patient indicated (a) the average anger level experienced that day, and (b) the maximum anger level experienced that day (0 to 10 scale).
Measurement of 24-Hour RR Interval Variability
Holter ECG signals were digitized by a Marquette 8000 or MARS scanner, and submitted to Marquette algorithms for the initial QRS labeling and editing. Errors not detected by Marquette editing software were corrected using editing algorithms developed at Columbia University (32, 33). Analyses were conducted on 5-min epochs of ECG data. A sampling interval of 292 msec was used to obtain a 1024-point time series from a series of RR intervals. Noisy segments or segments with ectopic QRS complexes were spanned by linear splines (34). For 5-minute segments of ECG, we calculated HR and spectral power in the following frequency bands: (1) 0.0033 to <0.04 Hz, very low frequency (VLF) power; (2) 0.04 to <0.15 Hz, low frequency (LF) power; and (3) 0.15 to 0.40 Hz, high frequency (HF) power. Time domain estimates (SDNN, rMSSD) also were computed. Separate estimates were generated for the full 24-hour period, daytime (7:30 – 21:30), and nighttime (00:00 – 5:00).
Statistical Analysis
Data were analyzed according to intention-to-treat principles, i.e., all subjects randomized to either condition were included in the statistical analysis regardless of whether or not they completed the study. For comparison of baseline characteristics between CBT and wait list participants, we used t-tests for continuous measures and Chi-square tests for categorical measures. These same analyses were performed for comparisons of dropouts to participants who returned.
Pearson’s two-tailed correlation was used to determine the strength and direction of the linear relationship between changes in trait measures and RRV measures. Regression lines were fit for each treatment group for visual representation of the relationships.
As descriptive analyses revealed that all three outcomes under study were positively skewed, the variable values were transformed prior to initiating the modeling procedures. An inverse probit transformation of the rank-ordered values was performed separately for each measure (pooled across sessions), ensuring that the transformed measures each had a mean of zero, standard deviation of one, and an approximately normal distribution.
To examine the effect of the intervention on psychological outcomes, a multivariate ANCOVA model was tested in which there were two within-person factors, outcome measure (3 levels: Cook Medley, Trait Anger, and IHAT) and time (2 levels: baseline, follow-up), one between-person factor (treatment group) and three between-person covariates (age, sex, BMI). Full information maximum likelihood (FIML) estimates of the model parameters were obtained, using the SAS PROC MIXED procedure, to use all available data. The resulting estimates are valid under the assumption that missing data are “missing at random”, which is a substantially less restrictive than that required by traditional least-squares estimation procedures that completely ignore those subjects that are missing any of the six outcome measures. We initially estimated a full factorial model in order to test if the treatment effect (Group*Time*Measure interaction) varied by measure. Estimates of the treatment effect for each outcome are based on the model. Given that the 3-way interaction term was non-significant, we estimated the pooled (across outcomes) treatment effect (Group*Time interaction) from a model that excludes the 3-way interaction.
RESULTS
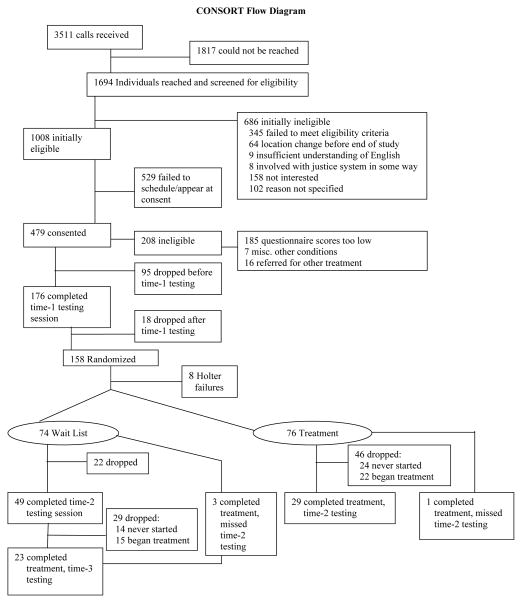
We received 3511 phone calls in response to the recruitment activities (Figure 1). Four hundred seventy nine participants provided written informed consent. Of these 479 participants, 208 were disqualified, mostly because they failed to meet Cook Medley or STAXI criteria. Ninety-five of those who met criteria dropped out of the study before initial testing. One hundred seventy six completed initial testing and of these, 18 dropped out before randomization. The remaining 158 participants were randomized to either the active treatment (N=80) or wait-list control (N=78) group. In four participants in each group, Holter recording failures were identified after randomization and start of treatment. In these participants, no pre-treatment RRV data were available and therefore, data analysis was based on 76 and 74 participants in the active treatment and wait list control condition respectively. Data collection began in November 2000 and ended in February 2005.
