Abstract
Background:
Ravenia spectabilis is a medium tall shrub found widespread in South America. It also found in India, Pakistan, Bangladesh etc. Few alkaloid and steroid compounds were reported from the plant previously.
Materials and Methods:
Methanol extract from the stems of Ravenia spectabilis were partitioned into n-hexane, carbon tetrachloride, chloroform and aqueous soluble fractions, respectively. The crude methanol extract, carbon tetrachloride fraction and chloroform fraction were fractionated by column chromatography of Silica gel and Sephadex LH-20 for isolation and purification of compounds. The structures of the isolated compounds were determined by extensive NMR spectral analysis, including 2D NMR, mass spectroscopy etc.
Results:
Ten compounds, γ-fagarine (1), ravenoline (2), N-methyl atanine (3),2,3,3,5-tetramethyl-2,3,4,5- tetrahydrofurano [3,2-c] quinolin-4-one (4), arborinine (5), 3-geranyl indole (6), atanine (7), steroids sitosta-4-en- 3-one (8), stigmasterol (9) and 3-methoxy-4-hydroxy cinnamic acid (10) were isolated from the stems of Ravenia spectabilis.
Conclusion:
Compounds N-methyl atanine (3), 2,3,3,5-tetramethyl-2,3,4,5-tetrahydrofurano [3,2-c] quinolin-4-one (4), 3-geranyl indole (6), sitosta-4-en-3-one (8) and 3-methoxy-4-hydroxy cinnamic acid (10) were isolated from this plant for the first time. 3-geranyl indole (6) was also isolated second time from natural sources.
Keywords: 3-methoxy-4-hydroxy cinnamic acid, alkaloids, Ravenia spectabilis, rutaceae, steroids
INTRODUCTION
Development of new drugs is essential due to the invasion of new threats or the formation of resistance against existing drugs. Medicinal plants are an ideal target for discovery of potential bioactive compounds or lead structures for new drugs. The main aim of this study was to identify and characterize the bioactive compounds from the stem of Ravenia spectabilis. Ravenia spectabilis is a medium tall shrub found widespread in South America. This species is cultivated in many districts of Bangladesh. It is included under the family Rutaceae. The Rutaceous plants contain a wide range of pharmacologically active compounds[1] including anti-inflammatory, anti-implantation, anti-neoplastic and anti-mutagenic activities. The family is well known for producing a wide range of secondary metabolites, such as phenanthridine, acridone and furo- and pyranoquinoline alkaloids, complex furo- and pyranocoumarins, flavonoids and various types of terpenoids, including limonoids.[2]
A literature survey indicated that R. spectabilis possesses antimicrobial and cytotoxic activities.[3,4] Previous phytochemical studies revealed the occurrences of a number of compounds such as paraensine,[5] ravesilone,[5] spectabiline,[6] ravenine,[7] ravenoline,[7] atanine,[7] γ-fagarine,[3] arborinine,[3,4] stigmasta-4,22-dien-3-one[3] and stigmasterol[3] from this plant and among them γ-fagarine, arborinine and atanine were found to be bioactive.[8–15]
As a continuation of our phytochemical studies on medicinal plants, this article reports the isolation of 7 alkaloids, namely γ-fagarine (1),[3,16,17] ravenoline (2),[7] N-methyl atanine (3),[18,19] 2,3,3,5-tetramethyl-2,,3,4,5-tetrahydrofurano [3,2-c] quinolin-4-one (4),[20] arborinine (5),[3,4,21] 3-geranyl indole (6),[22,23] atanine (7),[7] two steroids, namely sitosta-4-en-3-one (8),[24] stigmasterol (9)[3,25,26] and also the 3-methoxy-4-hydroxy cinnamic acid (10)[27] from the stem of R. spectabilis.
