Abstract
Little is known about the relationship between Major Depressive Disorder (MDD) and smoking-related behaviors such as cue-induced urges to smoke. The purpose of this pilot study was to examine: 1) differences in smoking cue reactivity by MDD history, and; 2) the association of a diagnosis of MDD, current depressive symptoms, and smoking variables to cue-induced urges to smoke. Participants (N=52) were n=31 smokers with no MDD history and n=21 smokers with past MDD. Participants completed a 2-hour laboratory session during which they were exposed to neutral (e.g., pencils) and smoking cues (e.g., cigarettes) after smoking one of their preferred brand cigarettes (Satiated Condition) and when it had been one hour since they smoked (Brief Deprivation Condition). Cue-induced urges increased with exposure to smoking cues and this increase did not significantly differ by diagnosis group. Current symptoms of depression, but not a diagnosis of MDD, were significantly and positively related to cue-induced cravings in satiated adult smokers. The association between depression symptoms and smoking urges was not significant in the Brief Deprivation Condition. Smoking cue reactivity may be a useful procedure for studying aspects of smoking behavior in adults with depression.
Keywords: smoking, cues, cravings, depression
INTRODUCTION
Adults with depression smoke at higher rates1, report greater nicotine dependence2, 3, and appear to have more difficulty quitting smoking4-8 than adults without depression (see Ziedonis et al8 for a review). Conversely, smokers report greater symptoms of depression, more frequent episodes of depression, and higher rates of suicidal ideation and suicide than nonsmokers9-11. Little is known about the reasons for the strong relationship of depression with both smoking and difficulty achieving smoking abstinence. Research has suggested that depression is associated with more severe withdrawal symptoms, including urges to smoke12-15. Urges to smoke play a critical role in both habitual smoking and smoking lapse behavior16 and therefore learning more about urges to smoke experienced by adults with depression might help to improve our understanding of this group’s smoking behavior.
Urges to smoke are commonly assessed in laboratory studies through cue reactivity procedures. The cue reactivity procedure is based on classical conditioning theories that cravings will be elicited by drug-related stimuli and that these cravings may contribute to relapse to drug use after cessation16, 17. A meta-analysis of cue-reactivity studies with a variety of drugs17 suggested that cue reactivity procedures produce a stable and robust increase in self-reported cravings across all substances (Cohen’s d=0.92) and specifically for smokers (d=1.18). More moderate associations to cues were found overall and for smokers related to physiological responses such as heart rate (d=0.26 and d=0.21, respectively) and skin temperature (d=-0.24 and d=-0.07, respectively). On the whole, the research on cue reactivity suggests that exposure to smoking-related cues increases cravings in smokers17 and response to smoking cues has been suggested to relate to smoking lapse and relapse after a quit attempt17, 18. In addition, some studies have found that higher pre-quit levels of cue reactivity predict worse smoking cessation outcomes19-21 (but see also Niaura et al22) extending the laboratory results to suggest the important clinical and intervention implications of cue reactivity research.
Few studies have used laboratory procedures to examine cue-induced urges in smokers with and without depression. Pomerleau and colleagues14 examined changes in smoking urges in 34 smokers with “high depression” (defined as having either current depression measured by the Center for Epidemiological Studies-Depression Scale23 or a diagnosis of lifetime depression from the Composite International Diagnostic Interview24) and 35 smokers with “low depression” who went through cue reactivity procedures after 3 days of smoking abstinence. There was a significant main effect of depression status with “high depression” smokers reporting higher overall levels of urges than “low depression” smokers. There was also a main effect of time with all smokers reporting an increase in urges after viewing smoking cues. The depression status by time interaction was not significant. To our knowledge, examining differences in cravings to smoke between smokers with and without depression during satiety and brief smoking deprivation has not yet been studied. Learning more about the ways in which smokers with depression respond to smoking-related cues may improve our understanding of the strong association of depression and smoking and provide information that can be used to tailor treatments with the goal of reducing smoking and improving cessation outcomes for these adults.
Aims of the current study
The primary purpose of this pilot study was to examine differences in cravings elicited by smoking-related cues in two groups of smokers defined by history of Major Depressive Disorder (MDD): 1) smokers with no history of MDD (MDD−), and 2) smokers with a history of MDD (MDD+). Cue-induced cravings were studied in two conditions: (1) no deprivation from smoking (Satiated Condition) and (2) after an hour of no smoking (Brief Deprivation Condition). The first aim of the laboratory study was to compare changes in smoking urges from pre- to post-exposure to smoking cues by diagnosis group. It was predicted that MDD+ smokers would show a greater increase in smoking urges after exposure to cues than MDD− smokers. The second aim of the study was to examine the association of a diagnosis of MDD, current symptoms of depression, demographics, and smoking variables to changes smoking urges after presentation of smoking cues. It was expected that both a diagnosis of MDD and current symptoms of depression would be associated with greater cue-induced urges to smoke. Little is known about the smoking-related behavior of adults receiving antidepressant treatment in comparison to other adults with depression; therefore, an exploratory aim of this study was to examine differences in smoking cue reactivity in smokers with no history of MDD (MDD−), smokers with a history of MDD and no current depression treatment (MDD+Tx−), and smokers with a history of MDD and current treatment with an antidepressant medication (MDD+Tx+).