Figure 1.
Participant flow diagram.
The mean age of subjects was 30.6 ± 6.7 years. 46.7% were male. Table 1 presents demographic, physical, and physiological characteristics at study entry. At study entry, subjects assigned to the wait list condition had significantly lower diastolic blood pressure, marginally greater HR, and marginally lower LF power but the groups did not differ on any other variable.
TABLE 1.
Demographic, Physical, and Psychological Characteristics (Mean ± SD) of Subjects at Study Entry
| Active Treatment (N=76) | Wait List Control (N=74) | P value for t-test of group difference | |
|---|---|---|---|
| Age (yr) | 30.46 ± 6.67 | 30.84 ± 6.85 | p = 0.74 |
| Gender | 49% Male | 45% Male | p = 0.62 |
| Weight (lbs) | 158.46 ± 33.99 | 152.25 ± 32.21 | p = 0.26 |
| Height (in) | 67.80 ± 4.22 | 67.53 ± 3.88 | p = 0.69 |
| BMI (kg/m2) | 24.06 ± 3.75 | 23.38 ± 3.57 | p = 0.27 |
| 24h HR (bpm) | 75.90 ± 8.15 | 78.57 ± 9.05 | p = 0.06 |
| 24h ln HF (msec2) | 6.11 ± 0.96 | 5.93 ± 0.94 | p = 0.22 |
| 24h ln LF (msec2) | 7.00 ± 0.65 | 6.82 ± 0.61 | p = 0.07 |
| Seated SBP (mmHg) | 114.79 ± 10.48 | 113.33 ± 12.14 | p = 0.44 |
| Seated DBP (mmHg) | 73.36 ± 8.86 | 69.27 ± 8.82 | p = 0.006 |
| Cook Medley | 32.83 ± 5.87 | 32.88 ± 5.44 | p = 0.96 |
| Trait Anger | 31.04 ± 4.65 | 30.81 ± 3.91 | p = 0.75 |
Effect of Treatment on Hostility and Anger
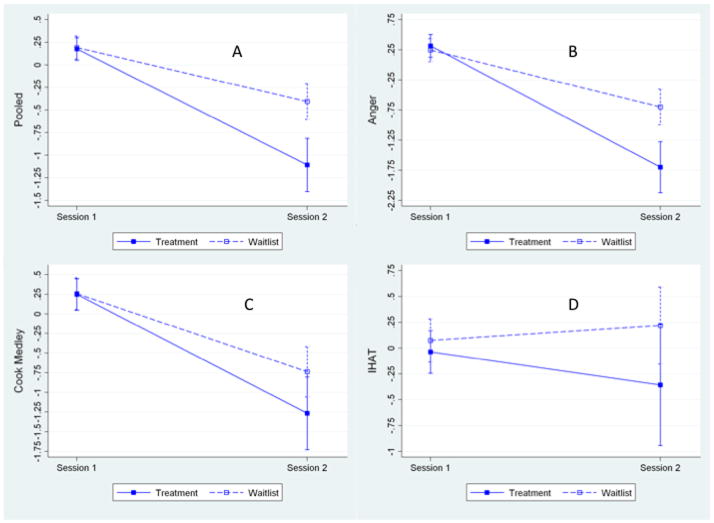
A series of mixed multivariate models (MANOVA) revealed a nonsignificant group X session X outcome measure interaction (p = .289) indicating that the effect of intervention on the groups did not differ among the three outcome variables. The group X session effect was highly significant (p < .001), with estimates indicating that the effect of the intervention in reducing outcomes was greater in the treatment group scores compared with the wait-list group. There was a significant treatment effect, with an average reduction across the three outcomes that was approximately .7 SD (ES=.685, se=. 184, p<.001) greater for the intervention group than for the control group..
