Abstract
INTRODUCTION
We investigated the expression of angiotensin receptors in early pregnancy and established whether normal pregnancy or preeclampsia alters the expression and distribution of the uteroplacental AT1R, AT2R and mas/AT1-7R at late gestation.
METHODS
The percentage of each receptor subtype present in tissues from virgin rats and from normotensive and RUPP hypertensive rats was established by in vitro receptor autoradiography. Receptor mRNA levels were determined by quantitative PCR at early and late pregnancy.
RESULTS
AT1R mRNA levels were up-regulated in the interimplantation (IIS) site at day 7 of gestation. AT2R mRNA levels were decreased at day 5 and 7 in the IIS but increased in the implantation site (IS) at day 5 and 7 as compared to the IIS at day 5. Mas/AT1-7R mRNA was increased in early pregnancy. In normal pregnancy and RUPP the mRNA for all angiotensin receptors was reduced in the uterus at late gestation. The AT1R accounted for the majority of binding in the uterus of virgin and the placenta of pregnant and RUPP. In RUPP pregnancy there was a significant competition with D-Ala in the placenta labyrinth.
DISCUSSION and CONCLUSION
The expression of angiotensin receptors suggests their involvement in the maintenance of early stages of pregnancy. During late gestation down-regulation of Ang receptors in the uterus may arise from feedback down-regulation by Ang II. In the placenta the levels of AT1Rs are equivalent in the RUPP model. The increased binding of mas/AT1-7R at late gestation in RUPP may represent a compensatory mechanism to reduce uteroplacental vascular resistance.
Keywords: angiotensin receptors, renin-angiotensin system, pregnancy, placenta, uterus, preeclampsia
INTRODUCTION
The renin-angiotensin system (RAS) plays an important role during pregnancy and in the development of preeclampsia [1; 2]. The counter-regulatory actions of angiotensin II (Ang II) and vasodilatory peptide angiotensin-(1-7) [Ang-(1-7)] are demonstrated in the regulation of blood pressure, vasoconstriction, cell proliferation and apoptosis [3; 4], suggesting opposing actions of these two peptides on hemodynamics and uteroplacental development. Normal human pregnancy is associated with an up-regulation of circulating renin, Ang II [5], and Ang-(1-7); however during preeclampsia the systemic levels of Ang II and Ang-(1-7) were reduced [5]. On the other hand, in the uteroplacental unit Ang II is increased with no change in Ang-(1-7) during preeclampsia [6; 1; 7; 8] suggesting that an enhanced local production of Ang II may have detrimental paracrine actions in this region.
Our studies previously demonstrated augmented Ang II at late gestation in the rat uterus [9]. However, in a rat model of preeclampsia (the reduced uterine perfusion pressure (RUPP)), Ang II was reduced in the placenta, while Ang-(1-7) was decreased in the uterus or placenta between RUPP and normal pregnant rats [9]. These studies uncovered the shifts in the angiotensin peptide profiles within the uteroplacental unit associated with hypertensive pregnancy. The significance of changes in angiotensin peptides is dependent on the distribution and expression of their receptors. Angiotensin receptors are differentially distributed in normal human pregnancy [6; 8]. In the report of Anton et al, [6] we used two methods to characterize Ang receptors, receptor autoradiography and RT-PCR. With autoradiography the AT1R was the predominate receptor subtype and the binding was not changed with preeclampsia; with the measurement of the AT1R mRNA expression in the placenta there was an increase in AT1R mRNA expression with preeclampsia. The difference in protein binding and gene expression has been observed previously. Nevertheless, our data which also included an increase in Ang II levels in the placenta with preeclampsia would suggest an activated RAS in preeclampsia with the high levels of Ang II binding at the AT1R [6; 8]. These data suggest that failure to suppress the placental RAS in preeclampsia may lead to vasoconstriction and fetal growth restriction [8]. A number of studies have described a non-AT1/non-AT2 receptor in the uteroplacental unit [10]. With the discovery of a selective Ang-(1-7) receptor antagonist, [D-alanine7-angiotensin-(1-7)] (D-Ala) that does not interact with AT1 nor AT2 receptors and specifically inhibits Ang-(1-7) effects, the mas/AT1-7 - G protein coupled receptor has been characterized [6]. However there are limited data on the expression of angiotensin receptors during early pregnancy and no data on spatial distribution and expression of angiotensin receptors in the RUPP model of preeclampsia. Therefore, the goals of this study were to determine the expression of angiotensin receptors in early pregnancy and to establish whether normal pregnancy or preeclampsia (RUPP) alters the expression and spatial distribution of the uteroplacental angiotensin receptors (AT1R, AT2R and mas/AT1-7R) at late gestation.
METHODS
Animals
Age matched female Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN, USA) and housed under a 12 h light/dark cycle in an AAALAC-approved facility. Day 0 of pregnancy was designated as the day when sperm were found on the vaginal smear. All protocols were approved by the Animal Care and Use Committee of Wake Forest School of Medicine and are in compliance with NIH guidelines.
