Abstract
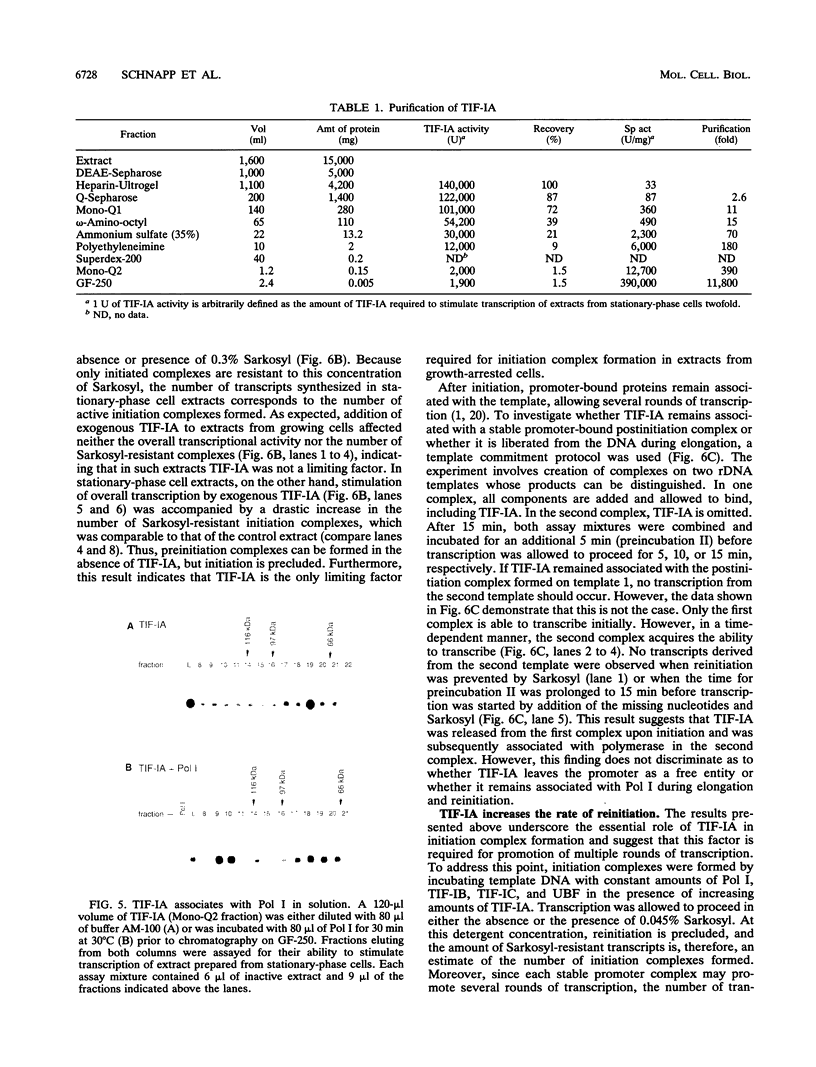
Alterations in the rate of cell proliferation are accompanied by changes in the transcription of rRNA genes. In mammals, this growth-dependent regulation of transcription of genes coding for rRNA (rDNA) is due to reduction of the amount or activity of an essential transcription factor, called TIF-IA. Extracts prepared from quiescent cells lack this factor activity and, therefore, are transcriptionally inactive. We have purified TIF-IA from exponentially growing cells and have shown that it is a polypeptide with a molecular mass of 75 kDa which exists as a monomer in solution. Using a reconstituted transcription system consisting of purified transcription factors, we demonstrate that TIF-IA is a bona fide transcription initiation factor which interacts with RNA polymerase I. Preinitiation complexes can be assembled in the absence of TIF-IA, but formation of the first phosphodiester bonds of nascent rRNA is precluded. After initiation, TIF-IA is liberated from the initiation complex and facilitates transcription from templates bearing preinitiation complexes which lack TIF-IA. Despite the pronounced species specificity of class I gene transcription, this growth-dependent factor has been identified not only in mouse but also in human cells. Murine TIF-IA complements extracts from both growth-inhibited mouse and human cells. The analogous human activity appears to be similar or identical to that of TIF-IA. Therefore, despite the fact that the RNA polymerase transcription system has evolved sufficiently rapidly that an rDNA promoter from one species will not function in another species, the basic mechanisms that adapt ribosome synthesis to cell proliferation have been conserved.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnosti D. N., Merino A., Reinberg D., Schaffner W. Oct-2 facilitates functional preinitiation complex assembly and is continuously required at the promoter for multiple rounds of transcription. EMBO J. 1993 Jan;12(1):157–166. doi: 10.1002/j.1460-2075.1993.tb05641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman E., Paule M. R. Regulation of eukaryotic ribosomal RNA transcription by RNA polymerase modification. Cell. 1986 Nov 7;47(3):445–450. doi: 10.1016/0092-8674(86)90601-x. [DOI] [PubMed] [Google Scholar]
- Bell S. P., Jantzen H. M., Tjian R. Assembly of alternative multiprotein complexes directs rRNA promoter selectivity. Genes Dev. 1990 Jun;4(6):943–954. doi: 10.1101/gad.4.6.943. [DOI] [PubMed] [Google Scholar]
- Bell S. P., Pikaard C. S., Reeder R. H., Tjian R. Molecular mechanisms governing species-specific transcription of ribosomal RNA. Cell. 1989 Nov 3;59(3):489–497. doi: 10.1016/0092-8674(89)90032-9. [DOI] [PubMed] [Google Scholar]
- Buttgereit D., Pflugfelder G., Grummt I. Growth-dependent regulation of rRNA synthesis is mediated by a transcription initiation factor (TIF-IA). Nucleic Acids Res. 1985 Nov 25;13(22):8165–8180. doi: 10.1093/nar/13.22.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh A. H., Gokal P. K., Lawther R. P., Thompson E. A., Jr Glucocorticoid inhibition of initiation of transcription of the DNA encoding rRNA (rDNA) in lymphosarcoma P1798 cells. Proc Natl Acad Sci U S A. 1984 Feb;81(3):718–721. doi: 10.1073/pnas.81.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh A. H., Thompson E. A., Jr Hormonal regulation of transcription of rDNA: glucocorticoid effects upon initiation and elongation in vitro. Nucleic Acids Res. 1985 May 10;13(9):3357–3369. doi: 10.1093/nar/13.9.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Clos J., Buttgereit D., Grummt I. A purified transcription factor (TIF-IB) binds to essential sequences of the mouse rDNA promoter. Proc Natl Acad Sci U S A. 1986 Feb;83(3):604–608. doi: 10.1073/pnas.83.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Tanese N., Tjian R. The TATA-binding protein and associated factors are integral components of the RNA polymerase I transcription factor, SL1. Cell. 1992 Mar 6;68(5):965–976. doi: 10.1016/0092-8674(92)90039-f. [DOI] [PubMed] [Google Scholar]
