Abstract
Host lectin-like recognition molecules may play an important role in innate resistance in Biomphalaria glabrata snails to larval schistosome infection, thus implicating parasite-expressed glycans as putative ligands for these lectin receptors. While host lectins may utilize specific glycan structures for parasite recognition, it also has been hypothesized that the parasite may use this system to evade immune detection by mimicking naturally-expressed host glycans, resulting in reduced immunorecognition capacity. By employing immunocytochemical (ICC) and Western blot assays using schistosome glycan-specific monoclonal antibodies (mABs) we sought to identify specific glycan epitopes (glycotopes) shared in common between larval S. mansoni and B. glabrata hemocytes, the primary immune effector cells in snails. Results confirmed the presence of selected larval glycotopes on subpopulations of hemocytes by ICC and association with numerous hemocyte proteins by Western blot analyses, including a trimannosyl core N-glycan (TriMan), and two fucosylated lacdiNAc (LDN) variants, F-LDN and F-LDN-F. Snail strain differences were seen in the prevalence of constitutively expressed F-LDN on hemocytes, and in the patterns of protein immunoreactivity with these mABs. In contrast, there was little to no hemocyte reactivity with mABs for Lewis X (LeX), LDN, LDN-F or LDN-DF. When intact hemocytes were exposed to larval transformation products (LTPs), distinct cell subpopulations displayed weak (LeX, LDN-DF) to moderate (LDN, LDN-F) glycotope reactivity by ICC, including snail strain differences in the prevalence of LDN-reactive cellular subsets. Far-Western blot analyses of the hemocytes following exposure to larval transformation proteins (LTPs) also revealed multiple mAB-reactive hemocyte protein bands for LeX, LDN, LDN-F, and LDN-DF. These results demonstrate the existence of complex patterns of shared larval glycan constitutively expressed on hemocytes and their proteins, as well as the ability or hemocytes to acquire shared glycans by the selective binding of parasite-released LTP. Unraveling the functional significance of these naturally expressed and acquired shared glycans on specific hemocyte populations represents an important challenge for future investigations.
Keywords: Biomphalaria glabrata, Schistosoma mansoni, hemocyte, molecular mimicry, glycotope sharing, sporocyst, larval transformation products (LTP), glycoconjugate
1. Introduction
The immunologic mechanisms underlying larval blood fluke-snail host compatibility are still poorly understood, although it has been recently demonstrations that members of the highly-diversified lectin family of fibrinogen-related proteins (Freps) are functionally-associated with host resistance to echinostome and schistosome infections in Biomphalaria glabrata (Hanington et al., 2010; 2012). This suggests that Freps, and possibly other lectin-like hemolymph proteins, may be serving as important pattern recognition receptors (PRRs) in this system (Stout et al., 2009; Hanington and Zhang, 2011; Mitta et al., 2012). Carbohydrate determinants (glycans) associated with the larval surface or secreted glycoconjugates by larval stages have been identified as possible pathogen-associated molecular patterns (PAMPS) for which the PRRs bind. Because these glycans are in abundance and structurally diverse (Nyame et al., 2002; Lehr et al., 2008; Peterson et al. 2009) and are expressed on glycoproteins exhibiting high genetic polymorphism (Roger et al., 2008a, b), it has been hypothesized that the molecular diversity exhibited in this host- receptors and parasite-ligand recognition system provides a mechanism to explain why some schistosome-snail combinations are compatible, leading to infection, while others are not, resulting in immune-mediated larval encapsulation and infection failure (Bayne, 2009; Mitta et al., 2012; Yoshino and Coustau, 2011).
The laboratory model consisting of inbred strains of the snail B. glabrata and various Schistosoma mansoni parasite strains has been extensively used to characterize the cellular and molecular events surrounding host recognition and reactivity to invading larvae. Hemocytic encapsulation, the primary effector mechanism characteristic of the resistant phenotype in B. glabrata (Sullivan and Richards, 1981), consists of hemocyte attraction towards, infiltration around and attachment to early developing mother sporocysts (Loker et al., 1982). Although soluble hemolymph (plasma) proteins may be involved in this process (Bayne et al., 1980; Hanington et al., 2010), it is the molecular signaling, presumably mediated by membrane receptors, that ultimately dictates the taxis and adhesion events characteristic of encapsulation responses, or the down-regulation of hemocyte response (Loker et al., 2004; Bayne, 2009). However, at present there is little known about the identity of these receptors, their putative functions or how their activities are regulated.
As alluded to above, there is mounting evidence supporting the involvement of a lectinglycan recognition mechanism(s) in determining the eventual outcome (compatibility/incompatibility) of a given snail-larval encounter. However, the molecular basis of this recognition system is complex, involving not only plasma lectin-larval glycoconjugate/glycan interactions (Mitta et al., 2012), but also interactions between hemocyte receptors directly with larval glycans or indirectly with soluble immune complexes comprised of glycoconjugate-lectin aggregates (Mone et al., 2010). Before a clear understanding of how hemocytic encapsulation is accomplished or how larvae manage to avoid or escape encapsulation reactions, a dissection of the specific molecules present at the host-parasite interface early in the invasion process is needed. Information regarding the molecules involved in presumably multiple receptor-ligand interactions will provide important insights into those reactivity profiles that lead to host resistance phenotypes (incompatibility) or those associated with susceptibility (compatibility).
In a lectin-based immunorecognition system, one of the mechanisms by which larval trematodes may avoid or modulate recognition responses is through expression and display of glycans that are identical to those of the snail host. This general strategy, termed molecular mimicry (Damian, 1964), poses simply that the possession of antigens shared in common between host and parasite leads to reduced immune recognition and, consequently, decreased host reactivity. Recently, widespread sharing of specific carbohydrate epitopes (glycotopes) between larval schistosomes and cell-free hemolymph (plasma) of B. glabrata snails has been reported (Lehr et al. 2007; Peterson et al., 2009; Lehr et al., 2010; Yoshino et al., 2012) for the first time identifying specific larval glycans associated with host plasma proteins. This latest study also demonstrated that treatment of plasma with glycoconjugates released during in vitro miracidium-to-sporocyst transformation (larval transformation products or LTPs) resulted in dramatic changes in glycotopes displayed by subsets of plasma proteins, presumably by complexing with LTP components. In the present study, we incorporated a panel of monoclonal antibodies that recognize specific terminal glycan elements expressed on glycoproteins of larval S. mansoni and those released in LTP (Peterson et al., 2009), to determine if B. glabrata hemocytes naturally express these same glycotopes and to assess the effects of LTP-exposure on the composition and display of shared glycans by hemocytes of S. mansoni-susceptible and –resistant snail strains.
2. Materials and Methods
2.1. Maintenance of Biomphalaria glabrata snails and larval Schistosoma mansoni
Two strains of B. glabrata, the S. mansoni-susceptible NMRI strain and -resistant BS-90 strain, were used in all experiments. Snails were maintained in 20-gal aquaria at 26°C under a 12hr light:12hr dark photoperiod. They were fed leaf-lettuce ad libitum supplemented with Tetramin® fish food and chalk. Mice, infected with the NMRI strain of S. mansoni, were provided by the Biomedical Research Institute (Rockville, MD) under a NIAID contract to BEI Resources (Manassas, VA). At 6–7 weeks post-infection, mice were euthanized and necropsied for removal of livers and and axenic egg isolation (Yoshino and Laursen, 1995). Mice were maintained in an AAALAC-approved animal housing facility (Animal Resource Center, University of Wisconsin-Madison) using standard-of-care cage housing and feeding. Animal health monitoring was provided by veterinary technicians with oversight by a full-time staff veterinarian. All animal handling and experimental protocols were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee (IACUC) under Animal Welfare Assurance no. A3368-01.
