Abstract
Physical activity is an important aspect of good health for everyone; it is even more important for psychiatric patients who usually live an unhealthy lifestyle. In recent years, there has been growing focus on the use of soccer as a vehicle to improve the health of subjects with severe mental illness. The aim of this study was to investigate the effects of soccer practice on the self-reported health quality of life (SRHQL) and sports performance (SP) in psychotic subjects. Eighteen male patients with diagnosis of schizophrenia were randomized into either a trained (TG) or a control group (CG). The TG was trained for 12 weeks using two soccer training sessions per week. The CG did not perform any regular sports activity during the experimental period. Anthropometric measurements, SRHQL, personal time records in a 30 meter sprint test and slalom test running with a ball were evaluated before and after the experimental period. SRHQL was assessed using Short Form-12 questionnaire measuring physical and mental component summary scores. After the training period, the TG showed a relevant decrease by 4.6% in bodyweight (BW) and body mass index compared to baseline. Conversely, the CG showed an increased BW and body mass index by 1.8% from baseline to posttest. Moreover, after 12 weeks we found that control patients increased their BW significantly when compared to trained patients (Δ = 5.4%; P < 0.05). After the training period, comparing the baseline TG’s Short Form-12-scores to posttest results, we found an improvement of 10.5% and 10.8% in physical component summary and mental component summary, respectively. In addition, performances on the 30 meter sprint test and slalom test running with a ball in the TG improved significantly (P < 0.01) from baseline to posttest when compared to CG. Soccer practice appears able to improve psychophysical health in individuals with diagnosis of schizophrenia. Indeed, our study demonstrated that programmed soccer physical activity could reduce antipsychotic medication-related weight gain and improve SRHQL and sports performance in psychotic subjects.
Keywords: schizophrenia, mental illness, psychotic subjects, sport, exercise, soccer
Introduction
Physical activity and mental health
The theoretical perspective for this study is the salutogenic model,1 which proposes a global approach aimed at considering health and illness as the two opposite extremes along a continuum; individuals move on this continuum in order to attain health and well-being. In this approach, health is not conceived as the absence of illness, but rather it involves, more globally, all the factors that lead to increasing well-being, such as physical activity. A variety of studies underlie the widespread consensus concerning the positive effects of motor activity on mental and physical health from childhood to senescence.2–4
To date, research has primarily focused on the positive effect of physical activity to decrease symptoms associated with depression and anxiety and, inversely, to increase levels of self-esteem and self-image. If physical activity is a pivotal component of good health for everyone, there is an increasing emphasis on its importance for psychiatric patients affected by schizophrenia, Alzheimer’s dementia, and major depressive disorders.5,6 People with mental disorders live an unhealthy lifestyle and take medications that tend to reinforce the metabolic syndrome;7 indeed, it is known that antipsychotic drugs induce weight gain and stimulate appetite.8,9 Several therapeutic strategies, including physical activity programs and psychoeducational behavioral interventions, have been elaborated in order to manage antipsychotic-induced weight gain.10,11 Wirshing et al10 elaborated a specific project based on oral presentations in order to educate psychotic subjects about lifestyle changes that they could adopt to reduce their weight gain. The authors showed that psychotic patients are able to benefit from educational presentations about nutrition and a healthy lifestyle. In the same way, Poulin et al11 elaborated on a prospective naturalistic study to investigate the effectiveness of a supervised exercise program and an educational activity about dietary and physical movement counseling in individuals with a diagnosis of schizophrenia, schizoaffective, or bipolar disorders. The researchers found that a weight control program including unstandardized physical activity could effectively manage body weight (BW) and metabolic risk profile in patients receiving atypical antipsychotic medications. However, only a few studies have investigated the effects of regular exercise training in definite psychiatric populations because of the small number of available investigations and methodological aspects, such as election criteria and adequate control conditions.12–14 In particular, Faulkner and Sparkes12 have demonstrated that a 10-week exercise training program can reduce auditory hallucinations and improve sleep patterns and levels of self-esteem in patients with a diagnosis of schizophrenia. However, limitations are recognized as this clinical population had a low level of participation in voluntary or prescribed physical activity and exhibited a sedentary lifestyle. With respect to this matter, Hutzler and Korsensky15 conducted a systematic review focusing on motivational correlates of physical activity in persons with disability and identified a list of barriers to the success of programs based on physical activity. This list includes the lack of motivation to maintain sports activity or to carry out a planned exercise protocol for sufficient periods of time, and low levels of motivational factors regarding self-efficacy, self-determination, mastery goals, and persistence, amongst other barriers.