Experimental Procedures
On day 5 of pregnancy (the day after implantation), implantation sites (IS) were identified by increased uterine vascular permeability at the site of the blastocyst attachment through intra-cardiac injection of 0.2 ml of 0.5% Evans blue dye under light anesthesia The animals were allowed to recover fully and then were sacrificed by decapitation. At day 5 and 7 of pregnancy IS or interimplantation sites (IIS) of the uterus were collected for mRNA analysis. Implantation sites (IS) were separated from interimplantation sites (IIS) and frozen at −80°C until processed for mRNA analysis. About 50 mg of tissue was used for the quantitative PCR (requiring a pool of IS (n = 2–3) and IIS n = 3–4 from the same animal for single determinations). Uterine tissue from virgin at estrus was used as a control for early gestation, as previously published [9], because this phase precedes ovulation and implantation.
Surgical Procedures
On day 14 of pregnancy, rats were anesthetized with isoflurane and were either sham operated or prepared for RUPP surgery [9; 11]. Briefly, silver clips were placed around the descending aorta just above the iliac bifurcation (0.203 mm) and around both right and left uterine arteries (0.221 mm).
Blood pressure recordings and tissue collection
Two days before sacrifice animals were anesthetized with 2% isoflurane and the carotid artery was cannulated. On day 19 of pregnancy blood pressures were recorded (Biopac System, Santa Barbara, CA) in conscious animals. Following decapitation the uterus and the placenta were rapidly removed and frozen at −80°C. Uterine tissues from virgin animals at diestrus phase of the estrus cycle were used in the late gestation study, since this is a stage at which the levels of 17-β estradiol are the lowest. Two days before sacrifice the animals were placed in metabolic cages for 24h urine collection.
Autoradiography
Tissues were sectioned at 14 μm and receptor autoradiography was performed using 125I-[Sarcosine1, Threonine8]-Ang II (125I-SarThran). 125I-SarThran was used at a concentration of 0.6 nM to determine the apparent maximal density of receptors [6]. A lower concentration of 125I-SarThran (0.2 nM) was used in the presence or absence of 3 μM losartan (AT1R antagonist), PD 123319 (AT2R antagonist) or D-Ala7-Ang-(1-7) (mas/AT1-7R antagonist) to determine the percentage of each receptor subtype present [12; 13]. Sections were exposed to film and were analyzed using a computerized densitometry system (MCID) [12; 13]. The film was quantified using standards and the amount of radioactivity bound to tissue sections was expressed in fmoles bound per milligram tissue equivalent [14]. Data for binding density were expressed as the amount of total binding attributed to each receptor subtype as determined by the competition study.
RNA isolation and quantitative PCR
Total RNA was isolated from tissue and reverse transcribed as described [15; 16]. The RNA concentration and integrity were assessed using an Agilent 2100 Bioanalyzer with an RNA 6000 Nano LabChip (Agilent Technologies, Palo Alto, CA). Approximately 1 μg of total RNA was reverse transcribed. The primer/probe sets were purchased from Applied Biosystems (Foster City, CA). The results were quantified as Ct values and defined as relative gene expression (the ratio of target/control).
Statistical analysis
Comparisons between the groups were performed using one way analysis of variance (ANOVA) followed by the Newman-Keul’s post-hoc test for multiple comparisons or unpaired Student’s t-test (GraphPad Software, San Diego, CA). A p value less than 0.05 was considered statistically significant different. All values are presented as mean ± SEM.
RESULTS
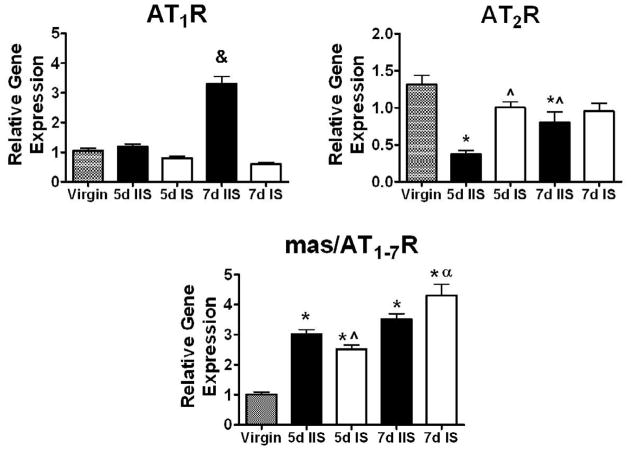
Fig. 1 shows the mRNA levels of AT1, AT2 and mas/AT1-7 receptors in the IS and IIS uterine sites at day 5 and 7 of early pregnancy. The AT1R mRNA levels were increased 3-fold at day 7 in the IIS as compared to virgin uterus and to pregnant uterus at day 5 in the IIS and at day 7 in the IS (p<0.0001). The AT2R was markedly decreased at day 5 and 7 in the IIS as compared to virgin rats (p<0.0001). There was a significant increase in the AT2R at day 5 in the IS compared to IIS (p<0.0001). There was an overall increase in mas/AT1-7R mRNA levels with pregnancy in a temporal and spatial manner as compared to virgin rats (p<0.0001). The mas/AT1-7R mRNA levels at day 7 in the IS were higher as compared to day 5 IS (p<0.0001).