- Conconi A., Widmer R. M., Koller T., Sogo J. M. Two different chromatin structures coexist in ribosomal RNA genes throughout the cell cycle. Cell. 1989 Jun 2;57(5):753–761. doi: 10.1016/0092-8674(89)90790-3. [DOI] [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Flores O., Lu H., Killeen M., Greenblatt J., Burton Z. F., Reinberg D. The small subunit of transcription factor IIF recruits RNA polymerase II into the preinitiation complex. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9999–10003. doi: 10.1073/pnas.88.22.9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokal P. K., Cavanaugh A. H., Thompson E. A., Jr The effects of cycloheximide upon transcription of rRNA, 5 S RNA, and tRNA genes. J Biol Chem. 1986 Feb 25;261(6):2536–2541. [PubMed] [Google Scholar]
- Gokal P. K., Mahajan P. B., Thompson E. A. Hormonal regulation of transcription of rDNA. Formation of initiated complexes by RNA polymerase I in vitro. J Biol Chem. 1990 Sep 25;265(27):16234–16243. [PubMed] [Google Scholar]
- Grummt I., Roth E., Paule M. R. Ribosomal RNA transcription in vitro is species specific. Nature. 1982 Mar 11;296(5853):173–174. doi: 10.1038/296173a0. [DOI] [PubMed] [Google Scholar]
- Hammond M. L., Bowman L. H. Insulin stimulates the translation of ribosomal proteins and the transcription of rDNA in mouse myoblasts. J Biol Chem. 1988 Nov 25;263(33):17785–17791. [PubMed] [Google Scholar]
- Hawley D. K., Roeder R. G. Functional steps in transcription initiation and reinitiation from the major late promoter in a HeLa nuclear extract. J Biol Chem. 1987 Mar 15;262(8):3452–3461. [PubMed] [Google Scholar]
- Killeen M. T., Greenblatt J. F. The general transcription factor RAP30 binds to RNA polymerase II and prevents it from binding nonspecifically to DNA. Mol Cell Biol. 1992 Jan;12(1):30–37. doi: 10.1128/mcb.12.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn A., Grummt I. Dual role of the nucleolar transcription factor UBF: trans-activator and antirepressor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7340–7344. doi: 10.1073/pnas.89.16.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn A., Stefanovsky V., Grummt I. The nucleolar transcription activator UBF relieves Ku antigen-mediated repression of mouse ribosomal gene transcription. Nucleic Acids Res. 1993 May 11;21(9):2057–2063. doi: 10.1093/nar/21.9.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learned R. M., Cordes S., Tjian R. Purification and characterization of a transcription factor that confers promoter specificity to human RNA polymerase I. Mol Cell Biol. 1985 Jun;5(6):1358–1369. doi: 10.1128/mcb.5.6.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan P. B., Gokal P. K., Thompson E. A. Hormonal regulation of transcription of rDNA. The role of TFIC in formation of initiation complexes. J Biol Chem. 1990 Sep 25;265(27):16244–16247. [PubMed] [Google Scholar]
- Mahajan P. B., Thompson E. A. Hormonal regulation of transcription of rDNA. Purification and characterization of the hormone-regulated transcription factor IC. J Biol Chem. 1990 Sep 25;265(27):16225–16233. [PubMed] [Google Scholar]