Soccer and mental health
In recent years, there has been a growing focus on the use of soccer as a vehicle for improving mental health. Local health organizations are realizing that soccer may be an effective way to promote good mental health and help increase access to – and uptake of – services for mental health service users.16,17 In particular, Pringle17 observed that some mental health service providers have begun to cooperate with football clubs to improve the psychophysical health of subjects with mental illness. More specifically, the aim of this cooperation was to involve their patients in playing or watching football. In a similar manner, Carter-Morris and Faulkner18,19 have reported that football, as the “national sport” in England, plays a key role promoting men’s engagement. Several recent initiatives have employed football to engage marginalized and stigmatized men, such as those with mental health problems.18,19
The involvement in soccer practice could provide particular positive benefits in people with severe mental health problems such as schizophrenia; these peculiar benefits are related to the achievement of a sense of meaning, purpose, optimism, and hope of obtaining a goal. In playing soccer, the phrase “It’s a goal” becomes a metaphor for the success in the complex process aimed at rediscovering personal identity.20
Moreover, from a psychoeducational perspective, it is important to acknowledge the positive effects derived from the inner features of team activities, such as social and motivational opportunities, when soccer is employed to increase mental health; examples of these effects include having opportunities of social interaction, cooperating with others, respecting rules, acquiring other skills suitable for everyday life, sharing leisure experiences, experiencing personal achievement, being part of a winning team, and receiving the encouragement of other people with the same disorder. However, there are no studies in the literature that have investigated the effects of soccer practice on the psychophysical condition of psychotic subjects. We chose to use soccer for its abovementioned benefits and because it is known to be the most popular and practiced team sport in Italy among men.21 For these reasons, the aim of this study is to analyze the effects of a 12-week soccer training program on self-reported quality of life (QOL), BW gain, and physical performance in psychiatric patients with a diagnosis of schizophrenia and/or schizoaffective disorders.
Materials and methods
Participants
Twenty-three male subjects were recruited in the Psychiatric Departments of the Local Health of Palermo (Sicily, Italy). All patients participated in the local project “Calciapensieri,” which was aimed at taking a whole approach centered on a more complete description of the “person” with a mental disorder. Patients were eligible for the study if they met all of the following inclusion criteria: (1) diagnosis of schizophrenia and/or schizoaffective disorders based on Diagnostic and Statistical Manual Version IV criteria,22 and without any other disorder in comorbidity (ie, intellectual disability, depression, anxiety); (2) ≥18 years of chronological age; (3) male sex, with a motivation to play soccer; (4) capacity to provide informed consent for study participation; (5) a stable antipsychotic pharmacological program composed of clozapine, olanzapine, risperidone for 6 weeks prior to and during the experimental period; (6) occupational therapy (pottery, carpentry, kitchen laboratories) by a psychiatrist in a community-based office setting to include active people; (7) suitability to practice physical activity, as attested by a medical certificate and their psychiatrist’s consent; (8) at least 1 year of soccer experience in order to train people with a basic level of technical–tactical skills; and (9) availability to attend at least 80% of the training period.
Patients were excluded from the study if they were considered urgent candidates for hospitalization and presented unstable pharmacological treatment during the intervention period. Recruited patients were randomly assigned to two groups entering a random numbers table, a trained group (TG) that was trained for 12 weeks with two soccer sessions/week, and a control group (CG) that did not perform any organized physical activity during the experimental period; 12 and eleven patients were included in the TG and CG groups, respectively. However, two subjects of the TG were excluded from study because they did not attend 80% of the training period for personal reasons; instead, three patients of the CG were not subjected to a second assessment. For these reasons, there was a total of 18 overweight and smoker, male subjects, of which ten psychotics were in the TG and eight were in the CG for consideration in this double-blinded study (Table 1). In particular, the examiner could not distinguish the trained subjects from the control ones because both groups wore the same sports uniform during test sessions.
Table 1.
Patients’ characteristics at baseline
| Subjects | Age (years) | Weight (kg) | Height (cm) | BMI (kg/m2) |
|---|---|---|---|---|
| TG (n = 10) | 36.00 ± 5.00 | 77.44 ± 13.60 | 164.44 ± 7.00 | 28.55 ± 4.06 |
| CG (n = 8) | 35.00 ± 4.00 | 76.71 ± 09.75 | 163.42 ± 4.99 | 28.65 ± 2.62 |
| Significance (P < 0.05) | NS | NS | NS | NS |
Abbreviations: BMI, body mass index; TG, trained group; n, number; CG, control group; NS, not significant.
Prior to the start of the study, appropriate local ethics committee approval and written informed consent from each patient were obtained. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Anthropometric measurements
BW and height were measured according to standardized procedures recommended at the Airlie Conference.23 In particular, height was measured using a standard stadiometer (maximum height recordable, 220 cm; resolution, 1 mm) with the subjects barefoot and standing upright. BW was measured using a Seca electronic scale (maximum weight recordable, 300 kg; resolution, 100 g) (Seca Deutschland, Hamburg, Germany) with the subjects wearing only underwear. Body mass index (BMI) was calculated as bodyweight divided by height squared (kg · m−2).
Physical performance evaluation
Physical performances of the subjects were evaluated by a 30 meter sprint run test (30-mST) and slalom test running with ball (STB) between five cones located 50 cm apart. During the 30-mST and STB, subjects performed three maximal performances interspersed with 3 minutes of passive recovery, and the fastest possible time achieved was recorded. Sprint distance was chosen according to the average sprint distance reported in football match-analysis studies.24 All running tests were performed on a synthetic football pitch, with the players wearing football boots with studs. Testing began after a 10-minute standardized preparation including exercises 1, 2, 3, and 4 of the warm-up phase (Table 2). The 30-mST and STB performance times were recorded by manual chronometer. The same assessor performed all test procedures before and after the experimental period. Before the start of the study, subjects were familiarized with all test procedures.