METHODS
Participants
Participants were non-treatment seeking daily cigarette smokers between the ages of 18 and 70 who consumed ≥5 cigarettes per day (CPD) with expired breath carbon monoxide (CO) levels of ≥10 ppm. Smokers were not eligible for the laboratory study if they reported a current major Axis I disorder (other than nicotine dependence and MDD), reported drug or alcohol abuse or dependence within the past 3 months, or had a positive urine drug screen at the screening or laboratory session. Participants with and without depression were recruited from the greater New Haven area through radio, television, newspaper, and flyer advertisements. Smokers with depression were also recruited through flyers and referrals from clinicians at the Connecticut Mental Health Center in New Haven, Connecticut. Written informed consent was obtained from all participants and the research protocols were approved by Yale Medical School’s Human Investigation Committee.
Based on a brief phone screen to assess initial eligibility, 172 adults were scheduled for in-person screening appointment. Fifty-two adults (MDD− n=31; MDD+ n=21) attended the appointment, were eligible for the study, and completed the laboratory appointment. Reasons for non-eligibility included discontinued participation (n=90), current drug use (n=19), Axis I diagnoses other than MDD and nicotine dependence (n=7), and ineligibility related to smoking criteria (e.g., CPD<5; n=4).
Procedure
During the in-person screening appointment, potential participants completed consent forms, measures of eligibility (e.g., urine drug screen, assessments of smoking), provided demographic information, and completed measures of current symptoms of depression. An assessment of Axis I disorders including MDD was completed by a doctoral level clinician. Eligible participants were invited to complete a 2-hour laboratory appointment that included two identical cue reactivity sessions. One cue reactivity session was completed immediately after the participants smoked one of their cigarettes (Satiated Condition) and the second cue reactivity session was completed an hour later (Brief Deprivation Condition).
See Figure 1 for a timeline of the laboratory appointment. At the beginning of the laboratory appointment, participants completed baseline measures of depression symptoms and smoking. Then participants smoked one of their cigarettes in order to standardize the time from their last cigarette to the two cue reactivity sessions. After finishing their cigarette, participants began the first cue reactivity session. The cue reactivity procedure was adapted from previous research25, 26. Participants were presented with both neutral cues to assess for nonspecific reactivity and smoking cues. In order to prevent carryover from smoking cues (see Sayette et al27 for a review), the conditions were not counterbalanced and neutral cues were presented first for all participants. Neutral cues were two unsharpened pencils, a bowl, and two pencil sharpeners. Smoking cues were two single unsmoked cigarettes of the participant’s preferred brand, an ashtray, and two lighters. Participants were instructed to examine the objects through scripted directions played on a tape recorder which standardized the time of exposure across the sample. Participants completed measures of smoking urges, mood, and physiological response (i.e., blood pressure, pulse, and temperature) before and after presentation of the neutral and the smoking cues.
Figure 1.
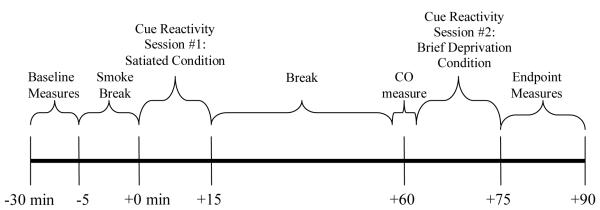
Timeline for the Laboratory Session
After completing the first cue reactivity session, participants remained in the laboratory until it had been one hour since they smoked their last cigarette. During this time, participants were permitted to read their own books or listen to music. After participants were deprived of smoking for a total of 60 minutes and non-smoking was confirmed with a CO reading (i.e., the CO level post-break was equal to or less than the CO level taken before the break), participants completed the second cue reactivity session which was identical in procedure to the first session. At the end of the session, participants completed measures of depression.
Measures
Demographic and Smoking Variables
Participants were asked to report demographics (e.g., gender, race, age), age of smoking onset, duration of smoking, and number of past attempts to quit smoking. Nicotine dependence was assessed using the 6- item Fagerström Test for Nicotine Dependence28 (FTND; range 0-10).
Cigarette Consumption
A 7-day timeline follow-back (TLFB; Sobell et al.29) was administered as a self-report measure of current daily cigarette consumption. Smoking was biochemically confirmed using expired breath CO levels (Bedfont EC50 Microsmokerlyzer II, Kent, UK).
Smoking Urges, Negative Affect, and Positive Affect
Using procedures from our previous study30, a Visual Analogue Scale31 (VAS) was used to assess the intensity of urge to smoke, negative affect, and positive affect during the cue reactivity sessions. Participants were asked to rate the intensity of their urges or affect “at that moment” on a scale from 0 to 100.
Assessment of Axis I Disorders
The presence of current and past Axis I disorders including MDD was assessed using The Structured Clinical Interview for DSM-IV32. Current symptoms of depression were assessed using the 21-item Beck Depression Inventory33 (BDI; range 0-63) and 21-item Hamilton Depression Rating Scale34 (HDRS).
Statistical Analyses
Depression Diagnosis
Participants were classified as MDD− if they did not meet criteria for either past or current MDD (n=31). Participants were classified as MDD+ if they met criteria for a lifetime diagnosis of MDD (n=21). MDD+ participants were further classified either as MDD+Tx− (i.e., “treatment negative”) if they met criteria for past MDD and did not report any current treatment with antidepressant medications (n=13) or as MDD+Tx+ (i.e., “treatment positive”) if they met criteria for a lifetime diagnosis of MDD and reported current antidepressant medication use (n=8).