Inspection of Figure 2 confirms the findings from the MANOVA, showing that for the pooled data (panel A), the reduction in outcome variables was significantly greater in the treatment group compared to the wait list group. The same pattern generally was seen for each individual outcome variable, shown in panels B–D.
Figure 2.
Change in hostility measured by the pooled estimate (panel A), trait anger (B), Cook Medley Hostility Scale (C), and IHAT (D) from time-1 (pre treatment) to time-2 (post treatment) in the treatment and wait-list control groups. IHAT = interpersonal hostility assessment technique.
We further explored the findings by computing post-hoc contrasts of the effect of the intervention in the treatment group only for each outcome variable separately. CBT treatment led to a significant reduction in Anger (−1.071 ± .286, p < .001). In the treatment group, hostility was marginally reduced (−0.519 ± .298, p = .084). The effect of treatment on the IHAT was nonsignificant (−0.464 ± .351, p = .188).
Forty-six of the 76 participants (61%) assigned to the treatment group were considered to be dropouts, either because they never began treatment (N=24) or began but failed to return for testing (N=22). Of the thirty who completed treatment, one failed to complete time-2 testing. Thus, among those randomized to treatment, the dropout rate was 61% (46/76).
Twenty-two of the 74 participants (30%) assigned to the wait list condition failed to return for the second testing session. Forty-nine participants completed time-2 testing and three missed this testing session but completed treatment. Of these 49, 14 who never started treatment and 15 who began but did not return for time-3 testing session were considered to be dropouts. Among those randomized to the wait list condition, the dropout rate was 59% 29/49).
Few differences between participants who returned for testing and those who dropped out were found. Compared to dropouts, those who returned had lower HF (p<0.001) and LF power (p=0.03), lower SDRR (p = .03), and DBP (p=0.02) at study entry. Those who returned had marginally higher HR and were marginally older. They did not differ on other variables.
Effect of Treatment on RR Interval Variability
Table 2 presents the data on HR and RR variability for the 24-hour recordings and for daytime and nighttime. The relationship between time (pre and post treatment) and HR was not significantly different for the two groups (p = 0.43). Similarly, the relationships between time (pre and post treatment) and mean 24-hour HF, LF, VLF power and the time domain indices SDNN and rMSSD did not significantly differ between the CBT and wait list groups. Mixed effect models adjusted for age, sex and BMI showed no significant group X session interaction.
TABLE 2.
LEAST SQUARE MEANS OF INTERACTION EFFECT FROM MIXED MODEL ANALYSES
| Treatment Group | Wait-List Controls | ||||
|---|---|---|---|---|---|
| Pre-treatment (N=76) | Post-treatment (N=30) | Pre-treatment (N=74) | Post-treatment (N=50) | ||
| 24-hours | HR (bpm) | 75.59 ± 0.96 | 75.45 ± 1.12 | 77.94 ± 0.98 | 76.61± 0.94 |