Table 2.
Battaglia and Inguglia’s model of training session
| Phase | Activities | Minutes | ||
|---|---|---|---|---|
| 1. Recording | Patients signed their presence in a daily log | ∼10 | ||
| 2. Social | Presentation of the activities | ∼10 | ||
| 3. Warm-up | Participants executed a standard sequence of exercises:
|
∼20 | ||
| 4. Central | Recovery | Drink (still water) and socializing | 3′ | ∼40–60 |
| Game | Patients played with nurses and/or medical doctors in a small-sided game (5 versus 5; 7 versus 7) 50%–85% HRmax | 20′–30′ | ||
| Recovery | Drink (still water) and socializing | 3′ | ||
| Game | Patients played with nurses and/or medical doctor in small-sided game (5 versus 5; 7 versus 7) 50%–85% HRmax | 20′–30′ | ||
| 5. Cool-down |
|
∼10 | ||
| 6. Feedback | Discussion of the activities and game; farewell | ∼10 | ||
| Total | ∼120 | |||
Abbreviations: CC, clockwise; ACC, anticlockwise; FC, forward circumduction; BC, backward circumduction; BA, both arms; BLL, both lower limbs; HRmax, maximum heart rate; DB, deep breathings; ′, minutes; ″, seconds.
Assessment of self-reported quality of life
Psychophysical health state perception was evaluated by the Short Form (SF)-12 Health Survey, an abbreviated version of the SF-36 Health Survey,25 which assesses patients’ self-reported health quality of life using physical and mental composite scores. This instrument has been shown to be a valid measure in individuals with severe mental illness such as schizophrenia.26,27 The measure investigated two dimensions: physical component summary (PCS-12, physical domain of the SF-12, scores ranging from 0 to 70) and mental component summary (MCS-12, mental domain of the SF-12, scores ranging from 0 to 70). In particular, this questionnaire measures health-related functions along eight subscales: physical functioning; role limitations caused by physical problems; bodily pain; general health (components of PCS); role limitations caused by emotional problems; social functioning; vitality; and mental health (components of MCS). PCS and MCS of the SF-12 showed good internal consistency and reliability, with alpha coefficients of 0.80 and 0.78, respectively. The MCS and the PCS were scored with norm-based methods.27 All data were acquired before (pretest) and after (posttest) the experimentation.
Heart rate analysis
To manage the intensities of efforts during the match, heart rate (HR) is often used in relation to the percentage of individuals’ theoretical HR.28 For this reason, the patients’ HRs were recorded at 5-second intervals during each game by means of telemetry (Polar Team Sport System; Polar Electro Oy, Kempele, Finland).
Training program
The experimental group was trained for 12 weeks with two sessions per week. Every training session was long, about 100–120 minutes and included the following: (1) a recording phase (∼10 minutes); (2) a social interaction phase (∼10 minutes) to enhance the participation effects; (3) a warm-up period (∼20 minutes); (4) a central training period (∼40–60 minutes) made up of two games (∼20–30 minutes) including soccer technical–tactical exercises and a small-sided soccer games;17 (5) a cool-down period (∼10 minutes); and (6) a feedback phase (∼10 minutes). Concerning the basic principles of exercise training, we progressively increased the duration of each game of the training period (20 minutes from week 1 to week 5; 25 minutes from week 5 to week 8; and 30 minutes from week 9 to week 10). We used Polar Team Sport System (Polar Electro, Kempele, Finland) to monitor cardiovascular workload during the central phase of the standard training unit (Table 2). We used several technical–tactical strategies (one-, two-, or three-touch play, free play, play with or without a goalkeeper) to promote a combination of moderate- and vigorous-intensity activity by managing patients’ mean HRs between 50%–85% of the estimated individual HRmax.29,30 Participants were allowed to break exercising whenever they felt the exercise was too difficult.
Statistical analysis
Results are expressed as means ± standard deviation. The group differences were analyzed using independent sample t-tests. Paired t-tests were used to analyze the pre–post changes. Statistica 8.0 Software (StatSoft®, Tulsa, OK, USA) was used for the analysis. The significance level adopted was P < 0.05.
Results
The participants’ baseline characteristics were not significantly different between the TG and the CG (Table 1). After the training period, the TG showed a significant decrease in BW (before training [BT]: 77.44 ± 13.60 versus after training [AT]: 73.89 ± 12.51 kg, Δ = −4.6%, P < 0.001) and BMI (BT: 28.55 ± 4.06 versus AT: 27.22 ± 3.70, Δ = −4.6%, P < 0.001) compared to baseline. On the contrary, CG showed an increased BW and BMI by 1.8% from baseline to posttest (BW = BT: 76.71 ± 9.75 versus AT: 78.14 ± 10.56 kg, P < 0.05; BMI = BT: 28.65 ± 2.62 versus AT: 29.17 ± 2.68, P < 0.05). Moreover, after 12 weeks we found that control patients increased their BW significantly when compared to trained ones (CG: 78.14 ± 10.56 versus TG: 73.89 ± 12.51 kg, Δ = 5.4%; P < 0.05).