Cue Reactivity
Cue Reactivity was defined as: (Urge to smoke after presentation of smoking cues) – (Urge to smoke before presentation of smoking cues). Using this formula, which was based on our previous work (Fonder et al.30), all participants were classified as either “cue reactive” (i.e., Cue Reactivity > 0) or “not cue reactive” (i.e., Cue Reactivity ≤ 0).
Demographics
Comparison of demographics, cigarette consumption, smoking history, and depressive symptoms between the two diagnosis groups (MDD−, MDD+), between the two MDD+ groups (MDD+Tx−, MDD+Tx+), and among the three diagnosis groups (MDD−, MDD+Tx−, MDD+Tx+) were examined using Chi-square tests for categorical data and independent samples t-tests for continuous data. Because MDD+Tx− and MDD+Tx+ participants did not significantly differ on any demographic, smoking, or depression symptom variables (all ps>0.05, data not shown), the two groups were combined into one MDD+ group for the primary analyses to increase our power to examine group differences in this pilot study.
Aim 1
Repeated Measures analyses of covariance (ANCOVA) were conducted to analyze changes in urge by diagnosis group. Diagnosis (MDD−, MDD+) was a between-subjects independent variable and urge to smoke before presentation of cues and after presentation of cues were the dependent variables. Demographic and smoking variables that significantly differed between the diagnosis groups (current depression symptoms, see Table 1) were included in the analyses as covariates.
Table 1.
Baseline demographics, smoking behavior, and depression symptoms for the full sample, participants with no history of Major Depressive Disorder (MDD−), and participants with a history of Major Depressive Disorder (MDD+).
| Total Sample (N=52) |
MDD− (n=31) |
MDD+ (n=21) |
p-value | |
|---|---|---|---|---|
| Age | 44.8 ± 10.9 | 44.6 ± 11.5 | 45.2 ± 10.1 | 0.83 |
| % Male | 58 | 61 | 52 | 0.52 |
| % Caucasian | 69 | 68 | 71 | 0.85 |
| Education (years) |
13.5 ± 1.7 | 13.7 ± 1.5 | 13.4 ± 2.0 | 0.54 |
| Age of smoking initiation |
16.1 ± 3.8 | 16.2 ± 6.1 | 16.1 ± 4.3 | 0.94 |
| Duration of smoking (years) |
28.4 ± 11.5 | 28.1 ± 12.8 | 29.0 ± 9.5 | 0.78 |
| Number of Quit Attempts |
4.9 ± 4.1 | 3.9 ± 3.0 | 6.2 ± 4.9 | 0.84 |
| FTND | 6.2 ± 1.4 | 5.8 ± 2.0 | 6.7 ± 2.0 | 0.13 |
| CPD | 18.6 ± 7.4 | 17.1 ± 5.6 | 20.8 ± 9.2 | 0.08 |
| CO (ppm) | 17.8 ± 8.3 | 16.5 ± 7.3 | 19.7 ± 9.3 | 0.18 |
| BDI | 4.8 ± 5.6 | 2.6 ± 2.9 | 8.2 ± 7.0 | <0.01 |
| HDRS-17 item | 4.2 ± 4.8 | 2.6 ± 2.8 | 6.6 ± 6.0 | <0.01 |
| HDRS-21 item | 4.5 ± 5.3 | 2.6 ± 2.8 | 7.4 ± 6.6 | <0.01 |
| Cue Reactive | 73% | 70% | 76% | 0.63 |
Key: MDD, Major Depressive Disorder; FTND, Fagerström Test for Nicotine Dependence; CPD, cigarettes per day; CO, expired breath carbon monoxide level; BDI, Beck Depression Inventory; HDRS, Hamilton Depression Rating Scale
Note: Cue Reactive was defined as: (Urge to smoke after presentation of the smoking cues) – (Urge to smoke before presentation of the smoking cues) > 0
The primary analysis described above was repeated with three diagnosis groups (MDD−, MDD+Tx−, MDD+Tx+) as the between-subjects variable as an exploratory analysis. Preliminary analyses showed that MDD+Tx+ participants significantly differed from MDD− in their number of past attempts to quit smoking (MDD+Tx+ M=8.6, SD=6.0) and current depression symptoms (MDD+Tx+ BDI M=11.3, SD=9.3; HDRS-17 M=8.1, SD=7.6; HDRS-21 M=8.8, SD=8.3). No other demographic or smoking differences between MDD− and MDD+Tx+ were found and no other pairwise comparisons (MDD− and MDD+Tx−; MDD+Tx− and MDD+Tx+) were significant. Past quit attempts and current depression symptoms were included as covariates for this analysis.
Aim 2
Linear regressions were run to examine associations between depression and smoking variables and changes in urges during the cue reactivity procedure. Demographic, smoking, and depression variables that were found by zero-order correlation to be significantly associated with change in urge were entered in a stepwise fashion as independent variables. The dependent variable was Cue Reactivity as defined above.
All ANCOVA and regression analyses were run separately for the Satiated Condition and the Brief Deprivation Condition and all analyses included response to neutral cues [calculated as (Urge to Smoke after presentation of neutral cues) – (Urge to Smoke before presentation of neutral cues)] as a covariate to control for nonspecific cue responsiveness. Based on research on alcohol and cocaine cues35, 36 suggesting the importance of studying both cue responders and non-responders and in order to provide as much information about the relationship of depression and cue reactivity, analyses were repeated twice for each condition: once with the full sample (N=52) which provided the greatest power for analyses and once with the subsample of participants who were “cue reactive” as defined above (n=37) in order to examine whether results differed after removing participants who did not initially respond to cues. Statistical analyses were performed using SPSS v.16.0 software for PC (SPSS Inc., Chicago, IL). Statistical tests were two-tailed and differences were considered significant when p<0.05.