| ln VLF | 8.00 ± 0.06 | 7.93 ± 0.08 | 7.88 ± 0.06 | 7.85 ± 0.07 | |
| ln LF | 7.16 ± 0.07 | 7.15 ± 0.07 | 6.99 ± 0.07 | 6.99 ± 0.06 | |
| ln HF | 6.30 ± 0.10 | 6.36 ± 0.11 | 6.15 ± 0.10 | 6.11 ± 0.10 | |
| ln SD | 5.00 ± 0.03 | 4.97 ± 0.04 | 4.97 ± 0.03 | 4.93 ± 0.03 | |
| ln rMSSD | 3.76 ± 0.05 | 3.77 ± 0.05 | 3.66 ± 0.05 | 3.69 ± 0.05 | |
| Daytime | HR (bpm) | 80.17 ± 1.01 | 80.52 ± 1.27 | 82.94 ± 1.03 | 81.04 ± 1.07 |
| ln VLF | 7.99 ± 0.06 | 7.91 ± 0.07 | 7.86 ± 0.06 | 7.85 ± 0.06 | |
| ln LF | 7.09 ± 0.06 | 7.09 ± 0.07 | 6.92 ± 0.06 | 6.94 ± 0.06 | |
| ln HF | 6.06 ± 0.09 | 6.10 ± 0.11 | 5.89 ± 0.10 | 5.92 ± 0.10 | |
| ln SD | 4.85 ± 0.03 | 4.81 ± 0.04 | 4.82 ± 0.03 | 4.79 ± 0.03 | |
| ln rMSSD | 3.64 ± 0.04 | 3.66 ± 0.05 | 3.53 ± 0.04 | 3.60 ± 0.05 | |
| Nighttime | HR (bpm) | 67.78 ± 1.13 | 67.02 ± 1.32 | 68.21 ± 1.17 | 67.90 ± 1.14 |
| ln VLF | 7.78 ± 0.07 | 7.76 ± 0.10 | 7.77 ± 0.08 | 7.74 ± 0.09 | |
| ln LF | 7.05 ± 0.09 | 7.11 ± 0.11 | 7.00 ± 0.10 | 6.94 ± 0.10 | |
| ln HF | 6.39 ± 0.12 | 6.50 ± 0.16 | 6.44 ± 0.13 | 6.25 ± 0.14 | |
| ln SD | 4.69 ± 0.04 | 4.56 ± 0.11 | 4.63 ± 0.04 | 4.49 ± 0.09 | |
| ln rMSSD | 3.84 ± 0.06 | 3.87 ± 0.11 | 3.83 ± 0.06 | 3.72 ± 0.09 | |
Separate analysis of daytime and nighttime RR interval and RRV yielded similar results. There were no significant group X session interactions. A single marginally significant interaction emerged for nighttime HF power (p = 0.09).
We conducted supplementary analyses of changes in RR interval and RRV only in subjects who returned for post-treatment (or wait list) testing. In these analyses, group X session interactions were not significant except for nighttime HF power (p = 0.06). On average, HF increased from pre- to post-treatment testing by 0.15 ln msec2 (95%CI=−0.11, 0.41) in the treatment group while it decreased by 0.19 ln msec2 (95%CI=−0.41, 0.04) in the wait-list group.
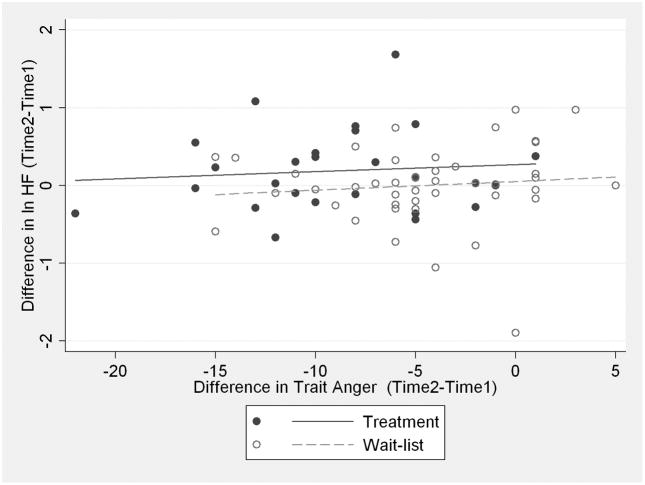
In a second set of supplementary analyses, we examined the relationship between changes in hostility and anger and changes in RRV. Pearson correlation coefficients were small, ranging from −.13 to .06. None was significant. Figure 3 presents data for HF power and trait anger.
Figure 3.
Scatterplot of changes in trait anger from time-1 to time-2 as they relate to changes in HF power from time-1 to time-2. Closed circles represent the treatment group. Open circles represent the wait list group.
DISCUSSION
The role of psychological factors in the development of coronary artery disease now is well established. Such characteristics as hostility, depression, and anxiety confer risk of CAD among healthy people and of cardiac events in patients with existing heart disease. Moreover, these psychological characteristics all appear to be associated with autonomic nervous system (ANS) dysregulation that may provide a mechanistic link between these traits and CAD. While many studies demonstrate that CBT programs can successfully reduce hostility and anger, there have been no studies examining the capacity of these hostility-reducing interventions to alter the ANS pathophysiologic mechanisms thought to link the ANS to heart disease.