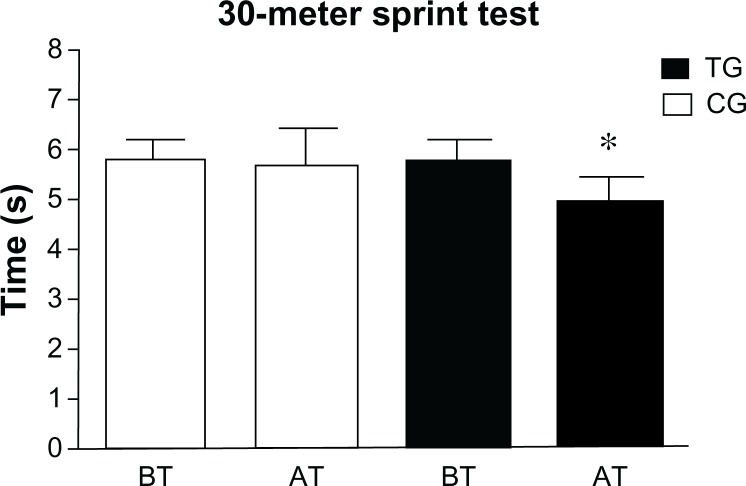
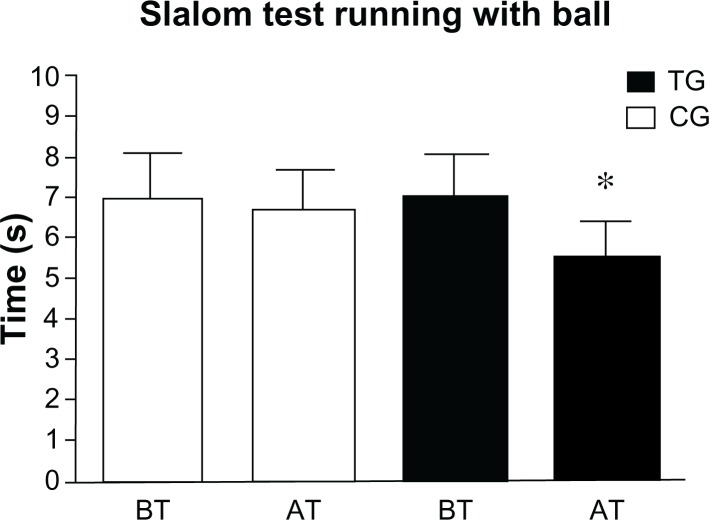
At baseline, the two groups were equivalent in their sports performance. We found that TG and CG patients did not show any significant difference in 30-mST (P = 0.075) and STB (P = 0.86). Instead, AT, the TG significantly improved their 30-mST (P < 0.01) and STB (P < 0.01) performance compared to CG (Figures 1 and 2).
Figure 1.
Patients’ 30-meter sprint test results at baseline and after the training period.
Notes: Means and standard deviations of patients’ best time records before and after the training period. *P < 0.01.
Abbreviations: TG, trained group; CG, control group; BT, before training; AT, after training.
Figure 2.
Patients’ slalom test running with ball results before and after the training period.
Notes: Means and standard deviations of patients’ best time records before and after the training period. *P < 0.01.
Abbreviations: TG, trained group; CG, control group; BT, before training; AT, after training.
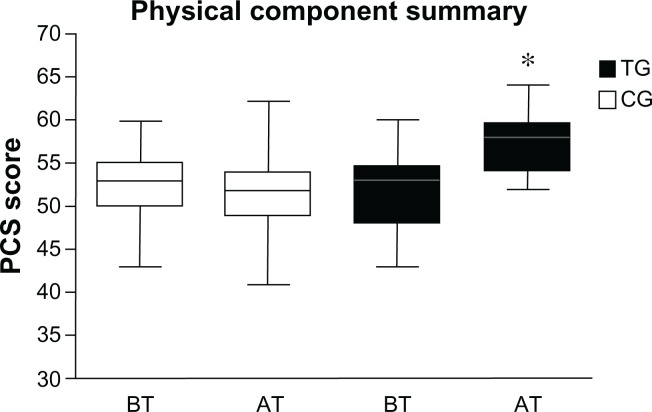
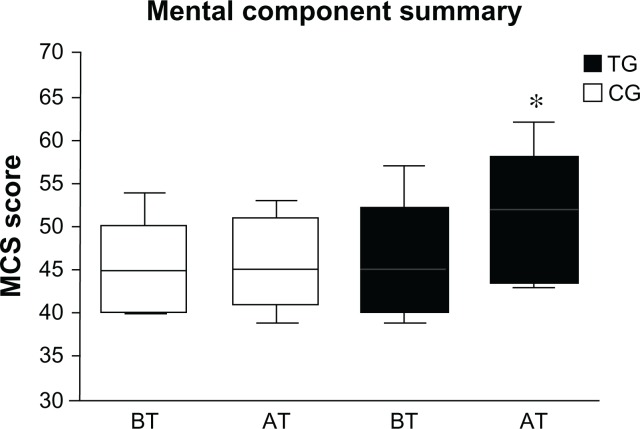
At baseline, no significant differences (P < 0.05) were found comparing CG’s PCS score to TG’s (P = 0.60), and comparing CG’s MCS score to TG’s scores (P = 0.68). After 12 weeks of training, TG patients showed higher PCS (P < 0.0001) and MCS (P < 0.0001) scores than CG (Figures 3 and 4). In particular comparing TG’s SF-12-scores from baseline to posttest, we found an improvement by 10.5% and 10.8% in PCS and MCS, respectively.