RESULTS
Baseline demographics, smoking, and depression symptoms (Table 1)
See Table 1 for baseline demographic variables, cigarette consumption, and depression symptoms for the two diagnosis groups (MDD−, MDD+). MDD+ and MDD− participants did not differ on age, gender, ethnicity, age of smoking initiation, nicotine dependence, or amount of daily smoking (all ps>0.05). MDD+ smokers reported greater current symptoms of depression than MDD− smokers. In total, 37 participants exhibiting positive cue reactivity and MDD+ and MDD− groups did not significantly differ in the proportion of participants showing positive cue reactivity. The majority of MDD+Tx+ adults reported currently taking sertraline as their antidepressant medication (sertraline n=6; citalopram n=1; duloxetine n=1).
Changes in urge to smoke after presentation of smoking cues (Table 2)
Table 2.
Mean urge ratings before and after cue exposure when undergoing no smoking deprivation (Satiated Condition) and after one hour of smoking deprivation (Brief Deprivation Condition) for the full sample, participants with no history of Major Depressive Disorder (MDD−), and participants with a history of Major Depressive Disorder (MDD+). by diagnosis group and cue exposure condition.
| Full Sample (N=52) |
MDD− (n=31) |
MDD+ (n=21) |
|
|---|---|---|---|
| Satiated Condition | |||
| Pre-Cue Urge | 10.1 ± 15.1 | 10.0 ± 15.7 | 10.3 ± 14.5 |
| Post-Cue Urge | 23.9 ± 24.9 | 20.3 ± 23.6 | 29.0 ± 26.4 |
| Change in Urge* | 13.8 ± 18.3 | 10.3 ± 12.9 | 18.7 ± 23.5 |
|
| |||
|
Brief Deprivation
Condition |
|||
| Pre-Cue Urge | 34.48 ± 26.6 | 30.6 ± 28.0 | 39.9 ± 24.2 |
| Post-Cue Urge | 42.0 ± 28.1 | 39.5 ± 30.2 | 45.4 ± 25.4 |
| Change in Urge* | 7.5 ± 12.2 | 8.9 ± 11.2 | 5.5 ± 13.4 |
Change in Urge = Post-Cue Urge – Pre-Cue Urge
Note: all comparisons of urges across rows were n.s. (all ps>0.05)
In the Satiated Condition, after controlling for current symptoms of depression, urges to smoke increased after presentation of the smoking-related cues (F=5.99, df=1,45, p<0.05) and this increase did not differ by diagnosis group (F=0.87, df=1,45, p=0.36). Similarly, in the Brief Deprivation condition, controlling for current symptoms of depression, urges to smoke increased after presentation of the smoking-related cues (F=4.34, df=1,44, p<0.05), but this increase did not differ by diagnosis group (F=0.91, df=1,44, p=0.35). Results for each condition did not change substantially when repeated in the subsample of participants who showed positive cue reactivity. Overall, participants showed a greater response to smoking cues in the Satiated Condition compared to the Brief Deprivation Condition in the full sample (F=9.86, df=1,47, p<0.01) and the subsample of participants showing cue reactivity (F=14.08, df=1,33, p<0.01).
Exploratory analysis examining three diagnosis groups (MDD−, MDD+Tx−, MDD+Tx+) did not differ from the primary analyses for the Satiated Condition. In the Brief Deprivation Condition, after controlling for number of past quit attempts and current symptoms of depression, the change in smoking urge from pre- to post-cue exposure showed a significant time by diagnosis group interaction (F=4.28, df=2,41, p<0.05). MDD+Tx+ smokers, who had the highest level of pre-cue urges, reported a smaller average increase in urges (M=4.0, SD=11.01) than the MDD− (M=10.5, SD=2.2) and MDD+Tx− (M=8.7, SD=10.7) smokers. When the analysis was repeated for the subsample of participants showing cue reactivity, neither the main effect of time (F=51.48, df=1,28, p=0.23) nor the time by diagnosis group interaction (F=2.40, df=2,28, p=0.11) were significant.
Association of smoking and depression variables with cue-induced changes in smoking urges (Table 3)
Table 3.
Factors significantly associated with cue-induced changes in smoking urges during the Satiated Condition for the full sample (n=52) and participants who were cue responders (n=37).
| r | R2 | Adjusted R2 | Beta | t | p-value | |
|---|---|---|---|---|---|---|
| Full Sample (n=52) | ||||||
| HDRS-21 | 0.41 | 0.17 | 0.13 | 0.39 | 2.91 | <0.01 |
| CPD | 0.50 | 0.25 | 0.20 | 0.29 | 2.23 | <0.05 |
|
| ||||||
| Cue Reactive* (n=37) | ||||||
| HDRS-21 | 0.53 | 0.29 | 0.24 | 0.58 | 3.67 | <0.001 |
Key: HDRS, Hamilton Depression Rating Scale; CPD, cigarettes per day; FTND, Fagerström Test for Nicotine Dependence; BDI, Beck Depression Inventory
Note: All analyses controlled for changes in smoking urges in response to the neutral cues defined as: (Urge to smoke after presentation of the neutral cues) – (Urge to smoke before presentation of the neutral cues)
Cue Reactive was defined as: (Urge to smoke after presentation of the smoking cues) – (Urge to smoke before presentation of the smoking cues) > 0
Satiated Condition
In the analysis of the full sample, zero-order correlations showed that changes in urges after exposure to smoking-related objects was significantly and positively associated with daily smoking as measured by CPD (r=0.29, p<0.05), nicotine dependence as measured by the FTND (r=0.32, p<0.05), and current depression symptoms as measured by the BDI (r=0.29, p<0.05), HDRS-17 item (r=0.39, p<0.01), and HDRS-21 item (r=0.40, p<0.01). Change in urge was not associated with a diagnosis of depression, demographics (age, gender, race, education), or other smoking variables (e.g., age of smoking onset, number of quit attempts). When the significant factors were entered into a regression analysis, current depression symptoms (HDRS-21 item) remained a significant predictor (see Table 3). Greater depressive symptoms were associated with greater increases in urge after seeing smoking-related cues.
