Abstract
Background:
Stem barks of Mangifera indica contain a rich content of mangiferin (xanthone glucoside), whereas Murraya koenigii leaves contain rich sources of mahanimbine (carbazole alkaloid) and used traditionally for the treatment of diabetes.
Objective:
To investigate the effects of mangiferin (xanthone glucoside) and mahanimbine (carbazole alkaloid) on glucose utilization in 3T3-L1 cells.
Materials and Methods:
Mangiferin was isolated from stem barks of Mangifera indica and mahanimbine was isolated from Murraya koenigii leaves. These isolated compounds were subjected to MTT assay and glucose utilization test with 3T3-L1 cells.
Results:
Treatment of the 3T3-L1 cells with mangiferin and mahanimbine increased the glucose utilization in a dose-dependent manner. At a concentration of 1 mM, mangniferin showed 2-fold increase in glucose utilization compared with untreated control. In case of mahanimbine, the observed effect at 1 mM was almost equivalent to positive control (insulin at 1 μM). Moreover, MTT assay showed that both of these compounds were less toxic at a concentration of 1 mM (nearly 75% cells are viable).
Conclusion:
The present results indicated that these natural products (mangiferin and mahanimbine) exhibited potential ethnomedical uses in management of diabetes.
Keywords: 3T3-L1, anti-diabetic, mahanimbine, mangiferin
INTRODUCTION
Diabetes is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. India is a major contributor to the global public health burden of diabetes. The number of Indians with diabetes will increase from 31.7 million in the year 2000 to 79.4 million by 2030.[1] Diabetes is divided in two broad etiopathogenic categories. In the first category, Type 1 diabetes occurs due to absolute deficiency of insulin and 5–10% of people are affected by this type of diabetes. In the other, much more prevalent category, Type 2 diabetes occurs due to combination of insulin resistance and an inadequate compensatory insulin secretory response. Type 2 diabetes mellitus is the most common form of diabetes, constituting 90% of the diabetic population.[2] The insulin resistance in Type 2 diabetes is due to decreased glucose uptake and decreased stimulation of muscle glycogen synthesis.[3] Oral hypoglycemic agents such as biguanides, sulphonylureas, thiozolidinediones, and insulin are available for the treatment of Type 2 diabetes. But they have some side effects associated with their uses.[4]
Medicinal plants or natural products have ability to stimulate glucose utilization in the body and it can be used for long-term treatment of diabetes. Several medicinal plants and their phyto-constituents (alkaloids, polyphenols, glycosides, triterpenes, polysaccharides, and saponins) have been reported for antidiabetic activities in animal model.[5] An example of such plants are Mangifera indica and Murraya koenigii. These plants are used traditionally for the management of diabetes. The in vivo antidiabetic potentials of Mangifera indica stem bark and Murraya koenigii leaves has showed lowering of blood sugar level in animal model.[6,7]
The Phytochemical compound such as mangiferin is rich content in the stem bark of Mangifera indica. Mahanimbine (carbazole alkaloid) is more in Murraya koenigii leaves. Glucose utilization test for mangiferin (xanthone glucoside) and mahanimbine (carbazole alkaloid) in the 3T3-L1 cells has not been established for antidiabetic activity. Based on this, the present study was designed to investigate the ability of mangiferin and mahanimbine to stimulate glucose utilization in the 3T3-L1 cells.
MATERIALS AND METHODS
Reagents
3T3-L1 cells procured from National Centre for Cell science (NCCS), Pune, India. Roswell Park Memorial Institute (RPMI) medium, thiazolyl blue tetrazolium bromide (MTT), fetal bovine serum (FBS), L-glutamine, penicillin, sodium pyruvate were procured from Hi-Media, India. Sterile 96-well plates were obtained from Tarson, India.
Isolation and characterization of mangiferin
The method of isolation and characterization (HPTLC, FTIR, ESI-MS, and NMR (13C and 1H NMR)) of mangiferin from Mangifera indica stem bark can be found from our earlier report.[8]
Isolation and characterization of mahanimbine
The method of isolation and characterization (HPTLC, FTIR, ESI-MS and NMR (13C and 1H NMR)) of mahanimbine from Murraya koenigii leaves can be found from our earlier report.[9]
Cell culture
3T3-L1 cells procured from NCCS was grown in RPMI medium supplemented with 10% FBS, L-glutamine, penicillin, sodium pyruvate, nonessential amino acids, and vitamin solution. Adherent monolayer cultures were maintained in T-25 flasks and incubated at 37°C in 5% carbon dioxide (CO2).