Figure 3.
Mean score of the physical component summary before and after the training period.
Notes: Mean score of physical component summary of the Short Form-12 health survey before and after the training period in the TG and CG. *P < 0.0001.
Abbreviations: TG, trained group; CG, control group; BT, before training; AT, after training.
Figure 4.
Mean score of the mental component summary before and after the training period.
Notes: Mean score of the mental component summary of the Short Form-12 health survey before and after the training period in the TG and CG. *P < 0.0001.
Abbreviations: TG, trained group; CG, control group; BT, before training; AT, after training.
Discussion
One of the most interesting and heuristic findings of this study is that patients with a diagnosis of schizophrenia are able to participate in a structured soccer training program and improve their psychophysical health and sports performance after a 12-week exercise period. Results show that psychological components measured by PCS-12 and MCS-12 scores, and physical components, evaluated by 30-mST and STB performances, were improved for only the TG subjects at 12 weeks compared to baseline (P < 0.05). Moreover after the training period, the TG decreased their BW and BMI when compared to baseline. These findings are in agreement with several literature studies that showed the favorable effects of physical activity on psychotic subjects’ health.11,13,31
In recent years, football has been used as a vehicle for improving psychotics’ mental health. McElroy et al32 have shown how several social agencies have actively supported interventions using soccer in several mental health initiatives. In particular, the Care Standard Improvement Program supports the Care Standard Improvement Program league in Manchester and regularly brings together over 200 users of mental health services to play competitions in a league of 7-a-side with matches lasting 20 minutes each.32 Soccer, indeed, appears to be a good complementary strategy for improving psychophysical health and performance in psychotic subjects.
Psychiatric patients can participate in exercise training programs in the same way as healthy subjects if they have no severe infectious and cardiovascular diseases.33 We found that the planning of work units in six subphases (recording, social, warm-up, central, cool-down, and feedback phases) was a suitable and safe strategy to conduct patients along a soccer training unit. Compared to previous similar programs, the inclusion of the social and feedback phases made our study innovative and original; our study was the first one aimed at improving the social interaction between players by promoting the ability to cope with new social situations or socially threatening events, using appropriate behavioral strategies. Particularly, this specific phase, which is not included in usual training programs, was planned to increase the social interaction and competence via peer modeling and to favor a positive climate primarily focused on mastery and collaboration, in contrast to competition. On the other hand, the feedback phase stimulated individuals to perceive their self-worth and to delineate adaptive self-beliefs. This phase was revealed to be particularly relevant in motivating subjects to participate and adhere to the training program,34 which is considered challenging for patients suffering from severe schizophrenia. We observed that in nearly every case, patients attended at least 80% of the soccer training period. Moreover, this was probably due to a strong soccer-related motivational component; it is particularly relevant in Italy, where soccer is the most popular sport among men.21 For this reason, we speculate that this fact was a key factor that promoted male subjects’ attendance and limited dropout rates (2/12 in TG, and 3/11 in CG) in our study.