When analyses were repeated with the subsample of cue responders, the same variables showed significant and positive correlations with changes in urges (CPD, r=0.33, p<0.05; FTND, r=0.40, p<0.05; BDI, r=0.36, p<0.05; HDRS-17 item, r=0.49, p<0.01; HDRS-21 item, r=0.52, p<0.01). When these variables were entered into a regression analysis, two variables remained as significant predictors of changes in urges: HDRS-21 item and CPD. Greater current depressive symptoms and a higher number of cigarettes smoked per day were associated with greater increases in urge after seeing smoking-related cues.
Brief Deprivation Condition
No demographic, depression, or smoking variables were significantly correlated with changes in urges in the Brief Deprivation Condition for either the full sample or for the subgroup of cue reactors (all ps>0.05).
Changes in positive mood, negative mood, and physiological responsiveness after presentation of smoking cues
There were no differences in either the Satiated Condition or the Brief Deprivation Condition by diagnosis group for changes in negative mood, positive mood, or physiological responsiveness (i.e., blood pressure, temperature, pulse) with exposure to smoking cues (all ps>0.05).
DISCUSSION
Only a few studies have examined smoking cue reactivity in adults who exhibit higher rates of smoking and lower rates of smoking cessation than the general population like adults with psychiatric disorders30, 37. In this pilot study, smokers with depression showed the expected increase in urges to smoke after exposure to their preferred brand of cigarettes, lighters, and an ashtray. These findings are consistent with one previous study of cue reactivity in smokers with depression14 and suggest that smokers with depression respond to smoking cues in a similar manner as other smokers. Therefore, cue reactivity procedures may be a useful tool for studying the relationship smoking and depression. Cue reactivity procedures can be used both to test mechanistic aspects of smoking and depression (e.g., serotonergic neurotransmission38) as well as the effects of pharmacological39, 40 and non-pharmacological41 treatments on urges to smoke.
While this pilot study suggested that the cue reactivity procedure might be useful for studying smokers with depression, it also provided information about the usefulness of two smoking conditions (immediately after smoking a cigarette and one hour after smoking). The period of smoking deprivation in this pilot study was brief and selected based on previous work by this research group30. In this study, administering the cue reactivity procedure to satiated smokers provided more informative research data than administering the procedure to smokers undergoing smoking deprivation of only one hour. Future studies could examine the response to smoking cues after longer periods of deprivation. Because this was a laboratory study, cue reactivity was not studied in relation to smoking cessation behavior such as the ability to quit smoking or the ability to avoid smoking relapse. Previous research found significant associations between cue reactivity and cessation outcomes in non-depressed adults19-21. It would be beneficial for future studies to examine smoking cue reactivity under conditions of satiety and varying periods of deprivation to determine the conditions that yield the most useful information for clinical outcomes.
There was less variation in increases in urges from pre-cue to post-cue exposure and less of an overall response to the smoking cues in the Brief Deprivation Condition compared to the Satiated Condition. This difference may have been due in part to the short time frame between the conditions, the within-subjects nature of the design, or other factors related to the structure of the session. For example, participants knew they would not be able to smoke for more than an hour while completing the Satiated Condition, but knew they would be able to smoke again shortly during the Brief Deprivation Condition. Further, all participants experienced the same cue reactivity session twice and in the same condition order (the Satiated Condition first, followed by the Brief Deprivation Condition without counterbalancing of the conditions). The greater urge response in the Satiated Condition may have been due in part to the novelty of experiencing the smoking cues for the first time. Also, pre-cue urges were much higher in the Brief Deprivation Condition than the Satiated Condition and the decreased variability in urges during the Brief Deprivation Condition may reflect a ceiling effect. Ceiling effects have been suggested in previous research to be a potential issue in the study of smoking urges especially for participants undergoing conditions of smoking deprivation who are likely to report high levels of cravings prior to cue exposure42, 43. While these decisions regarding the study design were made in response to the sample size of this pilot study, our past research30, and research reporting carryover effects when neutral cues are presented before smoking cues27, future studies with larger samples would have the ability to examine outcomes using random assignment to or counterbalancing of length of abstinence conditions.
Current symptoms of depression, but not a diagnosis of MDD, were significantly associated with cue-induces urges to smoke in satiated smokers. Symptoms of depression have been linked to less success in smoking cessation (e.g., Perez et al44, Niaura et al45, Cinciripini et al46). Further, negative affect, a primary symptom of depression, has been associated with many aspects of smoking (see Kassel et al47 for a review) including difficulty quitting smoking and smoking relapse18, 48-50. Smokers experiencing active depressive symptoms may have a stronger reaction to and find it more difficult to resist smoking around smoking cues. These findings suggest that valuable information could be gained by assessing depressive symptoms, in addition to a diagnosis of depression, when conducting research to better understand the relationship of depression to higher rates of smoking and lower rates of smoking cessation. Clinically, it may be more beneficial for health professionals and clinicians providing smoking cessation to adults to focus on current symptoms of depression, using validated measures of depression like the BDI and HDRS, rather than a diagnosis of depression per se.