MTT assay
Thiazolyl blue tetrazolium bromide (MTT) assay was carried out as follows: Cells were trypsinized, counted and 1000 cells were seeded per well in two different 96-well plates. The following day, 100 μl of medium containing the desired concentration of mahanimbine and mangiferin was added to the appropriate wells. The cells were then kept at 37°C in 5% CO2 for 48 h. Control used in these experiments was untreated cells kept for 48 h. At this point, 100 μl of (5 mg/ml) MTT reagent was added to each well, and the plate was placed at 37°C in the incubator for 2 h. Two hundred microliters of dimethyl sulfoxide was added to each well, after aspirating the supernatant. Colored formazan product was assayed spectrophotometrically at 570 nm using ELISA plate reader.
Glucose utilization Test with 3T3-L1 cells
The effects of mangiferin and mahanimbine on glucose utilization were performed using 3T3-L1 cells. For this, cells were maintained at 37°C. One hundred microliters of incubation medium (8 mM glucose RPMI + 0.1% Bovine Serum Albumin (BSA)) containing specific concentration of mangiferin and mahanimbine was added to appropriate wells. 1 μM of insulin served as positive control. Control wells contained incubation medium only. After 1.5 h incubation, 10 μl was removed from each well and placed in to a new 96-well plate. To this, 200 μl of glucose oxidase reagent was added and incubated for 15 min before measuring absorbance at 492 nm.
Statistical analysis
All glucose utilization and toxicity results were compared with relevant control using student's t-test and a P <0.05 was considered significant.
RESULTS AND DISCUSSION
MTT assay of mangiferin and mahanimbine
To study the toxicity of mangiferin [Figure 1] and mahanimnine [Figure 2] compounds, MTT assay were performed. It was found that both of these compounds were less toxic [Figures 3 and 4]. Even at a high concentration of 1 mM, nearly 75% cells were viable after 48 h for both compounds.
Figure 1.
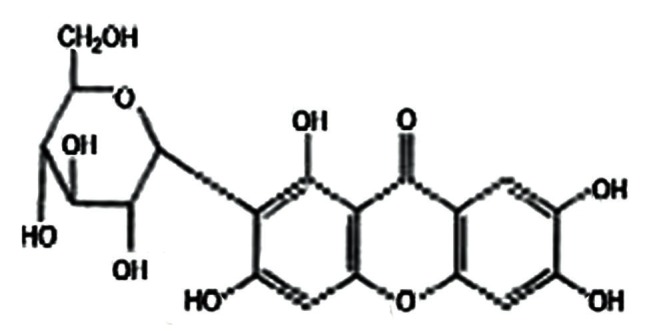
Mangiferin Structure
Figure 2.
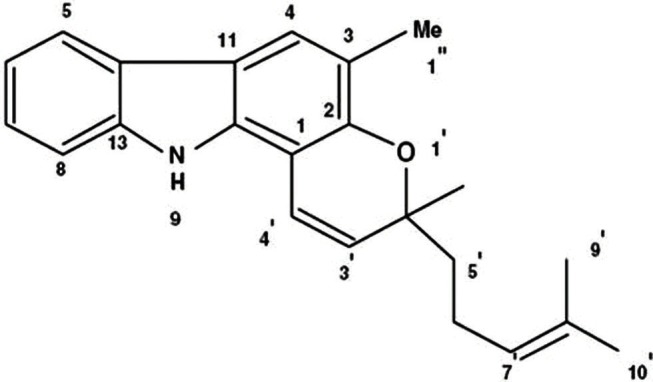
Mahanimbine structure
Figure 3.
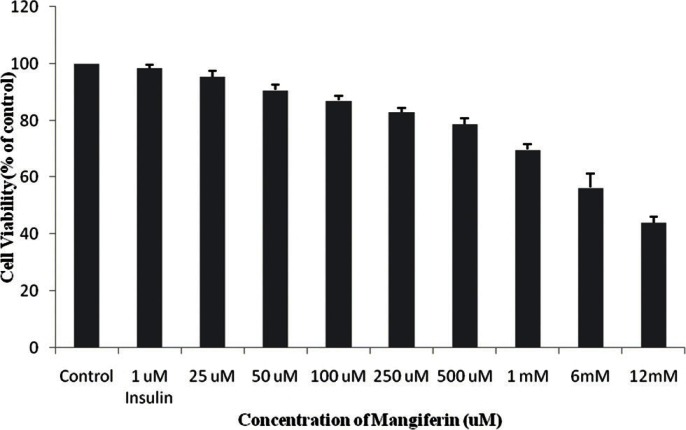
cytotoxic effects of mangiferin on the cell growth of 3T3-L1 cells as determined by MTT assay. The cells were treated with various concentrations (25 μM-1mM) of mangiferin for 48h. The results represent the mean ± SD of three independent experiments.
Figure 4.