Exercise is well accepted by people with mental illness and is often considered one of the most valued components of cure.35 In accordance with Poulin et al,11 we observed that a programmed physical activity can reduce weight gain and improve the psychophysical perception in psychotic subjects. In particular, using technical–tactical strategies (one-, two-, or three-touch play, free play, play with or without a goalkeeper), we were able to promote aerobic–anaerobic physical activity during soccer training. In addition to this, weight gain is a major collateral effect associated with modern antipsychotic drugs. In particular, second-generation antipsychotics can promote weight gain associated with abdominal obesity and enhanced adiposity,36,37 which causes a decreased quality and length of life.38
It is known that poor physical fitness and obesity, which are associated with a lack of exercise, are factors contributing to mortality among individuals with a diagnosis of schizophrenia.31 The performed soccer-training period appeared to improve movement coordination and speed in subjects with a diagnosis of schizophrenia. In particular, we found that the TG improved eye–foot coordination (P < 0.01) and 30-meter sprint run performance (P < 0.01) compared to CG after the 12-week soccer training period. This is likely because soccer is an open-skill interval sport, with a variable workload ranging from low-intensity, such as walking and running, to all-out intensity, such as jumps and sprints. During the game, players can have successful contact with the ball if every component of the team expresses a high level of motivation, technical–tactical concentration, speed, agility, and muscular strength.28
Enhanced psychomotor function is fundamental to improving health in psychotic subjects. In individuals with a diagnosis of severe schizophrenia, motor activities can be particularly slow and psychomotor activity is sometimes reduced to the bare minimum, hindering social communicative interactions and daily life activities.39 In addition to slowed actions, both fine and gross motor abilities are affected in subjects with a diagnosis of schizophrenia. Liddle40 identified this reduced psychomotor activity as the psychomotor poverty syndrome, including decreased spontaneous movements and paucity of speech and affect. Moreover, motor functioning is a key feature of schizophrenia and appears to be related to several psychological diseases. Silbersweig et al41 showed that hallucinations, for instance, were associated with blood flow to the motor region of brain. In the same way, Pajonk et al13 showed that hippocampal volume is plastic in response to aerobic exercise in patients with a diagnosis of schizophrenia and in healthy subjects. In particular, the authors quantified the increased hippocampal volume by 12% and 16% in exercised subjects with a diagnosis of schizophrenia and healthy individuals, respectively, compared to untrained subjects. A smaller volume of the hippocampus is typical of individuals with a diagnosis of schizophrenia and appears to be related to neuronal atrophy.42
In agreement with several studies,10,11 we showed an improved self-reported QOL in studied patients. In particular, we found an increase of 10.5% and 10.8% in PCS and MCS scores, respectively, in the TG compared to the CG. In recent years, improving the quality of life is a primary treatment goal in individuals with a diagnosis of schizophrenia.43 Psychosocial factors that are strictly related to emotional regulation – such as coping with stressful situations, feelings of self-efficacy, and social support – play a crucial role in the QOL of patients with a diagnosis of schizophrenia.44,45 For instance, Bechdolf et al46 argued that negative coping patterns and depressive symptoms, poor perceived social support, and low self-efficacy were determinant causes of a bad QOL. In our study, trained patients appeared to have high levels of motivational factors regarding self-efficacy, self-determination, mastery of goals, and persistence during the training period. However, even if we could not measure these components, we refer to the properties of soccer as a team sport that stimulates important social relations by uniting mates and preventing social isolation. Consequently, soccer enables each player to feel like an expert in self-determination, efficacy, and the player can be active in the process of rediscovering his/her identity.18 Participation in an organized sport, especially in a team sport, plays a crucial role in the stimulation of social and motivational profiles of players practicing in a social context with other same disorder mates.47 The process of competing and playing promotes a relationship between soccer team mates that is based on fair play, teamwork, and sports equality. In this way, the soccer practice appears to be a good add-on treatment to alleviate secondary symptoms such as social withdrawal and low self-esteem in individuals with a diagnosis of schizophrenia. In agreement with Pringle and Sayers,16 we consider soccer an organized social phenomenon able to promote a sporting, therapeutic, and healthy competition.
Conclusion
To sum up, the results of this study suggest some interesting implications on the educational and clinical field of psychiatric rehabilitation. We propose that it might be worth looking further into the need to plan multifaceted interventions aimed at combining traditional pharmacologic treatments and alternative and innovative behavioral methods such as physical activity. This proposal does not have the presumption of restricting the primary efficacy of pharmacological therapy in patients with diagnosis of schizophrenia. This is in line with the few results previously obtained in studies which found, for example, that health benefits, derived from regular participation in physical activities, are comparable to antidepressant medication in the treatment of major depressive disorder.48 Soccer appears to be able to improve mental health and performance in male individuals with a diagnosis of schizophrenia. In the same way, we could hypothesize that other national team sports such as volleyball might have beneficial effects on the health of female psychotic subjects. This is because we speculate that the national popularity of a sport could be a key factor promoting sports practice in a psychiatric population. Nonetheless, this pilot study needs to be extended on a larger scale in order to investigate the effects of several team sports.
It is important to acknowledge the limitations of this pilot study. Future studies are required to enlarge the sample and to provide a richer and more complete understanding of physiological variables mediating the combined efficacy of psychoeducational and pharmacological treatments in psychiatric disorders. However, our work is the first randomized controlled trial (that we are aware of) that has investigated the effects of soccer as a therapy for individuals with a diagnosis of schizophrenia. New studies could include more in-depth measures of motivation to sport and achievement to examine to what extent these factors contribute to understanding the role of sport in the treatment of psychiatric subjects. This work was supported by the Regional Sports School of CONI Sicilia, Italy.