In the current study, smokers taking antidepressant medications reported a greater number of quit attempts and current symptoms of depression than smokers with no history of depression. In addition, a significant interaction of time and depression diagnosis was found for cue reactors when smokers with antidepressant treatment were considered as a separate group in the analyses. Most of the research on smoking and antidepressants has been related to testing antidepressant medications as agents for smoking cessation (e.g., Hall et al51; Evins et al52; Spring et al53). Little is known about the ways in which smokers receiving antidepressants differ from other smokers. A study of 1,498 patients with coronary heart disease54 found that smokers using antidepressant medications, compared to smokers not using antidepressant medications, were less likely to quit smoking over a 9 month follow-up period and reported greater anxiety, depression, and insomnia.
Because the sample of smokers on antidepressant medications in this study was small, these results should be considered preliminary and exploratory. Additional research with larger samples, is needed to learn more about this rarely studied group of smokers and how antidepressant treatment might impact smoking behavior and smoking cessation outcomes. Smokers being treated for depression in the current study were primarily taking sertraline and limited information about treatment-related factors (e.g., duration of antidepressant treatment) was available for analyses. This research would benefit from including analysis of treatment-related variables such as type, dose, and duration of antidepressant treatment to determine whether these factors play a role in the association of depression symptoms to cue-induced urges to smoke and other smoking behavior.
Women experience higher rates of depression3 and exhibit a stronger relationship between depression and smoking behavior55. Further, gender differences in responsiveness to smoking cues have been reported in adult smokers in conditions of both satiety and deprivation14, 56-58. The size of the sample in this pilot study did not allow for analysis of the results by gender. Future studies should examine the role of gender in the relationship of depression and cue-induced smoking urges.
In addition to the brief period of time between conditions and the small sample size, other limitations should be noted. The current study was conducted in a laboratory setting. While the cue reactivity procedure reliably elicits urges to smoke and research suggests that in vivo cues, such as the ones used in the current study, elicit equal or greater levels of craving than imaginal or photographic cues56, 59, 60, the laboratory setting does not replicate urge-inducing experiences in the outside world. In addition, the cues in the current study did not elicit significant changes in physiological responses which may have been due to aspects of the laboratory setting and procedure. The laboratory setting has the advantage of allowing researchers to gain information about mechanisms of cravings and relapse than cannot easily be studied and which can be applied to future research in real world settings. Finally, participants in the study were primarily Caucasian, in their mid-40s; smoked approximately one pack of cigarettes per day; were not seeking treatment for smoking; were free from current substance and alcohol use, abuse, and dependence; and did not meet diagnostic criteria for any major Axis I disorder other than MDD or nicotine dependence. Thus, results may not generalize to other groups of smokers.
Smokers with depression respond to smoking cues with increased urges to smoke and current symptoms of depression were related to greater increase in cue-related urges. Cue reactivity procedures may provide useful information to gain a better understanding of the links between smoking and depression and to help improve smoking cessation outcomes and prevent smoking relapse for this group of smokers.
ACKNOWLEDGMENTS
We thank Taryn Allen, B.A., Cerissa L. Creeden, M.A., Erin L. Reutenauer, B.A., Kristi Sacco, Psy.D., Monica Solorzano, B.A., and Jennifer Vessicchio, L.C.S.W. for clinical and technical assistance with this study. This work was supported by the National Alliance for Research on Schizophrenia and Depression (Young Investigator Award to AHW); the National Institute on Drug Abuse (grants K12-DA000167 to AHW, R01DA14039 to TPG, and RL1DA024857 to SAM); and the Endowed Chair in Addiction Psychiatry at the University of Toronto and Operating Grants from the Canadian Institutes on Health Research and the Canadian Tobacco Control Research Initiative (to TPG).
Contributor Information
Andrea H. Weinberger, Division of Substance Abuse, Department of Psychiatry, Yale University School of Medicine, New Haven, Connecticut, USA.
Sherry A. McKee, Division of Substance Abuse, Department of Psychiatry, Yale University School of Medicine, New Haven, Connecticut, USA.
Tony P. George, Division of Addiction Psychiatry, Department of Psychiatry, University of Toronto and Schizophrenia Program, Centre for Addiction and Mental Health (CAMH), Toronto, Ontario, Canada.