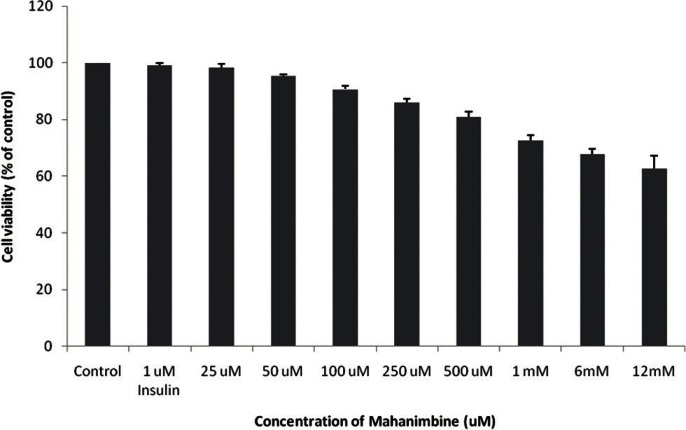
Cytotoxic effects of mahanimbine on the cell growth of 3T3-L1 cells as determined by MTT assay. The cells were treated with various concentrations (25 μM-1mM) of mahanimbine for 48h. The results represent the mean ± SD of three independent experiments.
Glucose utilization test for mangiferin and mahanimbine with 3T3-L1 cells
The effect of mangniferin and mahanimbine on glucose utilization is shown in Figures 5 and 6, respectively. The response of untreated control cells were considered as 100%. Both mangniferin and mahanimbine increased the glucose utilization in a dose-dependent manner. At a concentration of 1 mM, mangniferin showed 2-fold increase in glucose utilization compared with untreated control. In case of mahanimbine, the observed effect at 1 mM was almost equivalent to positive control (insulin at 1 μM).
Figure 5.
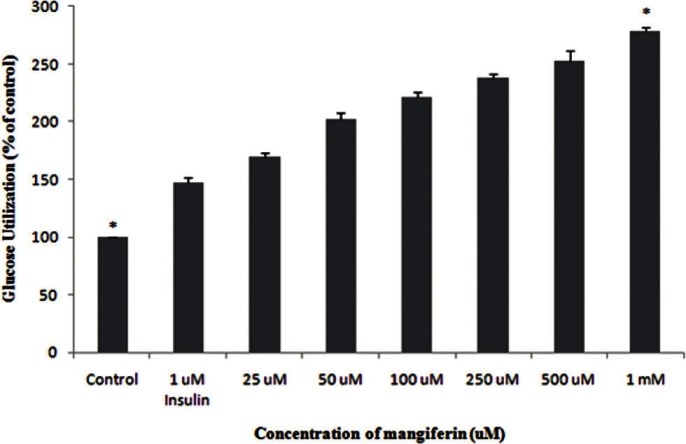
Glucose utilization of magiferin in 3T3-L1 cell line. 3T3-L1 cells were harvested in RPMI media, followed by incubation with different concentrations (25μM-1mM) of mangiferin. 1μM insulin was used as a standard drug. The results represent the mean ± Standard Deviation of three independent experiments.*(P <0.05) indicate significant difference from control.
Figure 6.
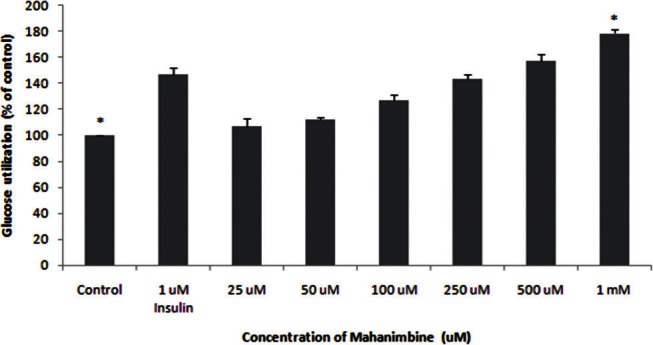
Glucose utilization of mahanimbine in 3T3-L1 cell line. 3T3-L1 cells were harvested in RPMI media, followed by incubation with different concentrations (25μM-1mM) of mahanimbine. 1μM insulin was used as a standard drug. The results represent the mean ± Standard Deviation of three independent experiments. *(P <0.05) indicate significant difference from control.