2.2. Preparation of snail hemocytes for Western/far-Western blot analyses
Snail hemolymph (HL) from S. mansoni-susceptible and –resistant B. glabrata snail strains (NMRI and BS90 strains, respectively) was collected by the headfoot retraction method (Sminia and Barendsen, 1980). HL from 5–6 snails (12–15 mm in diameter) was first pipetted into a petri dish on ice to permit the settling and removal of any shell debris. Blood was then transferred to a 15-mL sterile centrifuge tube containing cold, sterile snail phosphate-buffered saline (sPBS; 8.41 mM Na2HPO4, 1.65 mM NaH2PO4·H2O, 45.34 mM NaCl, pH 7.2). This process was repeated until HL from ~70 snails was collected, eventually yielding a 1:1 dilution of sPBS:HL on ice after the final collection. The sPBS/HL was then centrifuged at 1000 rpm (Eppendorf 5810R, Hauppauge, NY) for 9 min at 4°C to pellet hemocytes, the plasma was removed and the hemocyte pellet washed twice with 14 mL of cold sPBS followed by centrifugation. After washing, hemocytes were resuspended in ~1 mL sPBS, and transferred to a 2-mL microcentrifuge tube where they were washed two additional times by centrifugation at 1000 rpm for 3 min at 4°C. Following removal of the wash buffer, hemocytes were resuspended in 60 μL of sPBS containing protease inhibitors (Protease Inhibitor Cocktail Set III, EDTA free, Calbiochem) and stored at −20°C. After five samples were collected, hemocytes were thawed and pooled for Western and far-Western blot analyses as described below.
2.3. Preparation of S. mansoni larval transformation products (LTPs)
Macromolecules (mainly glycoproteins) released during in vitro miracidium-to-sporocyst transformation (LTPs; Wu et al., 2009) were incorporated into experiments to determine if hemocytes possess LTP-binding receptors and, if so, whether LTP binding affects the profile of shared larval glycotopes displayed by intact hemocytes (ICC) or hemocyte proteins (far-Western blot). To generate LTPs, eggs were obtained from infected mouse livers and processed as described in Yoshino and Laursen (1995). Miracidia, hatched from eggs under axenic conditions, were placed into in vitro culture in Chernin's balanced salt solution (CBSS, Chernin, 1963) supplemented with penicillin and streptomycin and 1 g/L each of glucose and trehalose (CBSS+). Culture supernatants containing LTPs were harvested after 24 hr and filtered with a Whatman Puradisc 0.45 uM syringe filter (GE Healthcare LTD, Buckinghamshire, UK) to remove any sporocysts, epidermal plates, and cellular debris. Supernatants were then concentrated by ultrafiltration (Amicon, 3 kDa nominal MW cut-off; Millipore Corp, Billerica, MA) and the protein concentration determined using a Nanodrop ND-1000 (Nanodrop, Wilmington, DE). Protease inhibitors were added (Protease Inhibitor Cocktail Set III, EDTA free, Calbiochem) and aliquots were stored at 4°C if used within a week, or at −80°C for longer-term storage.
2.4. Anti-S. mansoni glycan monoclonal antibodies
Mouse monoclonal antibodies (mABs) exhibiting reactivities to specific terminal di-, triand tetrasaccharides associated with schistosome glycans were generated from Schistosoma-infected mice and their glycan-specific reactivities identified and validated by rigorous screening against a panel of synthetic neoglycoproteins bearing defined schistosome-related glycan determinants (van Remoortere et al., 2000; Robijn et al., 2005; van Roon et al. 2005). Included in the present study were mABs with glycotope specificities to lacdiNAc [LDN: GalNAc(β1–4)GlcNAc; mAB#273-3F2]; fucosylated lacdiNAc variants [F-LDN: Fuc(α1–3)GalNAc(β1–4)GlcNAc; mAB#291-5D5; F-LDN-F: Fuc(α1–3)GalNAc(β1–4)[Fuc(α1–3)]GlcNAc; mAB#128-1E7-C; LDN-F: GalNAc(β1–4)[Fuc(α 1–3)]GlcNAc; mAB#114-4E8-A; LDN-DF: GalNAc(β1–4)[Fuc(α1–2)Fuc(α1–3)]GlcNAc; mAB#114-5B1-A; trimannosyl core N-glycan [TriMan: Man(α1–6)[Man(α1–3)]Man(β1–4)GlcNAc(β1–4)GlcNAc; mAB#100-4G11-A], and Lewis X [LeX: Galβ1–4[Fuc(α1–3)]GlcNAc; mAB#128-4F9-A].
2.5. Western and far-Western blot analyses
Antibody reactivity to glycotopes shared by hemocyte glycoproteins of susceptible NMRI and resistant BS-90 strains of B. glabrata snails were evaluated using Western and far-Western blot analyses of proteins derived from single populations of pooled cells. This allowed for simultaneous, direct comparisons of the effects of LTP exposure on mAB reactivity to cellular proteins of both snail strains. The technique of far-Western blot analysis is commonly used to test for protein-protein interactions (Edmondson and Dent, 2001; Hall, 2004), in which bait proteins are first separated by standard SDS-PAGE, blotted to a membrane, followed by exposure to a second protein mixture (serving as probe). This is followed by evaluation of bait vs. probe binding interactions, which, in the present experiments, employed glycan-specific mABs to detect the binding of LTP glycoconjugates to blotted hemocyte proteins.
2.5.1. “Normalization” of hemocyte samples
Hemocytes, isolated and pooled from the NMRI and BS-90 strains of B. glabrata as described above, were first subjected to SDS-PAGE by combining equal volumes of resuspended hemocytes and SDS sample loading buffer (EMD Chemcals, San Diego, CA), followed by incubation at 22°C for 10 min, heating at 95°C for 5 min, and separating on 12.5% polyacrylamide gels (Yoshino et al., 2012). Gels were then stained for protein with Coomassie blue and silver (Coomassie Brilliant Blue & SilverQuest, Invitrogen Co., Carlsbad, CA), and based on relative staining intensities between NMRI and BS-90 hemocyte samples, they were adjusted by volume to create approximately equivalent hemocyte loads between strains (Fig. 1).
Figure 1. “Normalized” hemocyte protein samples from NMRI and BS-90 Biomphalaria glabrata snail strains.
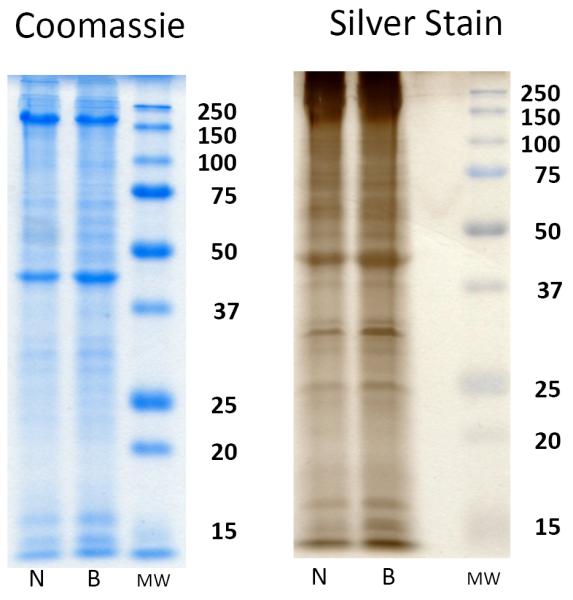
Prior to Western/far-Western blot analyses of shared glycotopes, single populations of pooled hemocytes were isolated from the NMRI (N) and BS-90 (B) strains of B. glabrata, subjected to SDS-PAGE, and stained with Coomassie brilliant blue or silver. Based on initial staining intensities, hemocyte samples were adjusted so NMRI and BS-90 snails exhibited similar intensities of protein banding indicating comparable sample loading between strains. Molecular weight markers are indicated on the right.