Acknowledgments
We would like to thank the Local Health Agency of Palermo and Dr Salvatore Rubino, Dr Lucia Nava, and Dr Antonio Sabatino for their support and assistance during the experimental period. Moreover, we want to acknowledge all patients that were involved in the local project “Calciapensieri.” This work was supported by the Regional Sports School of CONI Sicilia, Italy.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Antonovsky A. The salutogenic model as a theory to guide health promotion. Health Promot Int. 1996;11(1):11–18. [Google Scholar]
- 2.Loprinzi PD, Cardinal BJ, Loprinzi KL, Lee H. Benefits and environmental determinants of physical activity in children and adolescents. Obes Facts. 2012;5(4):597–610. doi: 10.1159/000342684. [DOI] [PubMed] [Google Scholar]
- 3.Bellafiore M, Battaglia G, Bianco A, Paoli A, Farina F, Palma A. Improved postural control after dynamic balance training in older overweight women. Aging Clin Exp Res. 2011;23(5–6):378–385. doi: 10.1007/BF03337762. [DOI] [PubMed] [Google Scholar]
- 4.Battaglia G, Bellafiore M, Bianco A, Paoli A, Palma A. Effects of a dynamic balance training protocol on podalic support in older women. Pilot Study. Aging Clin Exp Res. 2010;22(5–6):406–411. doi: 10.1007/BF03337736. [DOI] [PubMed] [Google Scholar]
- 5.Broocks A, Meyer TF, George A, et al. Value of sports in treatment of psychiatric illness. Psychother Psychosom Med Psychol. 1997;47(11):379–393. German. [PubMed] [Google Scholar]
- 6.Knöchel C, Oertel-Knöchel V, O’Dwyer L, et al. Cognitive and behavioural effects of physical exercise in psychiatric patients. Prog Neurobiol. 2012;96(1):46–68. doi: 10.1016/j.pneurobio.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Kopp M. Physical activity in persons with severe mental illness: research-based clinical recommendations. Neuropsychiatr. 2009;23(3):151–156. German. [PubMed] [Google Scholar]
- 8.Planansky P, Heilizer F. Weight changes in relation to the characteristics of patients on chlorpromazine. J Clin Exp Psychopathol. 1959;20(1):53–57. [PubMed] [Google Scholar]
- 9.Robinson RG, McHugh PR, Folstein MF. Measurement of appetite disturbances in psychiatric disorders. J Psychiatr Res. 1975;12(1):59–68. doi: 10.1016/0022-3956(75)90021-7. [DOI] [PubMed] [Google Scholar]
- 10.Wirshing DA, Smith RA, Erickson ZD, Mena SJ, Wirshing WC. A wellness class for inpatients with psychotic disorders. J Psychiatr Pract. 2006;12(1):24–29. doi: 10.1097/00131746-200601000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Poulin MJ, Chaput JP, Simard V, et al. Management of antipsychotic-induced weight gain: prospective naturalistic study of the effectiveness of a supervised exercise programme. Aust N Z J Psychiatry. 2007;41(12):980–989. doi: 10.1080/00048670701689428. [DOI] [PubMed] [Google Scholar]
- 12.Faulkner G, Sparkes A. Exercise as therapy for schizophrenia: an ethnographic study. J Sport Exerc Psychol. 1999;21(1):52–69. [Google Scholar]
- 13.Pajonk FG, Wobrock T, Gruber O, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67(2):133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- 14.Dodd KJ, Duffy S, Stewart JA, Impey J, Taylor N. A small group aerobic exercise programme that reduces body weight is feasible in adults with severe chronic schizophrenia: a pilot study. Disabil Rehabil. 2011;33(13–14):1222–1229. doi: 10.3109/09638288.2010.526162. [DOI] [PubMed] [Google Scholar]
- 15.Hutzler Y, Korsensky O. Motivational correlates of physical activity in persons with an intellectual disability: a systematic literature review. J Intellect Disabil Res. 2010;54(9):767–786. doi: 10.1111/j.1365-2788.2010.01313.x. [DOI] [PubMed] [Google Scholar]
- 16.Pringle A, Sayers P. It’s a goal!: Basing a community psychiatric nursing service in a local football stadium. J R Soc Promot Health. 2004;124(5):234–238. doi: 10.1177/146642400412400522. [DOI] [PubMed] [Google Scholar]
- 17.Pringle A. The growing role of football as a vehicle for interventions in mental health care. J Psychiatr Ment Health Nurs. 2009;16(6):553–537. doi: 10.1111/j.1365-2850.2009.01417.x. [DOI] [PubMed] [Google Scholar]
- 18.Spandler H, McKeown M. A critical exploration of using football in health and welfare programs: gender, masculinities, and social relations. Journal of Sport and Social Issues. 2012;36(4):387–409. [Google Scholar]
- 19.Carter-Morris P, Faulkner G. A football project for service users: the role of football in reducing social exclusion. Journal of Mental Health Promotion. 2003;2(1):24–30. [Google Scholar]
- 20.Carless D, Douglas K. Social support for and through exercise and sport in a sample of men with serious mental illness. Issues Ment Health Nurs. 2008;29(11):1179–1199. doi: 10.1080/01612840802370640. [DOI] [PubMed] [Google Scholar]
- 21.Battaglia G, Bellafiore M, Barberi E, Caramazza G, Bianco A, Palma A. Studio delle differenze di genere nel calcio a 5/Study of gender differences in futsal. Journal of Sport Sciences and Law. 2012;3–4:201–209. [Google Scholar]