REFERENCES
- 1.Lasser K, Boyd JW, Woolhander S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 2.Dierker L, Donny E. The role of psychiatric disorders in the relationship between cigarette smoking and DSM-IV nicotine dependence among young adults. Nicotine Tob Res. 2008;10(3):439–446. doi: 10.1080/14622200801901898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant BF, Hasin DS, Chou P, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 4.Walsh Z, Epstein A, Munisamy G, King A. The impact of depressive symptoms on the efficacy of naltrexone in smoking cessation. J Addict Dis. 2008;27(1):65–72. doi: 10.1300/J069v27n01_07. [DOI] [PubMed] [Google Scholar]
- 5.Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL. Depression and the dynamics of smoking: A national perspective. JAMA. 1990;264(12):1541–1545. [PubMed] [Google Scholar]
- 6.Wilhelm K, Wedgwood L, Niven H, Kay-Lambkin F. Smoking cessation and depression: Current knowledge and future directions. Drug Alcohol Rev. 2006;25(1):97–107. doi: 10.1080/09595230500459560. [DOI] [PubMed] [Google Scholar]
- 7.Kinnunen T, Henning L, Nordstrom BL. Smoking cessation in individuals with depression: Recommendations for treatment. CNS Drugs. 1999;11(2):93–103. [Google Scholar]
- 8.Ziedonis D, Hitsman B, Beckham JC, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008;10(12):1691–1715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- 9.John U, Meyer C, Rumpf H-J, Hapke U. Smoking, nicotine dependence and psychiatric comorbidity--a population-based study including smoking cessation after three years. Drug Alcohol Depend. 2004;76(3):287–295. doi: 10.1016/j.drugalcdep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Wiesbeck GA, Kuhl HC, Yaldizli O, Wurst FM. WHO/ISBRA Study Group on Biological State and Trait Markers of Alcohol Use and Dependence. Tobacco smoking and depression--results from the WHO/ISBRA study. Neuropsychobiology. 2008;57(1-2):26–31. doi: 10.1159/000123119. [DOI] [PubMed] [Google Scholar]
- 11.Breslau N, Johnson EO. Predicting smoking cessation and major depression in nicotine-dependent smokers. Am J Public Health. 2000;90(7):1122–1127. doi: 10.2105/ajph.90.7.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breslau N, Kilbey MM, Andreski P. Nicotine withdrawal symptoms and psychiatric disorders: Findings from an epidemiologic study of young adults. Am J Psychiatry. 1992;149(4):464–469. doi: 10.1176/ajp.149.4.464. [DOI] [PubMed] [Google Scholar]
- 13.Weinberger AH, Desai R, McKee SA. Nicotine withdrawal in U.S. smokers with current mood, anxiety, alcohol, and substance use disorders. Drug Alcohol Depend. 2010;108:7–12. doi: 10.1016/j.drugalcdep.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Ninowski R, Sen A. Nicotine dependence, depression, and gender: Characterizing phenotypes based on withdrawal discomfort, response to smoking, and ability to abstain. Nicotine Tob Res. 2005;7(1):91–102. doi: 10.1080/14622200412331328466. [DOI] [PubMed] [Google Scholar]
- 15.Niaura R, Britt DM, Borrelli B, Shadel WG, Abrams DB, Goldstein MG. History and symptoms of depression among smokers during a self-initiated quit attempt. Nicotine Tob Res. 1999;1(3):251–257. doi: 10.1080/14622299050011371. [DOI] [PubMed] [Google Scholar]
- 16.Piasecki TM. Relapse to smoking. Clinical Psychology Review. 2006;26(2):196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- 18.Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychology. 1996;64(2):366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- 19.Payne TJ, Smith PO, Adams SG, Diefenbach L. Pretreatment cue reactivity predicts end-of-treatment smoking. Addict Behav. 2006;31(4):702–710. doi: 10.1016/j.addbeh.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 20.Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI. Reactivity to smoking cues and relapse: Two studies of discriminant validity. Behav Res Ther. 1988;26(3):225–233. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- 21.Janes AC, Pizzagalli DA, Richardt SdeB, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67(8):722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niaura R, Abrams DB, Monti PM, Pedraza M. Reactivity to high risk situations and smoking cessation outcome. J Subst Abuse. 1989;1(4):393–405. [PubMed] [Google Scholar]
- 23.Weissman MM, Sholomakis D, Pottenger M, Prushoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: A validation study. Epidemiology. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 24.Robbins L, Wing J, Wittchen H, Helzer JE. The Composite International Diagnostic Interview: An epidemiologic instrument suitable for use in conjunction with difference diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- 25.Sayette MA, Hufford MR. Effects of cue exposure and deprivation on cognitive resources in smokers. J Abnorm Psychol. 1994;103:812–818. doi: 10.1037//0021-843x.103.4.812. [DOI] [PubMed] [Google Scholar]
- 26.Shadel WG, Niaura R, Abrams DB. Effect of different cue stimulus delivery channels on craving reactivity: Comparing in vivo and video cues in regular cigarette smokers. J Behav Ther Exp Psychiatry. 2001;32(4):203–209. doi: 10.1016/s0005-7916(01)00035-0. [DOI] [PubMed] [Google Scholar]
- 27.Sayette MA, Griffin KM, Sayers WM. Counterbalancing in smoking cue research: A critical analysis. Nicotine Tob Res. 2010;12(11):1068–1079. doi: 10.1093/ntr/ntq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 29.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 30.Fonder MA, Sacco KA, Termine A, et al. Smoking cue-reactivity in schizophrenia: Effects of a nicotinic receptor antagonist. Biol Psychiatry. 2005;57:802–808. doi: 10.1016/j.biopsych.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Hutchison KE, Niaura R, Swift R. Smoking cues decrease prepulse inhibition of the startle response and increase subjective craving in humans. Exp Clin Psychopharmacol. 1999;7(3):250–256. doi: 10.1037//1064-1297.7.3.250. [DOI] [PubMed] [Google Scholar]