Results of this randomized controlled trial demonstrate that as expected, cognitive behavior therapy reduced indices hostility and anger. This finding is consistent with other CBT-based hostility reduction treatments (26, 27). Nonetheless, successful hostility reduction treatment had no impact on autonomic regulation of the heart as measured by RR interval variability. This conclusion pertains to intention to treat analyses as well as analyses restricted to only those subjects who returned for post-treatment testing. Moreover, across all subjects, there was no relationship between changes in hostility and anger and changes in RRV.
There are several possible interpretations of the negative findings of our study. First, inverse associations between hostility and RRV are based on observational studies and as recent reports have shown, randomized trials sometimes fail to support findings from these studies. For example, epidemiologic evidence indicates that diets high in carotenoid-rich fruits and vegetables and high serum levels of vitamin E and beta-carotene are associated with a reduced risk of lung cancer. Nevertheless, a randomized trial of dietary supplementation with alpha-tocopherol or beta-carotene found no reduction in the incidence of lung cancer (35). Similarly epidemiological evidence of a cardioprotective effect of hormone replacement therapy has not been supported by randomized trials (36).
Of course, these failures to support the findings of observational studies involved tests of disease endpoints while our study tested the impact on a putative mechanism. Nevertheless, it is possible that associations between hostility and cardiac autonomic control are the products of residual confounding (37). Rather than being in the causal pathway to ANS dysfunction, hostility may function as a marker of it. If so, efforts to reduce hostility may succeed, as we have shown, yet have no impact on the ANS and as such, on the risk of CHD.
Second, the failure to support the hypothesis may be due to the very high dropout rate that impeded the ability to detect an effect of treatment. Indeed, the dropout rate for participants who did not return for the second RRV evaluation was 61% in the treatment group, in contrast to the much lower rate of 30% in the wait list group. However, because neither the analysis of the effect of treatment only in subjects who returned for post-treatment testing nor the correlational analysis of changes in hostility and anger and RRV showed any effect, it is unlikely that the high dropout rate is responsible for the failure to support the hypothesis.
Moreover, based on the results of the Proc Mixed analysis, the standard deviation of the change scores for HF power was .49 and the observed treatment effect (the differential change between groups) was .109. Based on this, even with zero attrition, the p values for this magnitude of difference would have been .18, and the power of the study to detect this magnitude of effect would have been well under 50%. Thus, our findings are likely due to the small magnitude of the effect of treatment on measures of autonomic regulation and not attrition.
Third, it is possible that although the treatment had a statistically significant effect on hostility, its true effect on RR interval variability was obscured by an insufficiently powerful intervention. To examine this possibility, we identified the four participants who had the greatest reduction in hostility and anger and compared them to the three participants who had the least reduction in these indices. In the former group, ln HF power increased by .23 msec2, compared to an increase of .01 msec2 in subjects in the latter group. For ln LF power, the increases were .31 msec2 and .04 msec2 respectively. Thus, it is possible that in order to achieve increases in cardiac autonomic control, very powerful anger and hostility-reducing effects are required, which are of greater magnitude than the hostility reduction effects achieved in this trial.
Fourth, it may be that the failure to alter indices of cardiac autonomic regulation occurred because the link between hostility and anger and the autonomic nervous system is at best weak or spurious. Two recently published reports based on large community samples demonstrated that while depression was inversely related to HF power, trait anger was not (38, 39). Yeragani and Kumar also found no relationship between hostility and RRV (40).
Limitations
The association between hostility and CHD is well established, even if our findings on the effect of hostility and anger reduction on cardiac autonomic regulation were negative. Other mechanisms, e.g., increased platelet aggregability, enhanced inflammatory activity, or increased expression of the metabolic syndrome have been proposed as pathophysiological factors linking hostility to CHD (41). Our failure to demonstrate an autonomic effect of hostility reduction tells us nothing about an effect of hostility reduction on these other possible mechanisms.