2.5.2. Western/far-Western blot experiments
After “normalizing” hemocyte extracts between snail strains, 15 μL of 4× SDS loading buffer were added to a 60 μL cell extract, followed by placement of 18 μL-aliquots of NMRI and BS-90 snail samples into alternating lanes of an SDS-PAGE slab gel to facilitate direct comparisons between strains. Separated proteins were transferred to a nitrocellulose membrane, cut to isolate individual NMRI/BS-90 snail pairs, and blocked overnight at 4°C in a Tris-buffered saline blocking solution (TBS; 20mM Tris-HCl, 150 mM NaCl, pH 7.4) containing 5% bovine serum albumin (TBS-BSA). After blocking and washing in TBS containing 0.05%Tween-20 (TBS-T), one NMRI/BS-90 paired blot was incubated in LTP (50 μg protein/mL blocking buffer) while a corresponding paired blot was incubated in blocking buffer only. Paired blots (LTP-treated and untreated) were then incubated overnight at 4°C, followed by 3 washes with TBS-T, incubation in blocking buffer with a glycan-specific mAB for 2 hr at 22°C, and again washed 3× with TBS-T. Finally blots were incubated in an alkaline phosphatase (AP)-conjugated rabbit anti-mouse Ig antiserum (Promega, Madison, WI) for 1 hr at 22°C, washed, and reactions visualized using AP color developing reagents (Pierce, Rockford, IL). Western and far-western analyses were performed a minimum of three times, each representing independent biological samples.
2.6. Preparation of snail hemocytes for Immunocytochemistry (ICC)
HL was collected from NMRI and BS90 snails on ice as described above. For ICC assays, however, HL from four snails of each strain was combined 1:1 with cold CBSS in 1.5 mL microcentrifuge tubes, followed by transfer of equal aliquots to wells of a 16-well chamber slide (CultureWell Chambered Coverglass, Invitrogen, Eugene, OR). Hemocytes were allowed to settle and attach to the slide surface for 1 hr at 22°C, after which time the plasma was removed and adherent cells carefully washed 7 times with CBSS. After the final wash, hemocytes were fixed with 4% buffered paraformaldehyde for 1 hr at 22°C, washed 5 times with sPBS, followed by incubation for 2 hr in a 1% BSA/sPBS blocking solution at 22°C. This design permitted the simultaneous testing and direct comparison of antibody reactivity on LTP-treated and untreated hemocyte samples from a single cell population. Three to six independent biological replicates were carried out for each anti-glycan antibody and treatment being tested (see below for details of experimental design).
2.6.1. Fluorescence microscopy
Following blocking of fixed hemocytes, one set of cells for each strain was incubated in an LTP/blocking buffer solution (1μg LTP/100μL) while an identical set remained in blocking buffer only. After incubating overnight at 4°C in a humidity chamber, hemocytes were washed 5 times with sPBS, incubated in mAB (diluted 1:25 in block buffer) for 2 hr at 22°C, and washed an additional 5 times with sPBS. Finally, cells were incubated in Hoechst 33342 (2.2 μg/mL) (Invitrogen, Eugene, OR) and AlexaFluor 488-conjugated goat anti-mouse antibody (4.2 μg/mL) (Invitrogen, Eugene, OR), both in block buffer, for 2 hr at 22°C in the dark. After washing five times with sPBS, wells were either viewed immediately for microscopic analysis or stored at 4°C in a light-tight humidity chamber for later inspection. For analysis, cells were visualized and epifluorescence images were collected at 200× and 400× total magnification using a Nikon inverted epifluorescence microscope (Eclipse TE300; Nikon Instruments, Inc., Melville, NY, USA) equipped with a CoolPix digital camera interfaced with MetaMorph v7.0 software. Cells were first visualized by brightfield/Nomarski illumination to assess morphology, then nuclear staining was used to confirm the presence of intact cells. Gain boundaries for mAB detection were preset through the MetaMorph program to normalize parameters at which all mAB staining was measured. Monoclonals to LDN, LDN-F, LDN-DF, and Lewis × were set at 100 and 200 for background and signal, respectively, while F-LDN, F-LDN-F and TriMan were set at 150 and 350, due to their increased staining intensities. These parameters allowed for detection of all positive mAB reactions, while yielding good contrast. Three sets of digital images, including a Nomarski/brightfield, nuclear stained and mAB stained, were captured for each 400× field and saved for later analysis. Over 100 cells were evaluated and recorded from at least 6 randomly chosen fields. Field selection routinely included imaging the middle and coordinate edges of each well.
2.6.2. Data analysis
Cell images, captured as .tif files, were analyzed using MetaMorph v7.0 software. Although mABs displayed different intensities and/or patterns of fluorescent staining, a cell was considered positive if a clear fluorescent signal was detected at the established gain settings. The total number of cells for a given population was quantified by counting the number of nuclei present in the images and the percentage of positive hemocytes was then calculated. A total of 3–6 independent replicates were performed for each mAB tested (N=3–6), and mean variances (+/−standard deviation) were statistically analyzed using a 2-factor ANOVA incorporating LTP treatment and snail strain as variables (AnalysisToolPak, Microsoft Excel).
3. Results
Preliminary characterization of hemocyte protein profiles and standardization of protein sample loading by SDS-PAGE analysis indicated that hemocytes derived from the susceptible NMRI and resistant BS-90 strains of B. glabrata possessed similar protein banding patterns following in-gel staining with Coomassie brilliant blue and silver (Fig. 1). In contrast to the general quantitative and qualitative uniformity of electrophoretically separated hemocyte proteins, the patterns of specific glycan expression, as defined by anti-glycan mAB reactivity to hemocyte extracts (Western blot) and intact cells (ICC) was highly variable depending on the glycotope tested, type of assay, treatment with LTP and snail strain.
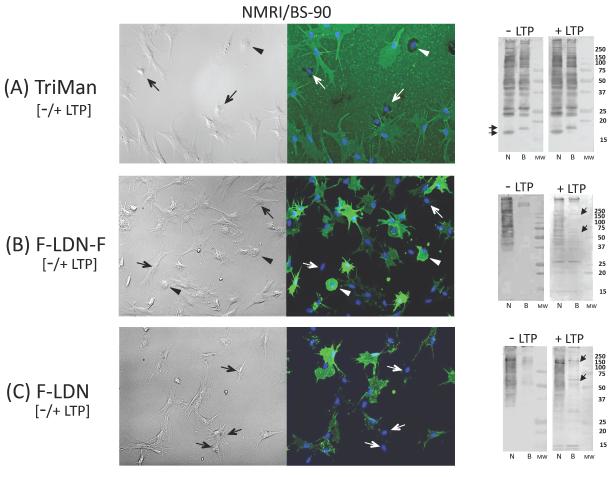
Of all of the mABs tested, the anti-trimannosyl core N glycan mAB (TriMan; Man(α1–6)[Manα1–3)]Man(β1–4)GlcNAc(β1–4)GlcNAc) exhibited the strongest reactivity to hemocytes, both by ICC and Western blot analyses (Fig. 2A), demonstrating an abundance shared TriMan glycotopes associated with multiple cellular glycoproteins. Uniform staining of spread granulocytic hemocytes suggested mAB recognition of cell surface glycans, while a high background staining indicated reactivity with plasma glycoproteins adhering to the glass substrate. However, approximately 10–15% of circulating hemocytes, mainly small spread cells, did not express the TriMan glycotope (Fig. 2A, arrows; Table 1), thereby appearing to represent a distinct subset of hemocytes. Treatment of intact hemocytes or hemocyte proteins on blots with LTP had no effect on anti-TriMan mAB reactivity, nor did snail strains differ in their prevalence of mAB-positive cells or their patterns of protein band reactivity, with the exception of 2 lower molecular weight bands between 15–20 kDa (Fig. 2A, arrows on Western/far-Western blots).
Figure 2. Immunocytochemical (ICC) and Western (−LTP)/far-Western (+LTP) blot analyses of hemocyte-associated glycotopes recognized by mABs binding to (A) trimannosyl core N-glycan [TriMan; Man(α1–6)[Man α1–3)]Man(β1–4)GlcNAc(β1–4)GlcNAc], and the fucosylated lacdiNAc variants (B) F-LDN-F; Fuc(α1–3)GalNAc(β1–4)[Fuc(α1–3)]GlcNAc and (C) F-LDN; Fuc[(α1–3)]GalNAc(β1–4)GlcNAc.