- 32.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Press; Washington, D.C.: 1997. [Google Scholar]
- 33.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 34.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szegedi A, Lörch B, Scheurich A, Ruppe A, Hautzinger M, Wetzel H. Cue exposure in alcohol dependent patients: Preliminary evidence for different types of cue reactivity. J Neural Transm. 2000;107(6):721–730. doi: 10.1007/s007020070073. [DOI] [PubMed] [Google Scholar]
- 36.Avants SK, Margolin A, Kosten TR, Cooney NL. Differences between responders and nonresponders to cocaine cues in the laboratory. Addict Behav. 1995;20(2):215–225. doi: 10.1016/0306-4603(94)00066-2. [DOI] [PubMed] [Google Scholar]
- 37.Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Subjective and physiological responses to smoking cues in smokers with schizophrenia. Nicotine Tob Res. 2005;7(3):421–429. doi: 10.1080/14622200500125724. [DOI] [PubMed] [Google Scholar]
- 38.Hitsman B, Spring B, Pingitore R, Munafo M, Hedeker D. Effect of tryptophan depletion on the attentional salience of smoking cues. Psychopharmacol. 2007;192(3):317–324. doi: 10.1007/s00213-007-0722-2. [DOI] [PubMed] [Google Scholar]
- 39.Hutchison KE, Monti PM, Rosenhow DJ, et al. Effects of naltrexone with nicotine replacement on smoking cue reactivity: preliminary results. Psychopharmacol. 1999;142:139–143. doi: 10.1007/s002130050872. [DOI] [PubMed] [Google Scholar]
- 40.Rohsenow DJ, Monti PM, Hutchison KE, et al. High-dose transdermal nicotine and naltrexone: Effects on nicotine withdrawal, urges, smoking, and effects of smoking. Exp Clin Psychopharmacol. 2007;15(1):81–92. doi: 10.1037/1064-1297.15.1.81. [DOI] [PubMed] [Google Scholar]
- 41.Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction. 2009;104(4):653–660. doi: 10.1111/j.1360-0443.2008.02448.x. [DOI] [PubMed] [Google Scholar]
- 42.Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95(Suppl 2):S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96(10):1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez GH, Nicolau JC, Romano BW, Laranjeira R. Depression: a predictor of smoking relapse in a 6-month follow-up after hospitalization for acute coronary syndrome. Eur J Cardiovasc Prev Rehabil. 2008;15(1):89–94. doi: 10.1097/HJR.0b013e3282f4b212. [DOI] [PubMed] [Google Scholar]
- 45.Niaura R, Britt DM, Shadel WM, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychol Addict Behav. 2001;15(1):13–17. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- 46.Cinciripini PM, Wetter DW, Fouladi RT, et al. The effects of depressed mood on smoking cessation: mediation by postcessation self-efficacy. J Consult Clin Psychol. 2003;71(2):292–301. doi: 10.1037/0022-006x.71.2.292. [DOI] [PubMed] [Google Scholar]
- 47.Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- 48.Strasser AA, Kaufmann V, Jepson C, et al. Effects of different nicotine replacement therapies on postcessation psychological responses. Addict Behav. 2005;30(1):9–17. doi: 10.1016/j.addbeh.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: Contrasting affective and physical models of dependence. J Consult Clin Psychol. 2002;70(1):216–227. [PubMed] [Google Scholar]
- 50.Strong DR, Kahler CW, Leventhal AM, et al. Impact of bupropion and cognitive-behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine Tob Res. 2009;11(10):1142–1153. doi: 10.1093/ntr/ntp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall SM, Reus VI, Munoz RF, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry. 1998;55:683–690. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]
- 52.Evins AE, Culhane MA, Alpert JE, et al. A controlled trial of bupropion added to nicotine patch and behavioral therapy for smoking cessation in adults with unipolar depressive disorders. J Clin Psychopharmacol. 2008;28(6):660–666. doi: 10.1097/JCP.0b013e31818ad7d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spring B, Doran N, Pagoto S, et al. Fluoxetine, smoking, and history of major depression: A randomized controlled trial. J Consult Clin Psychol. 2007;75(1):85–94. doi: 10.1037/0022-006X.75.1.85. [DOI] [PubMed] [Google Scholar]
- 54.Gravely-Witte S, Stewart DE, Suskin N, Grace SL. The association among depressive symptoms, smoking status and antidepressant use in cardiac outpatients. J Behav Med. 2009;32(5):478–490. doi: 10.1007/s10865-009-9218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Husky MM, Mazure CM, Paliwal P, McKee SA. Gender differences in the comorbidity of smoking behavior and major depression. Drug Alcohol Depend. 2008;93:176–179. doi: 10.1016/j.drugalcdep.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: Effects of gender and cue type. Addict Behav. 1998;23(2):209–224. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- 57.Field M, Duka T. Cue reactivity in smokers: The effects of perceived cigarette availability and gender. Pharmacol Biochem Behav. 2004;78(3):647–652. doi: 10.1016/j.pbb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 58.Heishman SJ, Lee DC, Taylor RC, Singleton EG. Prolonged duration of craving, mood, and autonomic responses elicited by cues and imagery in smokers: Effects of tobacco deprivation and sex. Exp Clin Psychopharmacol. 2010;18(3):245–256. doi: 10.1037/a0019401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: Physiological and self-report manifestations. J Abnorm Psychol. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- 60.Niaura R, Abrams DB, Pedraza M, Monti P, Rohsenow DJ. Smokers’ reactions to interpersonal interaction cues and presentation of smoking cues. Addict Behav. 1992;17:557–566. doi: 10.1016/0306-4603(92)90065-4. [DOI] [PubMed] [Google Scholar]