Attrition in the study was far greater than expected, raising the possibility that the lack of an effect of hostility reduction on cardiac autonomic regulation was due to insufficient statistical power. While attrition reduced the statistical power, this cannot account for the lack of a significant finding. The observed treatment effect was so small that even with zero attrition, this magnitude of effect would not have been significant; the p-value would still have exceeded 0.15.
Nevertheless, the high attrition rate merits some consideration. High dropout rates are common in behavioral treatment programs, suggesting that participants see them as too burdensome. At the same time, one possible account of the failure to demonstrate an effect of treatment on autonomic regulation was that the intervention was not sufficiently powerful. These two considerations – participant burden and intervention intensity – are at odds with each other and a challenge for behavioral medicine is to design future interventions that are sufficiently powerful while not excessively burdensome.
CONCLUSIONS
In this randomized controlled trial, cognitive behavioral treatment succeeded in reducing hostility and anger compared to a wait list control group but hostility reduction had no impact on autonomic regulation of the heart. Because evidence consistently has supported an association between hostility and CHD, these findings are consistent with several possibilities: that autonomic dysfunction is not a pathophysiological mechanism in the hostility-CHD pathway, i.e., is merely a marker of an elevated risk of CHD, or that CBT-based hostility reduction can achieve an autonomic enhancing effect only for participants with dramatic reduction in hostility.
Acknowledgments
This study was supported by grant R01 HL63872 from the National Heart, Lung, and Blood Institute (Dr. Sloan), by grant K02 MH01491 from the National Institute of Mental Health (Dr. Sloan), and by the Nathaniel Wharton Fund. We are grateful to Joseph Schwartz, Ph.D., for assistance in statistical analyses.
Abbreviations
- SD
Standard Deviation
- CBT
Cognitive Behavior Therapy
- RRV
RR interval variability
- ECG
Electrocardiogram
- HR
Heart rate
- HF
High frequency
- ln
Natural log
- CI
Confidence interval
- ANS
Autonomic nervous system
- CAD
Coronary artery disease
- MI
Myocardial infarction
- STAXI
State trait anger expression inventory
- VLF
Very low frequency
- LF
Low frequency
- rMSSD
Root mean squared successive differences
- SDNN
Standard deviation of normal to normal RR intervals
- BMI
Body mass index
- CHD
Coronary heart disease
References
- 1.Wilkins R. The Doctor’s Quotation Book. New York: Barnes and Noble Books; 1992. [Google Scholar]
- 2.Barefoot JC, Larsen S, Von der Lieth L, Schroll M. Hostility, incidence of acute myocardial infarction, and mortality in a sample of older Danish men and women. Am J Epidemiol. 1995;142:477–84. doi: 10.1093/oxfordjournals.aje.a117663. [DOI] [PubMed] [Google Scholar]
- 3.Everson SA, Kauhanen J, Kaplan GA, Goldberg DE, Julkunen J, Tuomilehto J, Salonen JT. Hostility and increased risk of mortality and acute myocardial infarction: The mediating role of behavioral risk factors. Am J Epidemiol. 1997;146:142–52. doi: 10.1093/oxfordjournals.aje.a009245. [DOI] [PubMed] [Google Scholar]
- 4.Matthews KA, Gump BB, Harris KF, Haney TL, Barefoot JC. Hostile Behaviors Predict Cardiovascular Mortality Among Men Enrolled in the Multiple Risk Factor Intervention Trial. Circulation. 2004;109:66–70. doi: 10.1161/01.CIR.0000105766.33142.13. [DOI] [PubMed] [Google Scholar]
- 5.Everson SA, Kauhanen J, Julkunen J, Kaplan GA, Goldberg DE, Salonen R, Salonen JT. Cynical hostility and the progression of carotid atherosclerosis. Annals of Behavioral Medicine. 1997;19 (Suppl):S073. [Google Scholar]