The ICC images of hemocytes (right panel) showing the heterogeneity of staining intensities and cell-types exhibiting specific glycotope reactivities within a single hemocyte population of both B. glabrata snail strains. TriMan-associated glycans are distributed mainly on granulocyte-type cells and substrate-adherent plasma proteins, although small spread cells (Fig. 2A; arrows) and distinctive rounded hemocytes (type “B” hyalinocyte [Schoenberg and Cheng, 1980; Yoshino and Granath, 1994]; Fig. 2A, arrowheads) completely lack TriMan glycotope expression. In contrast, granulocytes staining with F-LDN and F-LDN-F mABs were highly variable, ranging in reactivities from intensely fluorescent to negative (Figs. 2B, 2C; arrows). Moreover, the TriMan-negative type “B” hyalinocytes exhibited strong F-LDN-F mAB staining (Fig. 2B; arrowheads). Hemocyte treatment with LTPs had no effect on the prevalence or cellular distribution of glycotopes in either snail strain. Western blot analyses of shared glycotopes associated with hemocyte proteins (–LTP; left blot panels) showed a similar banding profile for TriMan glycotopes between NMRI (N) and BS-90 (B) snail strains, with the exception of bands between 15–20 kDa (Fig. 2A; arrows). A greater intensity and distribution of F-LDN-F (Fig. B) and F-LDN (Fig. C), however, was noted for N B. glabrata hemocyte proteins compared to the B snail strain. Using a far-Western blot approach, additional F-LDN-F and F-LDN-reactive bands (15–37 kDa range) were seen in blots exposed to LTP (“+LTP”, right blot panels) prior to specific mAB exposure. Arrows in Figs. 2B and 2C (“+LTP” panels) indicate appearance of reactive bands unique to the B snail strain.
Table 1.
Distribution of shared glycotopes expressed on hemocytes from the susceptible (NMRI) and resistant (BS-90) strains of Biomphalaria glabrata. Treatment of hemocytes with larval transformation products (LTPs) significantly modulates expression of shared glycans as determined by reactivity to specific anti-glycan monoclonal antibodies. Prevalence data represent the mean ± S.D. of 3–6 independent replicates. Significance levels were set at α < 0.05 (2-factor ANOVA; see Results)
| Glycotope | Prevalence Reactive Hemocytes (%) | |||
|---|---|---|---|---|
|
| ||||
| No LTP Treatment | LTP Treatment | |||
|
|
||||
| NMRI | BS-90 | NMRI | BS-90 | |
|
|
||||
| TriMan | 83.7 ± 3.1 | 92.6 ± 2.1 | 87.1 ± 5.2 | 89.6 ± 1.6 |
| F-LDN-F | 83.7 ± 6.5 | 89.2 ± 5.2 | 83.4 ± 7.1 | 90.0 ± 1.2 |
| F-LDN | 53.2 ± 5.0 | 77.4 ± 10.4* | 55.6 ± 7.8 | 77.7 ± 7.3* |
| LDN | 0.0 | 0.0 | 23.2 ± 7.7! | 46.6 ± 8.3! * |
| LDN-F | 3.7 ± 1.3 | 3.0 ± 4.1 | 45.9 ± 13.6! | 48.0 ± 11.5! |
| LDN-DF | 0.0 | 0.0 | 15.5 ± 2.5! | 22.9 ± 5.1! |
| LeX | 2.8 ± 3.4 | 1.6 ± 3.5 | 4.7 ± 5.5 | 7.4 ± 1.4 |
Significant difference between snail strains for a given glycotope
Significant difference between LTP-treated and untreated hemocytes for a given glycotope
Similar to the TriMan mAB, strong immunoreactivity was displayed by circulating hemocytes treated with mABs to F-LDN (Fuc[(α1–3)]GalNAc(β1–4)GlcNAc) and F-LDN-F (Fuc(α1–3)GalNAc(β1–4)[Fuc(α1–3)]GlcNAc). In this case, however, hemocytes exhibited a high degree of expressed glycan heterogeneity within a single cell population. Hemocytes displayed distinctive high, medium and low/no levels of staining intensity, with cells bearing FLDN-F glycotopes having greater mAB reactivity and higher prevalence than F-LDN-reactive hemocytes (Figs. 2B and 2C; Table 1). The percentage of F-LDN-positive BS-90 hemocytes was significantly greater than that of NMRI snails (F1,8= 22.3, Fcrit= 5.317; P=0.0014; Table 1), while no snail strain differences were found in the frequency of anti-F-LDN-F reactive cells. Moreover, LTP treatment had no effect on these ratios for either snail strain.
Contrary to our ICC finding of the same or higher prevalence of F-LDN-F and F-LDN-reactive cells in BS-90 hemocyte populations compared to those of the NMRI strain, Western blot analysis revealed stronger and more diverse reactivity against blotted NMRI hemocyte proteins compared to blotted BS-90 cell extracts (Figs. 2B and 2C). This dichotomy in ICC and Western blot results between snail strains was a consistent finding in all biological replicates. LTP treatment of blotted hemocyte proteins resulted in enhanced staining of multiple F-LDN-F and F-LDN reactive bands <37 kDa in both snail strains (Figs. 2B, 2C; “+LTP” far-Western blots). Several higher molecular weight bands also appeared in BS-90 blots (Figs. 2B and 2C; see arrows in “+LTP” blots) indicating possible binding of glycotope-bearing LTPs to BS-90-specific hemocyte proteins.
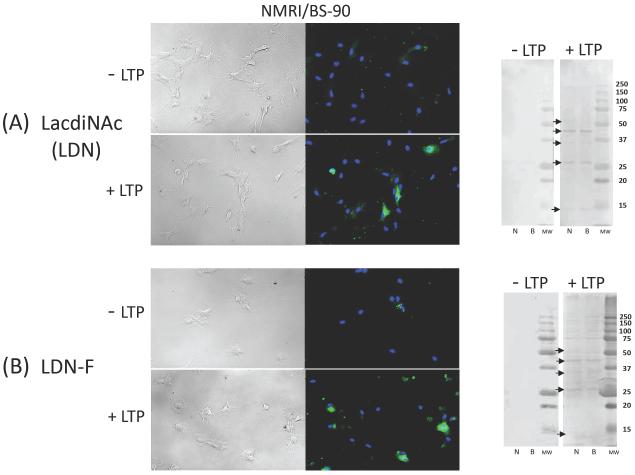
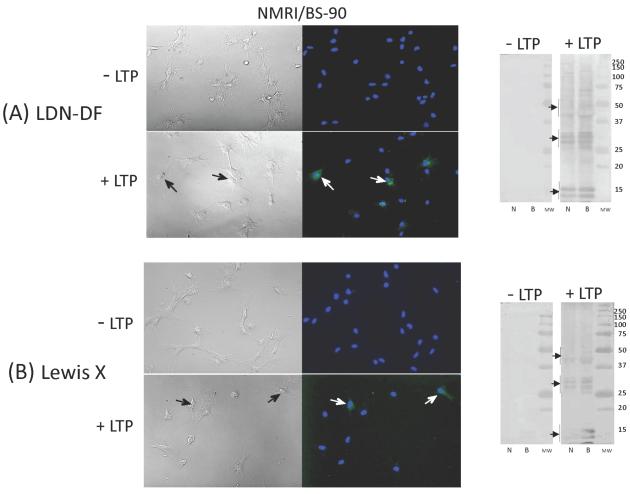
In contrast to the strong mAB reactivities demonstrated above, antibodies specific for LDN (GalNAc(β1–4)GlcNAc), LDN-F (GalNAc(β1–4)[Fuc(α1–3)]GlcNAc ), LDN-DF ([Fuc(α1–2)Fuc(α1–3)]GalNAc(β1–4)[Fuc(α1–2)Fuc(α1–3)]GlcNAc), and Lewis × (LeX; Galβ1–4[Fuc(α1–3)]GlcNAc) exhibited little to no immunoreactivity to native (untreated) hemocytes in both ICC and Western blot analyses (Figs. 3 and 4; “−LTP” ICC and Western blots). However, following LTP treatment (“+LTP”), mAB binding to distinct hemocyte subpopulations was observed by ICC, in which LTP-exposure resulted in a >10-fold increase in the prevalence of LDN-F mAB-reactive cells (F1,8= 67.9, Fcrit= 5.31, P=3.52E-05; Fig. 3B, Table 1) and the appearance of LDN, LDN-DF and LeX-positive hemocyte subpopulations (Table 1; Figs. 3A, 4A, and 4B; F values ranging from 6.34 (LeX) −160.4 (LDN-F) with P values from 0.02 to 9.34E-10). The percentage of LDN-positive BS-90 B. glabrata hemocytes was significantly higher than that of NMRI snails (F1,16=18.04, Fcrit= 4.49, P=0.0006), while no snail strain differences were found for LDN-F, LDN-DF, or LeX-positive cell subpopulations.