Fig. 1.
Uterine AT1R, AT2R and mas/AT1-7R mRNA in virgin and day (d) 5 and 7 pregnant Sprague-Dawley rats. AT1R, AT2R and mas/AT1-7R mRNA from the interimplantation (IIS) and implantation (IS) sites at day 5 and 7 of gestation are expressed relative to control. Values are mean ± SEM, &p<0.0001 vs. virgin (n=6), 5d IIS (n=9), 5d IS (n=9), and 7d IS (n=9), *p<0.0001 vs. virgin, ^p<0.0001 vs. 5d IIS, ap<0.0001 vs. 5d IS.
A significant increase of 12% was observed in MAP in RUPP as compared to normal pregnant animals (Table 1). RUPP animals also had 34% fewer fetuses with smaller fetal weight and length (p<0.001). There was also lower circulating estradiol in RUPP animals as compared with pregnant (p<0.0001), but levels were higher than in virgin rats (p<0.0001) [9]. Urinary protein excretion tended to increase in RUPP pregnant rats.
Table 1.
Basal Characterization of Virgin, 19-day Pregnant and RUPP Sprague-Dawley Rats.
| Virgin | Pregnant | RUPP | |
|---|---|---|---|
| Body weight (g) | 234±2.5 | 312±6.2* | 278±4.9*# |
| Litter size (n) | - | 11.5±0.7 | 7.6±0.8# |
| Pup crown to rump length (cm) | - | 2.92±0.04 | 2.63±0.05# |
| Pup weight (g) | - | 2.34±0.09 | 1.93±0.07# |
| # Pups reabsorbed | - | 0.31±0.17 | 5.86±0.92# |
| MAP (mmHg) | - | 108±1.6 | 121±1.0# |
| 17β-Estradiol (pmol/l) | 31±3.2 | 191±20* | 104±16.0*# |
| Urine Volume (ml) | - | 28.9±2.3 | 24.9±2.0 |
| Protein (mg/24h) | - | 8.1±1.3 | 11.9±3.3 |
| Urinary Protein/Creatinine Ratio (mg of protein/mg of urinary creatinine) | - | 0.7±0.14 | 1.2±0.30 |
Values are expressed as mean ± SEM.
p < 0.05 vs. virgin,
p < 0.05 vs. pregnant; n=14–16.
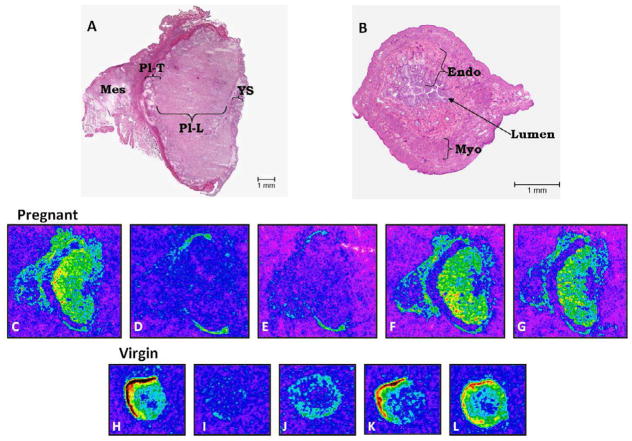
Fig. 2 shows the distribution of 125I-Sarthran binding in the uterus of virgin (Fig. 2B, H–L) and in the placenta of 19 days pregnant rats (Fig. 2A, C–G). Highest binding was observed in the myometrium of the uterus from virgin animals (Fig. 2B, H and Fig. 3). In pregnant rats, equivalent binding was observed between the labyrinth placenta and the mesometrial triangle (Fig. 2A and C) and there was no difference between normal pregnant and RUPP (Fig. 4). No receptor expression was found in the trophospongium region of the placenta (Fig. 2A and C).
Fig. 2.
Distribution of 125I-Sarthran binding in the uterus of virgin and in the uteroplacental unit of 19 day pregnant Sprague-Dawley rats. Panels A show H&E staining of the transverse section of the uteroplacental unit from pregnant rats. Panels B show H&E staining of the transverse section of the uterus from virgin rats. Panels C and H - Total binding; D and I - Non-specific binding in presence of 0.2 nM unlabeled Sarthran; E and J - AT1 receptors determined in the presence of 3 μM Losartan (amount of binding left is due to non-AT1 receptors); F and K - AT2 receptors determined in the presence of 3 μM PD123319; G and L - mas/AT1-7 receptors determined in the presence of 3 μM D-Ala. Pl-T - Trophospongium area of placenta. Pl-L - Labyrinth area of placenta. Mes - Mesometrial triangle. Endo - Endometrium. Myo - Myometrium. YS - Yolk Sac.