- Mahajan P. B., Thompson E. A., Jr Copurification of RNA polymerase I and the glucocorticoid-regulated transcription factor IC. Protein Expr Purif. 1992 Oct;3(5):410–416. doi: 10.1016/s1046-5928(05)80043-9. [DOI] [PubMed] [Google Scholar]
- McStay B., Hu C. H., Pikaard C. S., Reeder R. H. xUBF and Rib 1 are both required for formation of a stable polymerase I promoter complex in X. laevis. EMBO J. 1991 Aug;10(8):2297–2303. doi: 10.1002/j.1460-2075.1991.tb07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y., Matsui T., Muramatsu M. The mechanism of decrease in nucleolar RNA synthesis by protein synthesis inhibition. J Biochem. 1979 Mar;85(3):807–818. [PubMed] [Google Scholar]
- Paule M. R., Iida C. T., Perna P. J., Harris G. H., Knoll D. A., D'Alessio J. M. In vitro evidence that eukaryotic ribosomal RNA transcription is regulated by modification of RNA polymerase I. Nucleic Acids Res. 1984 Nov 12;12(21):8161–8180. doi: 10.1093/nar/12.21.8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paule M. R. Polymerase I transcription, termination, and processing. Gene Expr. 1993;3(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Schnapp A., Grummt I. Transcription complex formation at the mouse rDNA promoter involves the stepwise association of four transcription factors and RNA polymerase I. J Biol Chem. 1991 Dec 25;266(36):24588–24595. [PubMed] [Google Scholar]
- Schnapp A., Pfleiderer C., Rosenbauer H., Grummt I. A growth-dependent transcription initiation factor (TIF-IA) interacting with RNA polymerase I regulates mouse ribosomal RNA synthesis. EMBO J. 1990 Sep;9(9):2857–2863. doi: 10.1002/j.1460-2075.1990.tb07475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid A., Finsterer M., Grummt I. Limited proteolysis unmasks specific DNA-binding of the murine RNA polymerase I-specific transcription termination factor TTFI. J Mol Biol. 1992 Oct 5;227(3):635–647. doi: 10.1016/0022-2836(92)90213-4. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Pape L., Ryan K., Mougey E. B., Poretta R., Nikolov E., Paalman M. H., Lazdins I., Martin C. Expression of mouse and frog rRNA genes: transcription and processing. 1991 May 29-Jun 12Mol Cell Biochem. 104(1-2):149–154. doi: 10.1007/BF00229814. [DOI] [PubMed] [Google Scholar]
- Surmacz E., Kaczmarek L., Rønning O., Baserga R. Activation of the ribosomal DNA promoter in cells exposed to insulinlike growth factor I. Mol Cell Biol. 1987 Feb;7(2):657–663. doi: 10.1128/mcb.7.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N., Kato H., Ishikawa Y., Hisatake K., Tashiro K., Kominami R., Muramatsu M. Sequence-specific binding of a transcription factor TFID to the promoter region of mouse ribosomal RNA gene. J Biol Chem. 1990 Aug 15;265(23):13836–13842. [PubMed] [Google Scholar]
- Tower J., Culotta V. C., Sollner-Webb B. Factors and nucleotide sequences that direct ribosomal DNA transcription and their relationship to the stable transcription complex. Mol Cell Biol. 1986 Oct;6(10):3451–3462. doi: 10.1128/mcb.6.10.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J., Sollner-Webb B. Transcription of mouse rDNA is regulated by an activated subform of RNA polymerase I. Cell. 1987 Sep 11;50(6):873–883. doi: 10.1016/0092-8674(87)90514-9. [DOI] [PubMed] [Google Scholar]
- Zieve G. W., Turnbull D., Mullins J. M., McIntosh J. R. Production of large numbers of mitotic mammalian cells by use of the reversible microtubule inhibitor nocodazole. Nocodazole accumulated mitotic cells. Exp Cell Res. 1980 Apr;126(2):397–405. doi: 10.1016/0014-4827(80)90279-7. [DOI] [PubMed] [Google Scholar]