Figure 3. Immunocytochemical (ICC) and Western (−LTP)/far-Western (+LTP) blot analyses of hemocyte-associated glycotopes recognized by mABs generated to schistosome lacdiNAc (LDN; GalNAc(β1–4)GlcNAc) (Fig. 3A) and LDN-F (GalNAc(β1–4)[Fuc(α1–3)]GlcNAc) (Fig. 3B).
Untreated intact hemocytes display little to no anti-LDN or anti-LDN-F mAB reactivity in both ICC (−LTP; right ICC panel) and corresponding Western blot (−LTP; left blot panel) analyses of naturally shared LDN and LDN-F glycotopes. However, exposure of intact cells or blotted hemocyte proteins to LTP resulted in the appearance of glycotope-positive hemocyte subpopulations (+LTP; right ICC panel) and immunoreactive bands in far-Western blots (+LTP; right blot panel). Arrows indicate prominent NMRI (N) and BS-90 (B) hemocyte protein bands displaying both LDN and LDN-F glycotopes in LTP-treated blots.
Figure 4. Immunocytochemical (ICC) and Western (−LTP)/far-Western (+LTP) blot analyses of hemocyte-associated glycotopes recognized by mABs generated to schistosome LDN-DF (GalNAc(β1–4)[Fuc(α1–2)Fuc(α1–3)]GlcNAc) (Fig. 4A) and Lewis × (LeX; Galβ1–4[Fuc(α1–3)]GlcNAc) (Fig. 4B).
Using ICC (−LTP; right ICC panel) and Western blot (−LTP; left blot panel) analyses intact hemocytes and their blotted proteins exhibited no reactivity to LDN-DF and LeX mABs. By contrast, small subsets of intact hemocytes (arrows; +LTP; right ICC panel) and a range of cellular proteins (+LTP; right blot panel) became immunopositive following LTP treatment. Discrete groups of hemocyte proteins appear to bind both LDN-DF- and LeX-bearing LTP glycoconjugates in NMRI (N) and BS-90 (B) hemocytes (arrows; +LTP; right blot panel).
Consistent with ICC findings, LTP treatment of blotted hemocyte proteins in far-Western blot analyses resulted in the apparent binding of LDN, LDN-F, LDN-DF and LeX glycotope-bearing larval glycoconjugates to electrophoretically separated cellular proteins. Two general patterns of mAB reactivity were noted for these glycan-specific mABs: LDN and LDN-F had similar reactivities with LTP-exposed proteins between 50 and 25 kDa (Figs. 3A and 3B; arrows on “+LTP” far-Western blots), while LDN-DF and LeX had similar reactivites with three groups of LTP-exposed polypeptides from 50 to <15 kDa (Figs. 4A and 4B; arrows on “+LTP” far-Western blots). Although only minor snail strain differences in the overall glycotope-reactive banding profiles were seen (Figs. 3 and 4), the reactivity of specific mABs to subsets of hemocyte proteins suggests the presence of an array of cellular host proteins capable of binding larval glycoconjugates bearing shared glycan determinants.
4. Discussion
In the present study, using a combination of Western/far-Western blot and immunocytochemical (ICC) analyses, we have demonstrated that circulating hemocytes of B. glabrata snails naturally express carbohydrate determinants (glycotopes) that correspond to glycans commonly associated with the tegument and larval transformation products (LTPs) of S. mansoni miracidia and primary sporocysts (Nyame et al., 2001; Lehr et al. 2008; Peterson et al., 2009). The data presented here, which follow-up on previous reports of extensive sharing of defined larval glycans with various snail tissues (Lehr et al. 2008) including cell-free hemolymph (plasma) (Lehr et al., 2007, 2010; Yoshino et al., 2012), not only document the sharing of specific glycan structures, but also the capability of hemocytes to bind and display larval glycoconjugates bearing mAB-recognized glycotopes. Of particular note was the high degree of molecular heterogeneity in the distribution of innately shared glycans expressed within a given circulating hemocyte population, and the effect of LTP exposure in altering mAB reactivity with intact cells or individual hemocyte proteins, at times in a snail strain-specific fashion.
Antibody reactivities to the TriMan core, F-LDN-F and F-LDN glycotopes in ICC assays best illustrate the spectrum of shared glycotopes naturally expressed in distinct subpopulations of hemocytes. First, our finding that hemocytes from a single population differ significantly in the prevalence of mAB-reactive cells and/or glycotope localization indicates the existence of discrete cellular subsets that may express any combination of TriMan, F-LDN and F-LDN-F glycotopes. For example, based on the prevalence of F-LDN+ (53%) compared to F-LDN-F+ (83%) NMRI hemocytes, at least three hemocyte subpopulations can be predicted (F-LDN-F+/F-LDN+, F-LDN-F+/F-LDN− and F-LDN-F−/F-LDN−). Dual mAB labeling studies, however, would be needed to confirm this conjecture. Secondly, glycan-restricted hemocyte subpopulations are not confined solely to the substrate-adherent, morphologically distinct cell types designated as granulocytes and hyalinocytes (Cheng and Auld, 1977; Jeong and Heyneman, 1976; Johnson and Yoshino, 2001). Hemocytes exhibiting a spread granulocyte-type morphology were observed differentially expressing the F-LDN and F-LDN-F glycotopes, while smaller rounded, spread cells (Scheonberg and Cheng, 1980; Yoshino and Granath, 1985) differed in their reactivity to TriMan (− rxn) and F-LDN-F (+ rxn) mABs. Under “nonspreading” in vitro conditions, from 3 to 5 different morphological hemocyte types have been described from Biomphalaria spp., including B. glabrata (Maricon-Gondran and Letocart, 1999; Martins-Souza et al., 2006; Cavalcanti et al., 2012), and it is conceivable that some of these cell types may correspond to specific glycotope-bearing subpopulations.
The expression of shared TriMan, F-LDN and F-LDN-F glycotopes on hemocytes closely paralleled their occurrence on B. glabrata plasma proteins. For example, the TriMan mAB strongly reacted with a wide spectrum of glycoproteins from hemocytes (present study) and plasma (Yoshino et al., 2012) of both NMRI and BS-90 B. glabrata snail strains, while reactivities of F-LDN and F-LDN-F mABs were consistently greater in NMRI snail plasma and hemocyte proteins than those of the BS-90 strain (Lehr et al., 2010; Yoshino et al., 2012; present study). The high background staining by the TriMan mAB in ICC assays raises the possibility that hemocyte staining is merely the result of nonspecific plasma binding to cells. However, our finding that not all cell types were TriMan mAB-reactive, and the observation that banding patterns of anti-TriMan-reactive proteins by Western blot differed between plasma (Yoshino et al., 2012) and hemocyte samples provide evidence that hemocytes possess their own subset of TriMan-bearing glycoconjugates.