Figure 3.
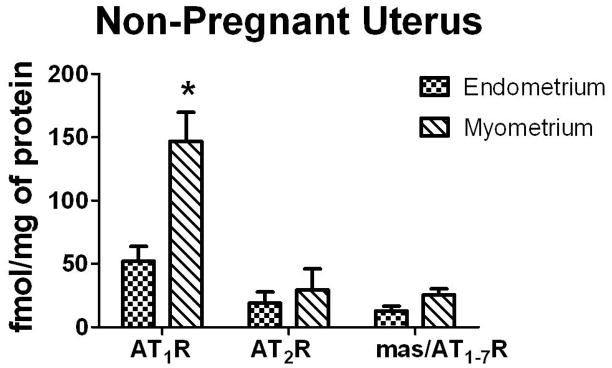
Angiotensin subtype receptor density in the endometrium and myometrium of the uterus of virgin Sprague-Dawley rats. Values are expressed as mean ± SEM, *p<0.001 vs. endometrium, n=9 in each group.
Fig. 4.
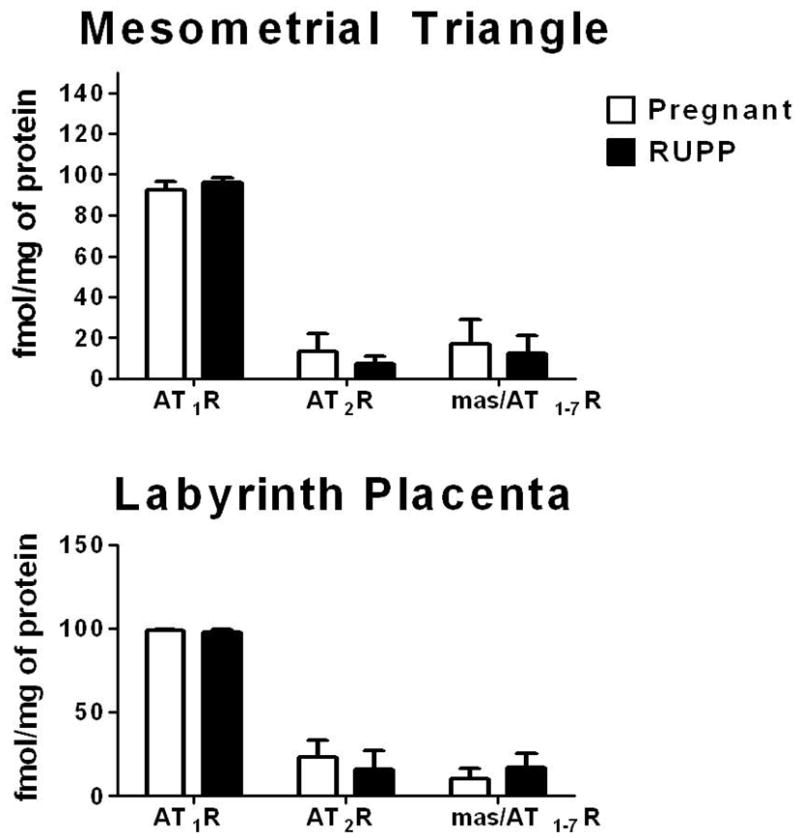
Subtype receptor density in the mesomertrial triangle and labyrinth placenta of 19 day pregnant and RUPP Sprague-Dawley rats. Values are expressed as mean ± SEM, n=7–10 (pregnant), n=10–11 (RUPP).
The myometrium of non-pregnant uterus had 182% higher AT1R density as compared to the endometrium (p<0.001) (Fig. 3). We observed a 92% competition with an AT1R antagonist in the myometrium and significant competition with D-Ala (p<0.0002) (Table 2). There was no difference in the density of AT2R or mas/AT1-7R in the myometrium as compared to the endometrium (Fig. 3). In the endometrium there was a 94%, 28% and 25% competition with AT1R, AT2R and mas/AT1-7R antagonists, respectively (Table 2).
Table 2.
AT1, AT2 and AT1-7 Receptors Binding in the Uterus of Virgin and the Uterus and Placenta of Normal Pregnant and RUPP 19-day Sprague-Dawley Rats.
| Uterus or Uteroplacental Unit | AT1R % competition with losartan | AT2R % competition with PD1233195 | Mas/AT1-7R % competition with D-Ala | |
|---|---|---|---|---|
| Virgin | Endometrium | 94 ± 3* | 28 ± 11# | 25 ± 7# |
| Myometrium | 92 ± 4* | 20 ± 11 | 18 ± 4# | |
| Pregnant | Mesometrial triangle | 90 ± 5 | 7 ± 7 | 22 ± 15 |
| Labyrinth placenta | 98 ± 1 | 20 ± 11 | 14 ± 8 | |
| RUPP | Mesometrial triangle | 96 ± 2 | 7 ± 4 | 13 ± 7 |
| Labyrinth placenta | 99 ± 1 | 7 ± 5 | 13 ± 5# |
Values represent % of total binding. AT1, AT2 and mas/AT1-7R values represent % competition with 3 μM of losartan, PD123319 and D-Ala7-Ang-(1-7) respectively. AT1R values were compared with the constant 100% to determine whether the AT1R subtype accounted for all binding observed. Values for AT2R and mas/AT1-7R were compared with the constant 0% to determine whether there was a significant contribution of AT2R and mas/AT1-7R to the total binding.
p<0.05 vs. 100%;
p<0.05 vs. 0%; n = 9–11.