MATERIALS AND METHODS
General experimental procedures
Accurate mass measurements were determined on JMS600H Mass Spectrometer. NMR spectra (both 1D and 2D) were obtained on a Bruker spectrometer (400 and 500 MHz for 1H and 100 and 125 MHz for 13C), using the residual solvent peaks as internal standard. J-modulated 13C spectra were acquired with a relaxation time (d1) of 4s. HMBC spectra were optimized for a long range JH-C of 7 Hz (d6 = 0.07s). Vacuum-liquid chromatography (VLC) was carried out using Merck Si gel 60 H. PTLC was carried out using Merck Si gel 60 PF254 on glass plates (20 cm X 20 cm) at a thickness of 0.5 mm. TLC was conducted on normal-phase Merck Si gel 60 PF254 plates. Spots on TLC and PTLC plates were visualised under UV light (254 and 366 nm) and by spraying with ceric sulphate and Vanillin-Sulfuric acid reagent.
Plant material
The stems of R. spectabilis were collected from Balda Garden (District-Dhaka) and the plant was identified by Professor Salar Khan of Bangladesh National Herbarium (BNH), Dhaka, where a voucher specimen has been deposited (DACB Accession no. 28090). The stems were collected again from the campus of Dhaka University (DACB Accession no. 34694) to undergo phytochemical work using a different method to isolate more compounds. The stems were first air dried and then ground into coarse powder using a grinding machine.
Extraction and isolation
About 1.0Kg of the powdered plant material of stem from the first collection was soaked in 2.5 litre of methanol for 7 days. The concentrated methanol extract (10.13g) was partitioned by the modified Kupchan partitioning procedure[28] into n-hexane, carbon tetrachloride, chloroform and aqueous soluble fractions, respectively.
Column fractionation of the carbon tetrachloride soluble fraction (1.42g) on Silica gel was performed using a mobile phase of petroleum ether, chloroform and methanol in order of increasing polarities. The column fraction of 20-60% petroleum ether in chloroform was subjected to further column chromatography on Silica gel using a mobile phase of petroleum ether, chloroform and methanol in order of increasing polarities. Preparative TLC (toluene-EtOAc-AcOH = 85:5:1) of the column fraction eluted with 25-35% petroleum ether in chloroform afforded compound 1 (28mg), compound 2 (4.2mg), compound 3 (5mg) and compound 4 (3mg). The chloroform soluble fraction (2.98g) was fractionated by VLC over Silica gel 60H using petroleum ether, chloroform and methanol mixtures of increasing polarity. The VLC fraction eluted with 90% chloroform in methanol was subjected to preparative TLC (toluene-EtOAc = 90:10) to obtain compound 5 (10.5mg).
About 1.5Kg of the powdered plant material of stem from the second collection was soaked in 4.0 litre of methanol for 7 days. The concentrated methanol extract (15.0g) was fractionated by VLC over Silica gel 60H using petroleum ether, ethyl acetate and methanol mixtures of increasing polarity. The VLC fraction of 8–15% ethyl acetate in petroleum ether was subjected to Gel Permeation Chromatography on Sephadex LH-20 using a mobile phase of petroleum ether, chloroform and methanol in order of increasing polarities to induce adsorption and partition quality like a normal phase column chromatography for better separation of components. The sephadex column fraction eluted with 80–90% petroleum ether in chloroform was subjected to preparative TLC (toluene-EtOAc = 98:2) to obtain compound 6 (6mg), compound 7 (10mg) and compound 8 (9mg). Compound 9 (8mg) was obtained as a white crystal directly from the VLC fraction of 20% ethyl acetate in petroleum ether by treatment with different solvents. The VLC fraction of 25% ethyl acetate in petroleum ether was subjected to further Gel Permeation Chromatography on Sephadex LH-20 using a mobile phase of petroleum ether, chloroform and methanol in order of increasing polarities. The sephadex column fraction eluted with 80–100% petroleum ether in chloroform was subjected to preparative TLC (toluene-EtOAc = 95:5) to obtain compound 10 (6mg).
RESULTS
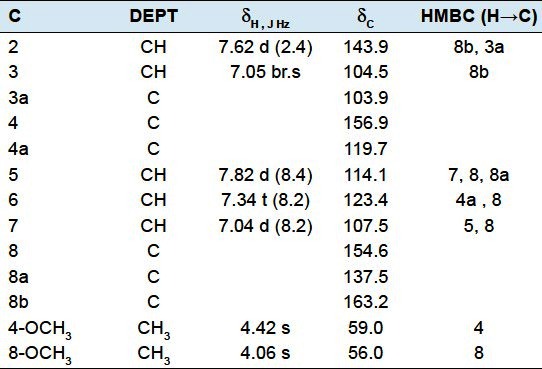
γ-fagarine (1): Pink gum. 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data have been given in Table 1.