One puzzling inconsistency in our data became apparent when comparing results of ICC and Western blot assays: namely that the prevalence of F-LDN and F-LDN-F positive cells were consistently higher (F-LDN) or the same (F-LDN-F) for the BS-90 snail strain, but exhibited much weaker reactivities in Western blots (Fig. 2B, 2C; Table 1) compared to NMRI snails. When the prevalence of high and moderate staining hemocytes was calculated separately (data not shown) and compared between strains, there was little change in relative proportions of these populations compared to the overall prevalence, indicating that this discrepancy was not due to differences in high vs. low staining positive cells. One explanation may be that these specific glycotopes are differentially expressed on cellular glycoconjugates (glycoproteins or glycolipids) in a snail strain-specific manner, but differ in mAB accessibility to reactive glycotopes in whole cell ICC (in situ) vs. Western blot preparations. In this case, certain glycoconjugates differing between strains (e.g., glycotope-bearing lipids or denatured proteins) may be lost during the Western cell extraction process. Regardless of this result, our finding of differential F-LDN expression in hemocyte subpopulations of resistant and susceptible B. glabrata strains is consistent with previous reports of Biomphalaria strain or species differences incorporating defined lectins as probe reagents (Schoenberg and Cheng, 1980; Martins-Souza et al., 2006).
In contrast to the constitutive expression of hemocyte TriMan, F-LDN and F-LDN-F glycotopes, there was little to no reactivity associated with anti-LDN, LDNF, LDN-DF and LeX mAB treatments in either ICC or Western blot experiments. The virtual absence of these glycan structures on circulating cells generally parallels the weak reactivity exhibited by these mABs to B. glabrata plasma glycans (Yoshino et al., 2012). However, exposure of cells to products released during in vitro miracidium-to-sporocyst transformation (LTPs), mainly comprised of glycoproteins (Wu et al., 2009), resulted in the apparent binding of glycotope-bearing LTPs to subsets of circulating hemocytes (ICC results) and to separated and blotted cell proteins (far-Western blot results). These findings support previous reports of larval S. mansoni excretory-secretory products (ESP; =LTP, Wu et al., 2009) binding to B. glabrata hemocytes (Johnson and Yoshino, 2001), thereby modulating various hemocyte functions including protein synthesis/secretion (Yoshino and Lodes, 1988; Lodes et al., 1991), ROS/NOS production (Connors and Yoshino, 1990; Zahoor et al., 2009), stress response (Zahoor et al., 2010) and MAPK (erk) signaling (Zahoor et al., 2008). Binding of glycotope-expressing LTP to hemocytes suggests the presence of putative receptors for LTP components, although from the data presented here, it cannot be determined whether these putative “receptors” are located at the cell surface or within the cytoplasm. Moreover, the finding that the prevalence of mAB-positive hemocyte subsets or the profile of LTP-reactive cellular proteins exhibit glycotope specificity, further suggests a multi-receptor system capable of recognizing shared glycan-bearing molecules released during early miracidium-to-sporocyst development. Notably, these larval glycoconjugates include TriMan+, F-LDN+ and F-LDN-F+ glycoproteins that overlap the molecular weight range corresponding to S. mansoni polymorphic mucins (Mone et al., 2010), which are hypothesized as playing a role in determining host-parasite compatibility in this system (Mitta et al., 2012).
The functional significance of the snail-schistosome shared glycans is not known, although it as long been speculated that one of the strategies used by parasites to avoid host immune recognition is molecular mimicry (Damian, 1964), in which the parasite, by expressing host-like antigens or molecules, is less likely to be recognized as foreign, thereby evading immune elimination. In B. glabrata a lectin-based immunorecognition system has been hypothesized to provide the basic mechanism(s) involved in initiating immune responses to invading pathogens, including digenetic trematodes (Loker, 2010; Yoshino and Coustau, 2011; Mitta et al., 2012). In such a system, glycan sharing between invading larvae and elements of the snail's immune system could play a prominent role in determining infection success or failure by any of several mechanisms: 1) by displaying host glycan structures at the surface tegument that completely masks the parasite from immune recognition throughout its development, including successive larval stages (long-term passive mechanism) (Yoshino and Boswell, 1985); 2) displaying shared glycans to temporarily delaying initial parasite recognition to allow time for other evasive strategies (e.g., adsorption of host “masking” molecules to avoid recognition (Loker, 2010; Yoshino and Coustau, 2011) or elaboration of immune interfering molecules (Lie, 1982; Loker et al., 1992) (short-term passive mechanism); 3) releasing soluble glycoconjugates during miracidial transformation (LTP) that serve as a protective molecular barrier (Guillou et al., 2007; Wu et al., 2009), which may include anti-immune factors such as anti-oxidants and proteases (Guillou et al., 2007; Vermeire and Yoshino, 2007; Wu et al., 2009) or glycoconjugates bearing shared glycan capable of binding to plasma proteins (Yoshino et al., 2012) and hemocytes (present study). With regards to these latter studies demonstrating a selective, but complex binding interaction between host immune elements and the parasite indicates that investigators must now consider not only the qualitative and quantitative expression of specific glycans innately shared between host and parasite (i.e., the degree of glycan mimicry), but also how binding interactions of larval glycoconjugates with hemocyte subpopulations or specific plasma proteins may impact positively or negatively on subsequent cellular immune reactivity against the early primary sporocyst stage.
Although the range of glycan binding specificities for the different Frep subfamilies has not been fully tested (Adema et al., 1997; Hanington et al., 2010), their capacity to bind S. mansoni sporocysts suggests the presence of carbohydrate recognition domains (CRDs) for one or more of the fucosylated glycans that are prominently displayed at the larval surface (Peterson et al., 2009). Given this senario, it is conceivable that specific shared glycans naturally expressed on, or acquired by, hemocytes may actually be serving as Frep “counter-receptors” that could then facilitate Frep-mediated crossbridging of hemocytes to the sporocyst surface during initial encapsulation reactions (Loker et al., 2004). Higher prevalence of LDN- and F-LDN-positive hemocytes in resistant BS-90 snails compared to the NMRI strain would be consistent with this mechanism. Since larval glycoproteins, such as the genetically-diverse polymorphic mucins (Roger et al., 2008a, b), may also differ quantitatively and/or qualitatively in their glycan content, this mechanism is also consistent with the compatibility polymorphism hypothesis (Mitta et al., 2012). Our current findings demonstrate that molecular heterogeneity, whether in larval glycotopes naturally expressed by snail hemocytes or hemocyte receptors capable of binding larval glycoconjugates, occurs in a potentially large repertoire of interacting molecules, the combination(s) of which may determine compatibility or incompatibility between individual parasites and hosts within a given strain of S. mansoni and B. glabrata. A major challenge confronting researchers now lies in the dissection and identification of the specific interactive components representing the snail host (lectin-like proteins) and the relevant larval stages (glycan and their associated glycoproteins).
Acknowledgments
We thank Mike Lindeke and Mike Gehring for maintaining snail colonies. Schistosome-infected mice and uninfected B. glabrata snails were supplied by Dr. Fred Lewis (Biomedical Research Institute, Rockville, MD) under NIH-NIAID Contract No. HHSN272201000005I. Supported by NIH, NIAID grant #2RO1AI015503 to TPY and Netherlands Science Foundation (NWO-CW) grant 700.58.003 to CHH.