We found 90–99% competition with the AT1R antagonist in the mesometrial triangle and labyrinth placenta of pregnant and RUPP rats (Table 2). In the labyrinth placenta of pregnant animals there was no significant competition with PD or D-Ala, however in the RUPP animals there was a significant 13% competition with D-Ala in the labyrinth placenta (p=0.03) but no significant competition with PD (Table 2). No significant competition with PD or D-Ala was observed in the mesometrial triangle of pregnant and RUPP rats (Table 2). The AT1R density was primarily expressed in the mesometrial triangle and labyrinth placenta of the pregnant and RUPP rats (Fig. 2 and Fig. 4). The placenta of pregnant and RUPP animals showed significantly higher AT1R density as compared with AT2R and AT1-7R (p<0.0001), but there was no difference in the AT1R density of RUPP as compared with normal pregnant animal (Fig. 4). There was no difference in AT2R and mas/AT1-7R densities in the uterus or placenta of RUPP or pregnant rats (Fig. 4).
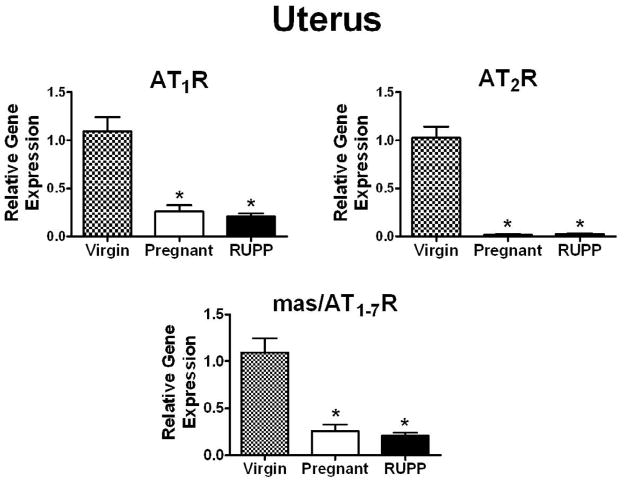
There were significantly lower AT1R, AT2R and mas/AT1-7R mRNA levels in the uterus during pregnancy as compared to virgin animals (Fig. 5). AT1R, AT2R and mas/AT1-7R mRNA levels in RUPP were not different from pregnant animals (Fig. 5). No difference was observed in the AT1R mRNA levels in the placenta of RUPP rats as compared with normal pregnant (Pregnant: 1.09 ± 0.18, RUPP: 1.22 ± 0.23; n=6 in each group). AT2R and mas/AT1-7R mRNA levels were not detectable in the placenta.
Fig. 5.
Uterus AT1R, AT2R and mas/AT1-7R mRNA in virgin, 19 day pregnant and RUPP Sprague-Dawley rats. The mRNA data are expressed relative to the virgin control. Values are mean ± SEM, *p<0.05 vs. virgin; n = 7–8 (virgin), n=7–8 (pregnant), n=8 (RUPP).
DISCUSSION
The components of local RAS are among the major mediators that can influence the uteroplacental environment [1; 2]. We previously found differential distribution and expression patterns of Ang II and Ang-(1-7) in the uteroplacental unit of pregnant and hypertensive RUPP animals [9]. Since the concentration and location of the receptor subtypes likely influence the action of the angiotensin peptides, we determined the expression and distribution of angiotensin receptors in the uterus of virgin animals and established whether the presence of the blastocyst or whether normotensive or RUPP pregnancy alters the expression and distribution of these receptors. The RUPP model of preeclampsia was chosen because of its similarity to human preeclampsia [10; 17]. Blood pressure was increased in RUPP animals [9] possibly due to high systemic vascular resistance and attenuated endothelium-dependent relaxation [18]. Maternal weight gain during pregnancy was reduced in RUPP compared to normotensive pregnant animals. In addition, fetal growth, crown to rump length, and litter size were also reduced [9], all features consistent with the RUPP model as previously published by Granger et al [19], In this model they reported that a decrease in uteroplacental blood flow and placental ischemia [19] accounted for the fetal growth restriction.. Our findings together with Granger’s [19] are consistent with placental insufficiency accounting for the reduction in fetal growth in RUPP rats.