Table 1.
1H- and 13C-NMR spectroscopic data for compound 1
Ravenoline (2): Yellow gum. 1H NMR (400 MHz, CDCl3): δ 1.38 (3H, d, J = 7.2 Hz, CH3 -1’), 1.82 (3H, s, CH3-2’), 3.72 (3H, s, N-CH3), 4.17 (1H, m, H-1’), 5.26 (1H, s, H-3’), 5.33 (1H, s, H-3’), 7.22 (1H, dd, J = 8.0, 1.2 Hz, H-6), 7.31 (1H, d, J = 8.2 Hz, H-8), 7.31 (1H, s, OH-4), 7.54 (1H, dd, J = 7.2, 1.5 Hz, H-7), 7.92 (1H, dd, J = 8.0, 1.2 Hz, H-5).
N-methyl atanine (3): Yellow gum, 1H NMR (400 MHz, CDCl3): δ 1.60 (3H, s, CH3-3’), 1.81 (3H, s, CH3-3’), 3.41 (2H, d, J = 6.8 Hz, -CH2-1’), 3.72 (3H, s, N-CH3), 3.92 (3H, s, OCH3-4), 5.25 (1H, m, H-2’), 7.24 (1H, tt, J = 7.2, 0.4 Hz, H-6), 7.36 (1H, d, J = 8.0 Hz, H-8), 7.53 (1H, ddd, J = 7.2, 7.2, 1.6 Hz, H-7), 7.82 (1H, dd, J = 8.0, 1.6 Hz, H-5).
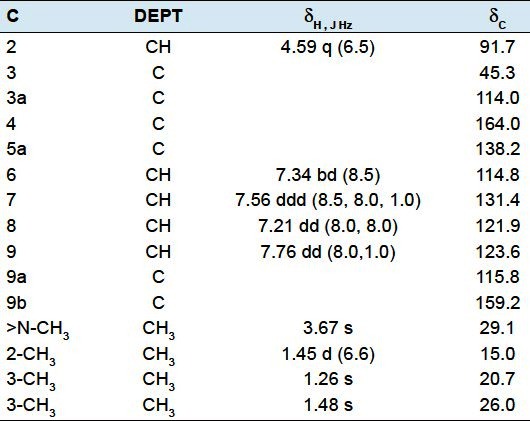
2,3,3,5-tetramethyl-2,3,4,5-tetrahydrofurano [3,2-c] quinolin-4-one (4): Yellow gum. EI-MS m/z (%): 243[M+] (30), 228 (100), 214 (10), 200 (13), 186 (8), 167 (15), 149 (32), 57 (20). 1H NMR (500 MHz, CDCl3) and 13C NMR (125 MHz, CDCl3) data have been given in Table 2.
Table 2.
1H- and 13C-NMR spectroscopic data for compound 4
Arborinine (5): Yellow gum. 1H NMR (400 MHz, CDCl3): δ 3.85 (3H, s, N-CH3), 3.93 (3H, s, OCH3-3), 4.02 (3H, s, OCH3-2), 6.29 (1H, s, H-4), 7.30 (1H, t, J = 7.4 Hz, H-7), 7.51 (1H, d, J = 8.8 Hz, H-5), 7.72 (1H, ddd, J = 7.2 Hz, H-6), 8.47 (1H, dd, J = 6.4 Hz, H-8).
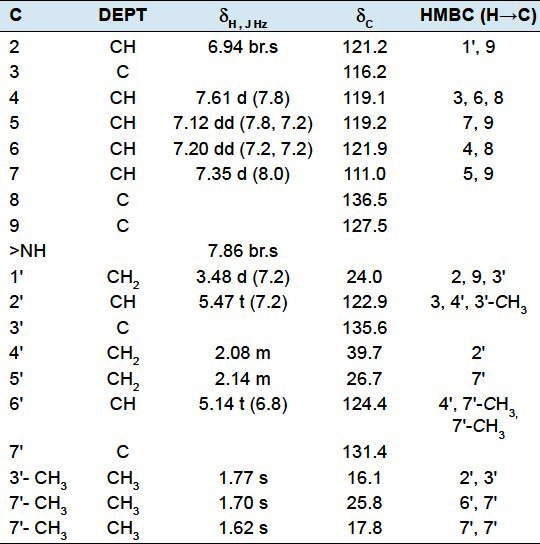
3-Geranyl indole (6): Yellow gum. 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data have been given in Table 3.