- 6.Lahad A, Heckbert SR, Koepsell TD, Psaty BM, Patrick DL. Hostility, aggression and the risk of nonfatal myocardial infarction in postmenopausal women. Journal of Psychosomatic Research. 1997;43:183–95. doi: 10.1016/s0022-3999(96)00369-8. [DOI] [PubMed] [Google Scholar]
- 7.Goodman M, Quigley J, Moran G, Meilman H, Sherman M. Hostility predicts restenosis after percutaneous transluminal coronary angioplasty. Mayo Clinic Proceedings. 1996;71:729–34. doi: 10.1016/S0025-6196(11)64836-2. [DOI] [PubMed] [Google Scholar]
- 8.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 9.Booth-Kewley S, Friedman HS. Psychological predictors of heart disease: A quantitative review. Psycholog Bull. 1987;101:343–62. [PubMed] [Google Scholar]
- 10.Miller TQ, Smith TW, Turner CW, Guijarro ML, Hallet AJ. A meta-analytic review of research on hostility and physical health. Psycholog Bull. 1996;119:322–48. doi: 10.1037/0033-2909.119.2.322. [DOI] [PubMed] [Google Scholar]
- 11.Chida Y, Steptoe A. The Association of Anger and Hostility With Future Coronary Heart Disease: A Meta-Analytic Review of Prospective Evidence. Journal of the American College of Cardiology. 2009;53:936–46. doi: 10.1016/j.jacc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 12.Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Rautaharju P, Heiss G. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1997;145:696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji H, Larson MG, Venditti FJ, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events: The Framingham Heart Study. Circulation. 1996;94:2850–5. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 14.Bigger JT, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–71. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 15.Kleiger RE, Miller JP, Bigger JT, Moss AJ the Multicenter Post-Infarction Research Group. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–62. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 16.La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. The Lancet. 1998;351:478–84. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 17.La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, Ferrari R, Franchini M, Gnemmi M, Opasich C, Riccardi PG, Traversi E, Cobelli F. Short-Term Heart Rate Variability Strongly Predicts Sudden Cardiac Death in Chronic Heart Failure Patients. Circulation. 2003;107:565–70. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- 18.Sloan RP, Shapiro PA, Bigger JT, Bagiella E, Steinman RC, Gorman JM. Cardiac autonomic control and hostility in healthy subjects. Am J Cardiol. 1994;74:298–300. doi: 10.1016/0002-9149(94)90382-4. [DOI] [PubMed] [Google Scholar]
- 19.Demaree HA, Everhart DE. Healthy high-hostiles: reduced parasympathetic activity and decreased sympathovagal flexibility during negative emotional processing. Personality and Individual Differences. 2004;36:457–69. [Google Scholar]
- 20.Sloan RP, Bagiella E, Shapiro PA, Kuhl JP, Chernikhova D, Berg J, Myers MM. Hostility, gender, and cardiac autonomic control. Psychosomatic Medicine. 2001;63:434–40. doi: 10.1097/00006842-200105000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Graham RE, Zeichner A, Peacock LJ, Dishman RK. Bradycardia during baroreflex stimulation and active or passive stressor tasks: cardiorespiratory fitness and hostility. Psychophysiology. 1996;33:566–75. doi: 10.1111/j.1469-8986.1996.tb02433.x. [DOI] [PubMed] [Google Scholar]
- 22.Fukudo S, Lane JD, Anderson NB, Kuhn CM, Schanberg SM, McCown N, Muranaka M, Suzuki J, Williams RB. Accentuated vagal antagonism of β-adrenergic effects on ventricular repolarization: Evidence of weaker antagonism in hostile type A men. Circulation. 1992;85:2045–53. doi: 10.1161/01.cir.85.6.2045. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz JM, Uchino BN, Smith TW. Hostility and sex differences in the magnitude, duration, and determinants of heart rate response to forehead cold pressor: Parasympathetic aspects of risk. International Journal of Psychophysiology. 2006;60:274–83. doi: 10.1016/j.ijpsycho.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Muranaka M, Monou H, Suzuki J, Lane JD, Anderson NB, Ku CM, Schanberg SM, McCown N, Williams RB. Physiologic response to catecholamine influsions in type A and type B men. Health Psychol. 1988;7:145–63. [PubMed] [Google Scholar]