AT1R, AT2R and mas/AT1-7R were present in virgin myometrium and endometrium (Fig. 1) in addition to previously observed detectable levels of Ang II and Ang-(1-7) [9]. In the uterus of virgin rats, most of the [125I]Sarthran binding was localized to the myometrium (Fig. 1 and 3). Competition studies showed higher density of AT1R in the myometrium compared to endometrium (Fig. 3), suggesting a role for Ang II in the maintenance of myometrial tone and other physiological functions of the non-pregnant uterus. We also show that AT1R is the predominant receptor in the uterus of virgin rat (Fig. 3). The activation of AT1R may oppose actions of mas/AT1-7R and AT2R on cell growth, proliferation and vasoactive responses in the uterus throughout the estrus cycle [20; 7]. Since the levels of estradiol were significantly higher in virgin rats at estrus vs. diestrus (99±17 vs. 37±3 pmol/l) [9] and Ang II levels are increased at estrus vs. diestrus (327.3 ± 28.5 vs 214 ± 21 fmol/g, n =10, p<0.005, Table 3), it is likely that angiotensin receptor expressions may also change with the estrus cycle in non-pregnant uterus [2; 21]. Unfortunately the design of the present study did not allow this comparison. Similar to the rat, the majority of total AT1R binding is localized to myometrium in human non-pregnant uterus [6]. However, in the human non-pregnant uterus there is a higher density of AT2R compared to AT1R and mas/AT1-7R, indicating a species specific difference [6; 22].
Table 3.
Angiotensin Peptide Content in the Uterus at Early Pregnancy and in the Uterus and Placenta of Normal Pregnant and RUPP Sprague-Dawley Rats at Late Pregnancy.
| A. Early Pregnancy (day 5 and 7 of gestation)
| |||||
|---|---|---|---|---|---|
| Virgin | 5d IIS | 5d IS | 7d IIS | 7d IS | |
| Ang II | 327.3 ± 28.5 | 195 ± 15& | 257 ± 17*& | 200 ± 12& | 148 ± 19#& |
| Ang-(1-7) | 234.5 ± 36.8 | 316 ± 31 | 288 ± 31 | 336 ± 36 | 231 ± 16# |
| B. Late Pregnancy (day 19 of gestation)
| |||
|---|---|---|---|
| Ang II | Ang-(1-7) | ||
| Uterus | Virgin | 214 ± 21 | 286 ± 22 |
|
| |||
| Uterus | Pregnant | 267 ± 20& | 372 ± 74 |
| RUPP | 300 ± 21& | 181 ± 16^& | |
| Placenta | Pregnant | 488 ± 27 | 197 ± 20 |
| RUPP | 382 ± 30^ | 143 ± 26^ | |
The data are expressed as fmol/g of tissue weight. Values are mean ± SEM.
p<0.05 vs. 5d IIS,
p<0.05 vs. 7d IIS,
p<0.05 vs. 19-day pregnant;
p<0.05 vs. virgin. n = 8–17. Data are modified with permission from our previous publication [9].
This is the first report demonstrating the regulation and distribution of the angiotensin receptors in early pregnancy in the rat. Previously [9], we showed that the Ang II and Ang-(1-7) were spatially and temporally regulated during implantation and decidualization. Ang II was decreased in both the IS and IIS at day 5 and 7 of gestation as compared with virgin rats, whereas Ang-(1-7) was unchanged [9]. Ang II was increased in the IS compared to IIS at day 5 of gestation, but at day 7 of gestation Ang II was increased in the IIS. (Table 3) [9]. The current findings demonstrating that the AT1R mRNA levels were 3-fold elevated in the IIS at day 7 of gestation in conjunction with the increased Ang II IIS levels (Fig. 1), suggest that an important Ang II-AT1R-mediated event related to decidualization is occurring at this time point. This change contrasts with the unchanged levels of AT1R mRNA levels within the IS at day 5 and 7 of gestation. The profile for the AT2R mRNA expression was markedly different from the AT1R with down-regulation of the AT2R mRNA at day 5 and 7 in the IIS, but up-regulation of the AT2R occurring in the IS at day 5 of gestation as compared to IIS. This pattern in conjunction with increased levels of Ang II at day 5 of gestation suggests that Ang II may activate the AT2R in the IS possibly to enhance pregnancy progression at early stages. The mas/AT1-7R mRNA levels were up-regulated in the IS and IIS sites at both time points in association with maintained Ang-(1-7) levels, and thus would suggest an enhanced function of Ang-(1-7) during early pregnancy.
There was a striking reduction in all three angiotensin receptor mRNA levels at late pregnancy in the uterus of pregnant normotensive females which persisted in the hypertensive pregnancy as compared to virgin animals (Fig. 5). We can only speculate that reduced receptor expression at late pregnancy may be explained by the negative feedback regulation by Ang II (Table 3). Interestingly, Anton et al., showed significantly lower angiotensin receptor expressions in uterine placental bed of normal pregnant and preeclamptic women compared to virgin uterine tissue [6] revealing similarities between RUPP and human preeclamptic uterus. In our study, the AT2R and mas/AT1-7R mRNA levels were undetectable at late gestation in the labryinth placenta; however we were able to detect receptor binding by autoradiography, suggesting different sensitivities of the two methods.