- 22.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 23.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 24.Stølen T, Chamari K, Castagna C, Wisløff U. Physiology of soccer: an update. Sports Med. 2005;35(6):501–536. doi: 10.2165/00007256-200535060-00004. [DOI] [PubMed] [Google Scholar]
- 25.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Salyers MP, Bosworth HB, Swanson JW, Lamb-Pagone J, Osher FC. Reliability and validity of the SF-12 health survey among people with severe mental illness. Med Care. 2000;38(11):1141–1150. doi: 10.1097/00005650-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Faulkner G, Cohn T, Remington G, Irving H. Body mass index, waist circumference and quality of life in individuals with schizophrenia. Schizophr Res. 2007;90(1–3):174–178. doi: 10.1016/j.schres.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Tessitore A, Meeusen R, Tiberi M, Cortis C, Pagano R, Capranica L. Aerobic and anaerobic profiles, heart rate and match analysis in older soccer players. Ergonomics. 2005;48(11–14):1365–1377. doi: 10.1080/00140130500101569. [DOI] [PubMed] [Google Scholar]
- 29.Donnelly JE, Blair SN, Jakicic JM, et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 30.De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10(2):138–151. doi: 10.1002/j.2051-5545.2011.tb00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wildgust HJ, Beary M. Are there modifiable risk factors which will reduce the excess mortality in schizophrenia? J Psychopharmacol. 2010;24(Suppl 4):37–50. doi: 10.1177/1359786810384639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McElroy P, Evans P, Pringle A. Sick as a parrot or over the moon: an evaluation of the impact of playing regular matches in a football league on mental health service users. Practice Development in Health Care. 2008;7(1):40–48. [Google Scholar]
- 33.Meyer T, Broocks A. Therapeutic impact of exercise on psychiatric diseases: guidelines for exercise testing and prescription. Sports Med. 2000;30(4):269–279. doi: 10.2165/00007256-200030040-00003. [DOI] [PubMed] [Google Scholar]
- 34.Maltese A, Alesi M, Alù AGM. Self-esteem, defensive strategies and social intelligence in the adolescence. Procedia – Social and Behavioral Sciences. 2012;69:2054–2060. [Google Scholar]
- 35.O’Kelly JG, Piper WE, Kerber R, Fowler J. Exercise groups in an insight-oriented, evening treatment program. Int J Group Psychother. 1998;48(1):85–98. doi: 10.1080/00207284.1998.11491523. [DOI] [PubMed] [Google Scholar]
- 36.Stedman T, Welham J. The distribution of adipose tissue in female in-patients receiving psychotropic drugs. Br J Psychiatry. 1993;162:249–250. doi: 10.1192/bjp.162.2.249. [DOI] [PubMed] [Google Scholar]
- 37.Eder U, Mangweth B, Ebenbichler C, et al. Association of olanzapine-induced weight gain with an increase in body fat. Am J Psychiatry. 2001;158(10):1719–1722. doi: 10.1176/appi.ajp.158.10.1719. [DOI] [PubMed] [Google Scholar]
- 38.Allison DB, Mackell JA, McDonnell DD. The impact of weight gain on quality of life among persons with schizophrenia. Psychiatr Serv. 2003;54(4):565–567. doi: 10.1176/appi.ps.54.4.565. [DOI] [PubMed] [Google Scholar]
- 39.Morrens M, Hulstijn W, Sabbe B. Psychomotor slowing in schizophrenia. Schizophr Bull. 2007;33(4):1038–1053. doi: 10.1093/schbul/sbl051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liddle PF. Schizophrenic syndromes, cognitive performance and neurological dysfunction. Psychol Med. 1987;17(1):49–57. doi: 10.1017/s0033291700012976. [DOI] [PubMed] [Google Scholar]
- 41.Silbersweig DA, Stern E, Frith C, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378(6553):176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- 42.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 43.Karow A, Naber D. Subjective well-being and quality of life under atypical antipsychotic treatment. Psychopharmacology (Berl) 2002;162(1):3–10. doi: 10.1007/s00213-002-1052-z. [DOI] [PubMed] [Google Scholar]
- 44.Ritsner M, Modai I, Endicott J, et al. Differences in quality of life domains and psychopathologic and psychosocial factors in psychiatric patients. J Clin Psychiatry. 2000;61(11):880–889. doi: 10.4088/jcp.v61n1113. [DOI] [PubMed] [Google Scholar]
- 45.Modestin J, Caveng I, Wehrli MV, Malti T. Correlates of coping styles in psychotic illness – an extension study. Psychiatry Res. 2009;168(1):50–56. doi: 10.1016/j.psychres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Bechdolf A, Klosterkötter J, Hambrecht M, et al. Determinants of subjective quality of life in post acute patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2003;253(5):228–235. doi: 10.1007/s00406-003-0436-3. [DOI] [PubMed] [Google Scholar]
- 47.Wipfli BM, Rethorst CD, Landers DM. The anxiolytic effects of exercise: a meta-analysis of randomized trials and dose-response analysis. J Sport Exerc Psychol. 2008;30(4):392–410. doi: 10.1123/jsep.30.4.392. [DOI] [PubMed] [Google Scholar]
- 48.Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]