Several plant extract and plant bioactive compounds (alkaloids, polyphenols) have been reported for antidiabetic activity in 3T3-L1 cell lines. Berberine alkaloid from Cortidis Rhizoma decreased the triglycerides accumulation in 3T3-L1 cells and also berberine increased glucose-stimulated insulin secretion in Min6 cells via an enhanced insulin/insulin-like growth factor-1 signaling cascade. This study suggested that berberine could be used as antidiabetic agents for obese diabetic patients.[10] Akuammicine, an indole alkaloid was isolated from the chloroform extract of the seeds of Picralima nitida (Apocynaceae) stimulated glucose uptake activity in differentiated 3T3-L1 adipocytes cell line.[11] Green tea polyphenol epigallocatechin gallate showed inhibition on adipogenesis in 3T3-L1 cell line. This study suggested that epigallocatechin gallate showed direct inhibition on differentiation of preadipocytes and could be an important adjunct in the treatment of diabetes with obesity.[12] Hence, screening of medicinal plants with antidiabetic potentials have been performed using glucose utilization test in 3T3-L1 cell line.[13]
Although various antidiabetic agents exist, still we could not find a single drug effective in all ways. Since in each individual the effect of diabetes is not same, search for new drugs seems inconclusive. Already extracts of Murraya koenigii leaves and Mangifera indica stem bark have been shown to have antidiabetic activity. But no research provided confirmation for the active compound responsible for it. In the present study, we had provided antidiabetic evidence for the isolated active compounds namely: mahanimbine and mangiferin from Murraya koenigii leaves and Mangifera indica stem bark extracts respectively. Both compounds exhibited less toxicity in the 3T3-L1 cell line.
CONCLUSION
The present study indicated that both mahanimbine and mangiferin showed dose-dependent glucose utilization in the 3T3-L1 cell line. At a concentration of 1 mM, mangniferin showed 2-fold increase in glucose utilization compared to untreated control. In case of mahanimbine, the observed effect at 1 mM was almost equivalent to positive control (insulin at 1 μM). Therefore, results of this study have validated the use of natural products like mangiferin and mahanimbine in the management of diabetes.
Footnotes
Source of Support: None
Conflict of Interest: No
REFERENCES
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31:S55–60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 3.Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. New Engl J Med. 1999;341:240–5. doi: 10.1056/NEJM199907223410404. [DOI] [PubMed] [Google Scholar]
- 4.Fowler MJ. Diabetes Treatment, Part 2: Oral agents for glycemic management. Clin Diabetes. 2007;25:131–4. [Google Scholar]
- 5.Atta-ur-Rahman, Zaman K. Medicinal plants with hypoglycemic activity. J Ethnopharmacol. 1989;26:1–55. doi: 10.1016/0378-8741(89)90112-8. [DOI] [PubMed] [Google Scholar]
- 6.Ojewole J. Anti-inflammatory, analgesic, and hypoglycemic effects of Mangifera indica Linn (Anacardiaceae) stem-bark aqueous extract. Methods Find Exp Clin Pharmacol. 2005;27:547–54. doi: 10.1358/mf.2005.27.8.928308. [DOI] [PubMed] [Google Scholar]
- 7.Kesari AN, Gupta RK, Geeta W. Hypoglycemic effects of Murraya koenigii on normal and alloxan-diabetic rabbits. J Ethnopharmacol. 2005;97:247–51. doi: 10.1016/j.jep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Dineshkumar B, Mitra A, Manjunatha M. Studies on the antidiabetic and hypolipidemic potentials of mangiferin (xanthone glucoside) in streptozotocin-induced type 1 and type 2 diabetic model rats. Int J Adv Pharma Sci. 2010;1:75–85. [Google Scholar]
- 9.Dineshkumar B, Mitra A, Manjunatha M. Antidiabetic and hypolipidemic effects of mahanimbine (carbazole alkaloid) from Murraya koenigii (Rutaceae) Leaves. Int J Phytomed. 2010;2:22–30. [Google Scholar]
- 10.Ko BS, Choi SB, Park SK, Jang JS, Kim YE, Park S. Insulin sensitizing and insulinotropic action of berberine from Cortidis Rhizoma. Biol Pharm Bull. 2005;28:1431–7. doi: 10.1248/bpb.28.1431. [DOI] [PubMed] [Google Scholar]
- 11.Shittu H, Gray A, Furman B, Young L. Glucose uptake stimulatory effect of akuammicine from Picralima nitida (Apocynaceae) Phytochemistry. 2010;3:53–5. [Google Scholar]
- 12.Lin J, Della-Fera MA, Baile CA. 2005. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Obesity Res. 2005;13:982–90. doi: 10.1038/oby.2005.115. [DOI] [PubMed] [Google Scholar]
- 13.van de Venter M, Roux S, Bungu LC, Louw J, Crouch NR, Grace OM, et al. Antidiabetic screening and scoring of 11 plants traditionally used in South Africa. J Ethnopharmacol. 2008;119:81–6. doi: 10.1016/j.jep.2008.05.031. [DOI] [PubMed] [Google Scholar]