Competition studies revealed that AT1R is the predominant receptor in the mesometrial triangle and labyrinth placenta of pregnant normotensive rats (Fig. 4). The presence of renin, angiotensinogen, Ang I, ACE, and ACE2 in the placenta [9] suggests local production of Ang II and Ang-(1-7) that may alter the late pregnancy events. We previously showed increased levels of Ang II in the mesometrial triangle and labyrinth placenta of a different preeclamptic transgenic rat model [23; 24], suggesting that Ang II may act on AT1R to elicit vasoactive effects [21]. The Ang II could be increased due to local production in the mesometrial triangle or due to diffusion from the placenta. As we reported previously [9], the uterine levels of Ang II were increased in both normotensive and RUPP pregnancy at late gestation compared with virgin uterus. However Ang-(1-7) was decreased only in RUPP animal. Therefore, the ratio of Ang II/Ang-(1-7) did not change with pregnancy but decreased with the RUPP, suggesting a diminished influence of Ang-(1-7) on uterine physiology at late pregnancy in the RUPP [9]. These studies suggest a protective role for Ang-(1-7) in late gestation. Since Ang-(1-7) is a vasodilatory agent, the heptapeptide hormone may participate in uterine and placental blood flow regulation and its absence may reduce its conterregulatory role in opposing the vasoconstriction [20; 7].
The lack of binding in the trophospongium region of the placenta at late pregnancy, which contains trophoblast cells that can release multiple hormones and cytokines involved in placentation at late pregnancy [25] suggests that Ang II or Ang-(1-7) have limited actions in this region. This contrasts with the much higher binding in the adjacent labyrinth placenta and mesometrial triangle regions, where Ang II and Ang-(1-7) may exert their strongest influence.
RUPP preeclampsia had no effect on AT1R expression in the placenta. In contrast to normotensive pregnancy both Ang II and Ang-(1-7) were reduced in the placenta of RUPP females. This would suggest a diminished influence of the local placental Ang II/AT1R axis in the RUPP. The Ang II/Ang-(1-7) ratio did not change in the placenta of hypertensive pregnancy, suggesting the maintained influence of Ang-(1-7). In addition, there was more competition with D-Ala in the labyrinth placenta of RUPP females compared to competition with PD compound suggesting an enhanced expression of the mas/AT1-7R. These findings suggest that Ang-(1-7) is probably exerting a counter-regulatory role in the RUPP placenta compared to normal pregnancy.
CONCLUSIONS
We conclude that both peptides, Ang-(1-7) acting on mas/AT1-7R and Ang II acting on either the AT2R, and AT1R may contribute to the maintenance of early pregnancy possibly via regulation of vascular changes or decidualization [21]. During late gestation the reduction of Ang receptors in the uterus as compared to virgin may arise from the feedback down-regulation by Ang II. In the placenta there may be diminished influence of the local placental Ang II/AT1R axis in the RUPP. The increased binding of mas/AT1-7R at late gestation in RUPP pregnancy may represent a compensatory mechanism to reduce uteroplacental vascular resistance.
Acknowledgments
Support was provided in part by the National Institutes of Health, NHLBI-P01 HL51952, HL62489, American Heart Association, Wake Forest University Venture grant, Unifi, Inc. Greensboro, NC and Farley-Hudson Foundation, Jacksonville, NC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.August P, Sealey JE. The renin-angiotensin system in normal and hypertensive pregnancy and in ovarian function. In: Laragh JH, Brenner BM, editors. Hypertension Pathophysiology, Diagnosis, and Management. New York: Raven Press, Ltd; 1990. pp. 1761–78. [Google Scholar]
- 2.James PR, Nelson-Piercy C. Management of hypertension before, during, and after pregnancy. Heart. 2004 Dec 1;90(12):1499–504. doi: 10.1136/hrt.2004.035444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrario CM, Ahmad S, Joyner J, Varagic J. Advances in the Renin Angiotensin System: Focus on Angiotensin-Converting Enzyme 2 and Angiotensin-(1–7) In: Paul MV, editor. Advances in Pharmacology Cardiovascular Pharmacology Heart and Circulation. 59. Academic Press: 2010. pp. 197–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos RAS, Ferreira AJ, Simoes e Silva AC. Recent advances in the angiotensin-converting enzyme 2 angiotensin-(1–7)–Mas axis. Experimental Physiology. 2008 May 1;93(5):519–27. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 5.Merrill DC, Karoly M, Chen K, Ferrario CM, Brosnihan KB. Angiotensin-(1-7) in normal and preeclamptic pregnancy. Endocrine. 2002;18:239–45. doi: 10.1385/ENDO:18:3:239. [DOI] [PubMed] [Google Scholar]