- 25.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: The emerging field of behavioral cardiology. Journal of the American College of Cardiology. 2005;45:637–51. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Del Vecchio T, O’Leary KD. Effectiveness of anger treatments for specific anger problems: a meta-analytic review. Clin Psychol Rev. 2004;24:15–34. doi: 10.1016/j.cpr.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 27.DiGiuseppe R, Tafrate RC. Anger treatment for adults: a meta-analytic review. Clinical Psychology: Science & Practice. 2003;10:70–84. [Google Scholar]
- 28.Cook WW, Medley DM. Proposed hostility and pharisiac-virtue scales for the MMPI. Journal of Applied Psychology. 1954;38:414–8. [Google Scholar]
- 29.Spielberger CD. State-Trait Anger Expression Inventory. Tampa: Psychological Assessment Resources; 1988. [Google Scholar]
- 30.Gorenstein EE, Tager F, Shapiro PA, Monk C, Sloan RP. Cognitive-Behavior Therapy for Reduction of Persistent Anger. Cognitive and Behavioral Practice. 2007;14:168–84. [Google Scholar]
- 31.Borkovec TD, Costello E. Efficacy of applied relaxation and cognitive-behavioral therapy in the treatment of generalized anxiety disorder. Journal of Consulting and Clinical Psychology. 1993;61:611–9. doi: 10.1037//0022-006x.61.4.611. [DOI] [PubMed] [Google Scholar]
- 32.Birman KP, Rolnitzky LM, Bigger JT. A shape oriented system for automated holter ecg analysis. Computers in Cardiology. 1978:217–20. [Google Scholar]
- 33.Rottman JN, Steinman RC, Albrecht P, Bigger JT, Rolnitzky LM, Fleiss JL. Efficient estimation of the heart period power spectrum suitable for physiologic or pharmacologic studies. Am J Cardiol. 1990;66:1522–4. doi: 10.1016/0002-9149(90)90551-b. [DOI] [PubMed] [Google Scholar]
- 34.Albrecht P, Cohen RJ. Estimation of heart rate power spectrum bands from real-world data: Dealing with ectopic beats and noisy data. Computers in Cardiology. 1988;15:311–4. [Google Scholar]
- 35.Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 36.Beral V, Banks E, Reeves G. Evidence from randomised trials on the long-term effects of hormone replacement therapy. Lancet. 2002;360:942–4. doi: 10.1016/S0140-6736(02)11032-4. [DOI] [PubMed] [Google Scholar]
- 37.Lawlor DA, Davey Smith G, Kundu D, Bruckdorfer KR, Ebrahim S. Those confounded vitamins: what can we learn from the differences between observational versus randomised trial evidence? Lancet. 2004;363:1724–7. doi: 10.1016/S0140-6736(04)16260-0. [DOI] [PubMed] [Google Scholar]
- 38.Bleil ME, Gianaros PJ, Jennings JR, Flory JD, Manuck SB. Trait Negative Affect: Toward an Integrated Model of Understanding Psychological Risk for Impairment in Cardiac Autonomic Function. Psychosomatic Medicine. 2008 doi: 10.1097/PSY.0b013e31816baefa. PSY.0b013e31816baefa. [DOI] [PubMed] [Google Scholar]
- 39.Ohira T, Roux AV, Prineas RJ, Kizilbash MA, Carnethon MR, Folsom AR. Associations of psychosocial factors with heart rate and its short-term variability: multi-ethnic study of atherosclerosis. Psychosomatic Medicine. 2008;70:141–6. doi: 10.1097/PSY.0b013e318160686a. [DOI] [PubMed] [Google Scholar]
- 40.Yeragani VK, Kumar HV. Heart period and QT variability, hostility, and type-A behavior in normal controls and patients with panic disorder. Journal of Psychosomatic Research. 2000;49:401–7. doi: 10.1016/s0022-3999(00)00185-9. [DOI] [PubMed] [Google Scholar]
- 41.Williams RB, Barefoot JC, Schneiderman N. Psychosocial Risk Factors for Cardiovascular Disease: More Than One Culprit at Work. JAMA. 2003;290:2190–2. doi: 10.1001/jama.290.16.2190. [DOI] [PubMed] [Google Scholar]