Abbreviations
- mAB
monoclonal antibody
- LTP
larval transformation product
- ICC
immunocytochemistry (-ical)
- CBSS
Chernins balanced salt solution
- sPBS
snail phosphate-buffered saline
- Frep
fibrinogen-related protein
- PRR
pattern recognition receptor
- PAMP
pathogen-associated molecular pattern
- TriMan
trimannosyl- core N-glycan
- LeX
Lewis X
- LDN
lacdiNAc
- F-LDN-(F)(DF)
fucosylated variants of lacdiNAc
References
- Adema CM, Hertel LA, Miller RD, Loker ES. A family of fibrinogen-related proteins that precipitate parasite-derived molecules is produced by an invertebrate after infection. Proc. Natl. Acad. Sci. (USA) 1997;94:8691–8696. doi: 10.1073/pnas.94.16.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne CJ. Successful parasitism of vector snail Biomphalaria glabrata by the human blood fluke (trematode) Schistosoma mansoni: A 2009 assessment. Mol. Biochem. Parasitol. 2009;165:8–18. doi: 10.1016/j.molbiopara.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne CJ, Buckley PM, DeWan PC. Schistosoma mansoni: cytotoxicity of hemocytes from susceptible snail hosts for sporocysts in plasma from resistant Biomphalaria glabrata. Exp. Parasitol. 1980;50:409–146. doi: 10.1016/0014-4894(80)90043-0. [DOI] [PubMed] [Google Scholar]
- Cavalcanti MGS, Filho FC, Mendonca AM, Duarte GR, Barbosa CC, De Castro CM, Alves LC, Brayner FA. Morphological characterization of hemocytes from Biomphalaria glabrata and Biomphalaria straminea. Micron. 2012;43:285–291. doi: 10.1016/j.micron.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Cheng TC, Auld KR. Hemocytes of the pulmonate gastropod Biomphalaria glabrata. J. Invertebr. Pathol. 1977;30:119–122. doi: 10.1016/0022-2011(77)90052-0. [DOI] [PubMed] [Google Scholar]
- Chernin E. Observations on hearts explanted in vitro from the snail Australorbis glabratus. J. Parasitol. 1963;49:353–364. [PubMed] [Google Scholar]
- Connors VA, Yoshino TP. In vitro effect of larval Schistosoma mansoni excretorysecretory products on phagocytosis-stimulated superoxide production in hemocytes from Biomphalaria glabrata. J. Parasitol. 1990;76:895–902. [PubMed] [Google Scholar]
- Damian RT. Molecular mimicry: antigen sharing by parasite and host and its consequences. Amer. Nat. 1964;98:129–149. [Google Scholar]
- Edmondson DG, Dent SYR. Identification of protein interactions by far-Western analysis. Curr Protocols Prot Sci. 2001:19.7.1–19.7.10. doi: 10.1002/0471140864.ps1907s25. Online publication, Wiley and Sons, Inc. DOI: 10.1002/0471140864.ps1907s25. [DOI] [PubMed] [Google Scholar]
- Guillou F, Roger E, Mone Y, Rognon A, Grunau C, Theron A, Mitta G, Coustau C, Gourbal BE. Excretory-secretory proteome of larval Schistosoma mansoni and Echinostoma caproni, two parasites of Biomphalaria glabrata. Mol. Biochem. Parasitol. 2007;155:45–56. doi: 10.1016/j.molbiopara.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Hall RA. Protein-protein interactions: methods and applications. Meth. Mol. Biol . 2004;261:167–174. doi: 10.1385/1-59259-762-9:167. [DOI] [PubMed] [Google Scholar]
- Hanington PC, Forys MA, Dragoo JW, Zhang SM, Adema CM, Loker ES. Role for a somatically diversified lectin in resistance of an invertebrate to parasite infection. Proc. Natl. Acad. Sci. USA. 2010;107:21087–21092. doi: 10.1073/pnas.1011242107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanington PC, Forys MA, Loker ES. A somatically diversified defense factor, FREP3, is a determinant of snail resistance to schistosome infection. PLoS Negl. Trop. Dis. 2012;6(3):e1591. doi: 10.1371/journal.pntd.0001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanington PC, Zhang SM. The primary role of fibrinogen-related proteins is invertebrate defense, not coagulation. J. Innate Immun. 2011;3:17–27. doi: 10.1159/000321882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong KH, Heyneman D. Leukocytes of Biomphalaria glabrata: morphology and behavior of granulocytic cells in vitro. J. Invertebr. Pathol. 1976;28:357–362. doi: 10.1016/0022-2011(76)90011-2. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Yoshino TP. Larval Schistosoma mansoni excretory-secretory glycoproteins (ESPs) bind to hemocytes of Biomphalaria glabrata (Gastropoda) via surface carbohydrate binding receptors. J. Parasitol. 2001;87:786–793. doi: 10.1645/0022-3395(2001)087[0786:LSMESG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lehr T, Geyer H, Maass K, Doenhoff MJ, Geyer R. Structural characterization of N-glycans from the freshwater snail Biomphalaria glabrata cross-reacting with Schistosoma mansoni glycoconjugates. Glycobiology. 2007;17:82–103. doi: 10.1093/glycob/cwl048. [DOI] [PubMed] [Google Scholar]
- Lehr T, Beuerlein K, Doenhoff MJ, Grevelding CG, Geyer R. Localization of carbohydrates common to Biomphalaria glabrata as well as to sporocysts and miracidia of Schistosoma mansoni. Parasitology. 2008;135:931–942. doi: 10.1017/S0031182008004514. [DOI] [PubMed] [Google Scholar]
- Lehr T, Frank S, Natsuka S, Geyer H, Beuerlein K, Doenhoff MJ, Hase S, Geyer R. N-Glycosylation patterns of hemolymph glycoproteins from Biomphalaria glabrata strains expressing different susceptibility to Schistosoma mansoni infection. Exp. Parasitol. 2010;126:592–602. doi: 10.1016/j.exppara.2010.06.031. [DOI] [PubMed] [Google Scholar]
- Lie KJ. Survival of Schistosoma mansoni and other trematode larvae in the snail Biomphalaria glabrata. A discussion of the interference theory. Trop. Geogr. Med. 1982;34:111–122. [PubMed] [Google Scholar]
- Lodes MJ, Connors VA, Yoshino TP. Isolation and functional characterization of snail hemocyte-modulating polypeptide from primary sporocysts of Schistosoma mansoni. Mol. Biochem. Parasitol. 1991;49:1–10. doi: 10.1016/0166-6851(91)90124-o. [DOI] [PubMed] [Google Scholar]
- Loker ES. Gastropod immunobiology. Adv. Exp. Med. Biol. 2010;708:17–43. doi: 10.1007/978-1-4419-8059-5_2. [DOI] [PubMed] [Google Scholar]
- Loker ES, Adema CM, Zhang SM, Kepler TB. Invertebrate immune systems-not homogeneous, not simple, not well understood. Immunol. Rev. 2004;198:10–24. doi: 10.1111/j.0105-2896.2004.0117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loker ES, Bayne CJ, Buckley PM, Kruse KT. Ultrastructure of encapsulation of Schistosoma mansoni mother sporocysts by hemocytes of juveniles of the 10-R2 strain of Biomphalaria glabrata. J. Parasitol. 1982;68:84–94. [PubMed] [Google Scholar]
- Loker ES, Cimino DF, Hertel LA. Excretory-secretory products of Echinostoma paraensei sporocysts mediate interference with Biomphalaria glabrata hemocyte functions. J. Parasitol. 1992;78:104–115. [PubMed] [Google Scholar]
- Matricon-Gondran M, Letocart M. Internal defenses of the snail Biomphalaria glabrata I. Characterization of hemocytes and fixed phagocytes. J. Invertebr. Pathol. 1999;74:224–234. doi: 10.1006/jipa.1999.4876. [DOI] [PubMed] [Google Scholar]
- Martins-Souza RL, Pereira CAJ, Martins Filho OA, Coelho PMZ, Correa A, Jr., Negrao-Correa D. Differential lectin labeling of circulating hemocytes from Biomphalaria glabrata and Biomphalaria tenagophila resistant or susceptible to Schistosoma mansoni. Mem. Inst. Oswaldo Cruz. 2006;101(Suppl. 1):185–192. doi: 10.1590/s0074-02762006000900029. [DOI] [PubMed] [Google Scholar]