- 6.Anton L, Merrill DC, Neves LAA, Stovall K, Gallagher PE, Diz DI, et al. Activation of local chorionic villi angiotensin II levels but not angiotensin (1-7) in preeclampsia. Hypertension. 2008;51:1066–72. doi: 10.1161/HYPERTENSIONAHA.107.103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komukai K, Mochizuki S, Yoshimura M. Gender and the renin–angiotensin–aldosterone system. Fundamental & Clinical Pharmacology. 2010;24(6):687–98. doi: 10.1111/j.1472-8206.2010.00854.x. [DOI] [PubMed] [Google Scholar]
- 8.Anton L, Merrill DC, Neves LA, Diz DI, Corthorn J, Valdes G, et al. The uterine placental bed Renin-Angiotensin system in normal and preeclamptic pregnancy. Endocrinology. 2009 Sep;150(9):4316–25. doi: 10.1210/en.2009-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neves LAA, Stovall K, Joyner J, Valdes G, Gallagher PE, Ferrario CM, et al. ACE2 and Ang-(1-7) in the uterus during early and late gestation. Am J Physiol Regul Integr Comp Physiol. 2007;294:R151–R161. doi: 10.1152/ajpregu.00514.2007. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Shams M, Zhu J, Khalig A, Wilkes M, Whittle M, et al. Cellular localization of AT1 receptor mRNA and protein in normal placenta and its reduced expression in intrauterine growth restriction. J Clin Invest. 1998;101:442–54. doi: 10.1172/JCI119881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, et al. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–5. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 12.Diz DI, Block CH, Barnes KL, Ferrario CM. Correlation between angiotensin II binding sites and substance P in the canine brain stem. J Hypertens (Suppl 6) 1986;4:S468–S471. [PubMed] [Google Scholar]
- 13.Li P, Morris M, Diz DI, Ferrario CM, Ganten D, Callahan MF. Role of paraventricular angiotensin AT-1 receptors in salt sensitive hypertension in MREN-2 transgenic rats. Am J Physiol. 1996;270(39):R1178–R1181. doi: 10.1152/ajpregu.1996.270.5.R1178. [DOI] [PubMed] [Google Scholar]
- 14.Grone HJ, Simon M, Fuchs E. Autoradiographic characterization of angiotensin receptor subtypes in fetal and adult human kidney. Am J Physiol. 1992;262(31):F326–F331. doi: 10.1152/ajprenal.1992.262.2.F326. [DOI] [PubMed] [Google Scholar]
- 15.Yamaleyeva LM, Gallagher PE, Vinsant S, Chappell MC. Discoordinate regulation of renal nitric oxide synthase isoforms in ovariectomized mRen2. Lewis rats. Am J Physiol Regul Integr Comp Physiol. 2007 Feb;292(2):R819–R826. doi: 10.1152/ajpregu.00389.2006. [DOI] [PubMed] [Google Scholar]
- 16.Pocock SJ, McCormack V, Gueyffier F, Boutitie F, Fagard RH, Boissel JP. A score for predicting risk of death from cardiovascular disease in adults with raised blood pressure, based on individual patient data from randomised controlled trials. BMJ. 2001 Jul 14;323(7304):75–81. doi: 10.1136/bmj.323.7304.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. American Journal of Physiology - Heart and Circulatory Physiology. 2012 Jul 1;303(1):H1–H8. doi: 10.1152/ajpheart.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension. 2001;35(Part 2):367–72. doi: 10.1161/01.hyp.35.1.367. [DOI] [PubMed] [Google Scholar]
- 19.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, et al. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–92. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 20.Brosnihan KB, Senanayake PS, Li P, Ferrario CM. Bi-directional actions of estrogen on the renin-angiotensin system. Braz J Med Biol Res. 1999;32:373–81. doi: 10.1590/s0100-879x1999000400001. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen AH, Schauser KH, Poulsen K. Current topic: the uteroplacental renin-angiotensin system. Placenta. 2000 Jul;21(5–6):468–77. doi: 10.1053/plac.2000.0535. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed A, Li XF, Dunk C, Whittle MJ, Rushton DI, Rollason T. Colocalisation of vascular endothelial growth factor and its Flt-1 receptor in human placenta. Growth Factors. 1995;12(3):235–43. doi: 10.3109/08977199509036883. [DOI] [PubMed] [Google Scholar]
- 23.Hering L, Herse F, Geusens N, Verlohren S, Wenzel K, Staff AC, et al. Effects of circulating and local uteroplacental angiotensin II in rat pregnancy. Hypertension. 2010 Aug;56(2):311–8. doi: 10.1161/HYPERTENSIONAHA.110.150961. [DOI] [PubMed] [Google Scholar]
- 24.Brosnihan KB, Hering L, Dechend R, Chappell MC, Herse F. Increased angiotensin II in the mesometrial triangle of a transgenic rat model of preeclampsia. Hypertension. 2010 Feb;55(2):562–6. doi: 10.1161/HYPERTENSIONAHA.109.145656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997 May 1;99(9):2139–51. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]