- Mitta G, Adema CM, Gourbal B, Loker ES, Theron A. Compatibility polymorphism in snail/schistosome interactions: From field to theory to molecular mechanisms. Dev. Comp. Immunol. 2012;37:1–8. doi: 10.1016/j.dci.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mone Y, Gourbal B, Duval D, Du Pasquier L, Kieffer-Jaquinod S, Mitta G. A large repertoire of parasite epitopes matched by a large repertoire of host immune receptors in an invertebrate host/parasite model. PLoS Negl. Trop. Dis. 2010;4(9):e813. doi: 10.1371/journal.pntd.0000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyame AK, Yoshino TP, Cummings RD. Differential expression of LacdiNAc, fucosylated LacdiNAc, and Lewis x glycan antigens in intramolluscan stages of Schistosoma mansoni. J. Parasitol. 2002;88:890–897. doi: 10.1645/0022-3395(2002)088[0890:DEOLFL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Peterson NA, Hokke CH, Deelder AM, Yoshino TP. Glycotope analysis in miracidia and primary sporocysts of Schistosoma mansoni: differential expression during the miracidium-to-sporocyst transformation. Int. J. Parasitol. 2009;39:1331–1344. doi: 10.1016/j.ijpara.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robijn ML, Wuhrer M, Kornelis D, Deelder AM, Geyer R, Hokke CH. Mapping fucosylated epitopes on glycoproteins and glycolipids of Schistosoma mansoni cercariae, adult worms, and eggs. Parasitology. 2005;130:67–77. doi: 10.1017/s0031182004006390. [DOI] [PubMed] [Google Scholar]
- Roger E, Mitta G, Mone Y, Bouchut A, Rognon A, Grunau C, Boissier J, Theron A, Gourbal BEF. Molecular determinants of compatibility polymorphism in the Biomphalaria glabrata/Schistosoma mansoni model: new candidates identified by a global comparative proteomics approach. Mol. Biochem. Parasitol. 2008a;157:205–216. doi: 10.1016/j.molbiopara.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Roger E, Grunau C, Pierce RJ, Hirai H, Gourbal B, Galinier R, Emans R, Cesari IM, Cosseau C, Mitta G. Controlled chaos of polymorphic mucins in a metazoan parasite Schistosoma mansoni interacting with its intermediate host Biomphalaria glabrata. PLoS Negl, Trop, Dis. 2008b;211:e330. doi: 10.1371/journal.pntd.0000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Richards CS. Schistosoma mansoni, NIH-SM-PR-2 strain, in susceptible and nonsusceptible stocks of Biomphalaria glabrata: comparative histology. J. Parasitol. 1981;67:702–708. [PubMed] [Google Scholar]
- Schoenberg DA, Cheng TC. Lectin-binding specificities of hemocytes from two strains of Biomphalaria glabrata as determined by microhemadsorption assays. Dev. Comp. Immunol. 1980;4:617–628. doi: 10.1016/s0145-305x(80)80064-4. [DOI] [PubMed] [Google Scholar]
- Sminia T, Barendsen L. A comparative morphological and enzyme histochemical study on blood cells of the fresh water snails Lymnaea stagnalis, Biomphalaria glabrata and Bulinus truncates. J. Morphol. 1980;165:31–39. doi: 10.1002/jmor.1051650104. [DOI] [PubMed] [Google Scholar]
- Stout BA, Adema CM, Zhang SM, Loker ES. Biology of FREPs: diversified lectins with fibrinogen-related domains from freshwater snail Biomphalaria glabrata. In: Vasta G, Ahmed H, editors. Animal lectins: a functional view. CRC Press Taylor & Francis; Boca Raton: 2009. pp. 471–491. [Google Scholar]
- van Remoortere A, Hokke CH, van Dam GJ, van Die I, Deelder AM, van den Eijnden DH. Various stages of Schistosoma express LewisX, LacdiNAc, GalNAcβ1–4 (Fucα1–3)GlcNAc and GalNAcβ1–4(Fucα1–2Fucα1–3)GlcNAc carbohydrate epitopes: detection with monoclonal antibodies that are characterized by enzymatically synthesized neoglycoproteins. xGlycobiology. 2000;10:601–609. doi: 10.1093/glycob/10.6.601. [DOI] [PubMed] [Google Scholar]
- van Roon AMM, Aguilera B, Cuenca F, van Remoortere A, van der Marel GA, Deelder AM, Overkleeft HS, Hokke CH. Synthesis and antibody binding of a series of parasite fuco-oligosaccharides. Bioorg. Med. Chem. 2005;13:3553–3564. doi: 10.1016/j.bmc.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Vermeire JJ, Yoshino TP. Antioxidant gene expression and function in in vitro-developing Schistosoma mansoni mother sporocysts: possible role in self-protection. Parasitology. 2007;134:1369–1378. doi: 10.1017/S0031182007002697. [DOI] [PubMed] [Google Scholar]
- Wu XJ, Sabat G, Brown JF, Zhang M, Taft A, Peterson NA, Harms A, Yoshino TP. Proteomic analysis of Schistosoma mansoni proteins released during in vitro miracidium-to-sporocyst transformation. Mol. Biochem. Parasitol. 2009;164:32–44. doi: 10.1016/j.molbiopara.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino TP, Boswell CA. Antigen sharing between larval trematodes and their snail hosts: how real a phenomenon in immune evasion? In: Lackie AM, editor. Immune mechanisms in Invertebrate vectors. Clarendon Press; Oxford, UK: 1986. pp. 221–238. [Google Scholar]
- Yoshino TP, Coustau C. Immunobiology of Biomphalaria-trematode interactions. In: Toledo R, Fried B, editors. Biomphalaria snails and larval trematodes. Springer; New York: 2011. pp. 159–189. [Google Scholar]
- Yoshino TP, Granath WO., Jr. Surface antigens of Biomphalaria glabrata (Gastropoda) hemocytes: Functional heterogeneity in cell subpopulations recognized by a monoclonal antibody. J. Invertebr. Pathol. 1985;45:174–186. doi: 10.1016/0022-2011(85)90007-2. [DOI] [PubMed] [Google Scholar]
- Yoshino TP, Laursen JR. Production of Schistosoma mansoni daughter sporocysts from mother sporocysts maintained in synxenic culture with Biomphalaria glabrata embryonic Bge cells. J. Parasitol. 1995;81:714–722. [PubMed] [Google Scholar]
- Yoshino TP, Lodes MJ. Secretory protein biosynthesis in snail hemocytes: in vitro modulation by larval schistosome excretory-secretory products. J. Parasitol. 1988;74:538–547. [PubMed] [Google Scholar]
- Yoshino TP, Wu XJ, Liu HD, Gonzalez LA, Deelder AM, Hokke CH. Glycotope shareing between snail hemolymph and larval schistosomes: Larval transformation products alter shared glycan patterns of plasma proteins. PLoS Negl. Trop. Dis. 2012;6(3):e1569. doi: 10.1371/journal.pntd.0001569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahoor Z, Davies AJ, Kirk RS, Rollinson D, Walker AJ. Disruption of ERK signalling in Biomphalaria glabrata defence cells by Schistosoma mansoni: implications for parasite survival in the snail host. Dev. Comp. Immunol. 2008;32:1561–71. doi: 10.1016/j.dci.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Zahoor Z, Davies AJ, Kirk RS, Rollinson D, Walker AJ. Nitric oxide production by Biomphalaria glabrata haemocytes: effects of Schistosoma mansoni ESPs and regulation through the extracellular signal-regulated kinase pathway. Parasit. Vectors. 2009;2:18. doi: 10.1186/1756-3305-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahoor Z, Davies AJ, Kirk RS, Rollinson D, Walker AJ. Larval excretory-secretory products from the parasite Schistosoma mansoni modulate HSP70 protein expression in defence cells of its snail host, Biomphalaria glabrata. Cell Stress Chaperones. 2010;15:636–650. doi: 10.1007/s12192-010-0176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SM, Zeng Y, Loker ES. Expression profiling and binding properties of fibrinogen-related proteins (FREPs), plasma proteins from the schistosome snail host Biomphalaria glabrata. Innate Immun. 2008;14:175–189. doi: 10.1177/1753425908093800. [DOI] [PMC free article] [PubMed] [Google Scholar]