Table 3.
1H- and 13C-NMR spectroscopic data for compound 6
Atanine (7): Yellow gum. 1H NMR (400 MHz, CDCl3): δ 1.69 (3H, s, CH3-3’), 1.82 (3H, s, CH3-3’), 3.56 (2H, d, J = 6.9 Hz, H-1’), 5.28 (1H, br.t, J = 6.9 Hz, H-2’), 7.20 (1H, ddd, J = 8.1, 7.2, 1.0 Hz, H-6), 7.27 (1H, d, J = 8.1 Hz, H-8), 7.45 (1H, ddd, J = 8.1, 7.2, 1.2 Hz, H-7), 7.76 (1H, dd, J = 8.1, 1.1 Hz, H-5), 10.82 (1H, br.s, NH).
Sitosta-4-en-3-one (8): Needle like crystal. 1H NMR (400 MHz, CDCl3): δ 0.70 (3H, s, CH3-18), 0.81 (3H, d, J = 6.6 Hz, CH3-26), 0.83 (3H, d, J = 6.6 Hz, CH3-27), 0.84 (3H, t, J = 6.8 Hz, CH3-29), 0.91 (3H, d, J = 6.6 Hz, CH3-21), 1.15 (3H, s, CH3-19), 5.70 (1H, s, H-4).
Stigmasterol (9): White crystal. 1H NMR (500 MHz, CDCl3): δ 0.70 (3H, s, CH3-13), 0.82 (3H, t, CH3-28), 0.84 (3H, d, J = 6.6 Hz, CH3-25), 0.86 (3H, d, J = 6.6 Hz, CH3-25), 0.94 (3H, d, J = 6.6 Hz, CH3-20), 1.02 (3H, s, CH3-10), 3.53 (1H, m, H-4), 5.03 (1H, dd, J = 15.0, 9.0 Hz, H-23), 5.16 (1H, dd, J = 15.0, 6.5 Hz, H-22), 5.36 (1H, m, H-6).
3-Methoxy-4-hydroxy cinnamic acid (10): Yellow powder. 1H NMR (400 MHz, CDCl3): δ 3.92 (3H, s, OCH3- 3), 5.83 (1H, br.s, OH-4), 6.29 (1H, d, J = 15.9 Hz, H-8), 6.91 (1H, d, J = 8.1 Hz, H-5), 7.03 (1H, d, J = 1.8 Hz, H-2), 7.07 (1H, dd, J = 8.1, 1.8 Hz, H-6), 7.61 (1H, d, J = 15.9 Hz, H-7).
DISCUSSION
The air-dried and ground stem of the first collection of R. spectabilis were macerated with methanol and partitioned with n-hexane, carbon tetrachloride and chloroform. Column fractionation of the carbon tetrachloride soluble fraction followed by preparative TLC afforded compound 1 (28mg), compound 2 (4.2mg), compound 3 (5mg) and compound 4 (3mg), as well as VLC fractionation of the chloroform soluble fraction followed by preparative TLC afforded compound 5 (10.5mg). Again, VLC fractionation of the crude methanol extract obtained from the second collection followed by Gel Permeation Chromatography on Sephadex LH-20 and preparative TLC afforded compound 6 (6mg), compound 7 (10mg) and compound 8 (9mg). Compound 9 (8mg) was obtained as a white crystal directly from the VLC fraction of the crude methanolic extract. Another VLC fractionation of the crude methanolic extract and further Gel Permeation Chromatography on Sephadex LH-20 followed by preparative TLC afforded compound 10 (6mg). The structures of 1 - 10 were determined by extensive NMR spectral analysis, including 2D NMR, mass spectroscopy and comparison with published literatures [Figure 1].
Figure 1.
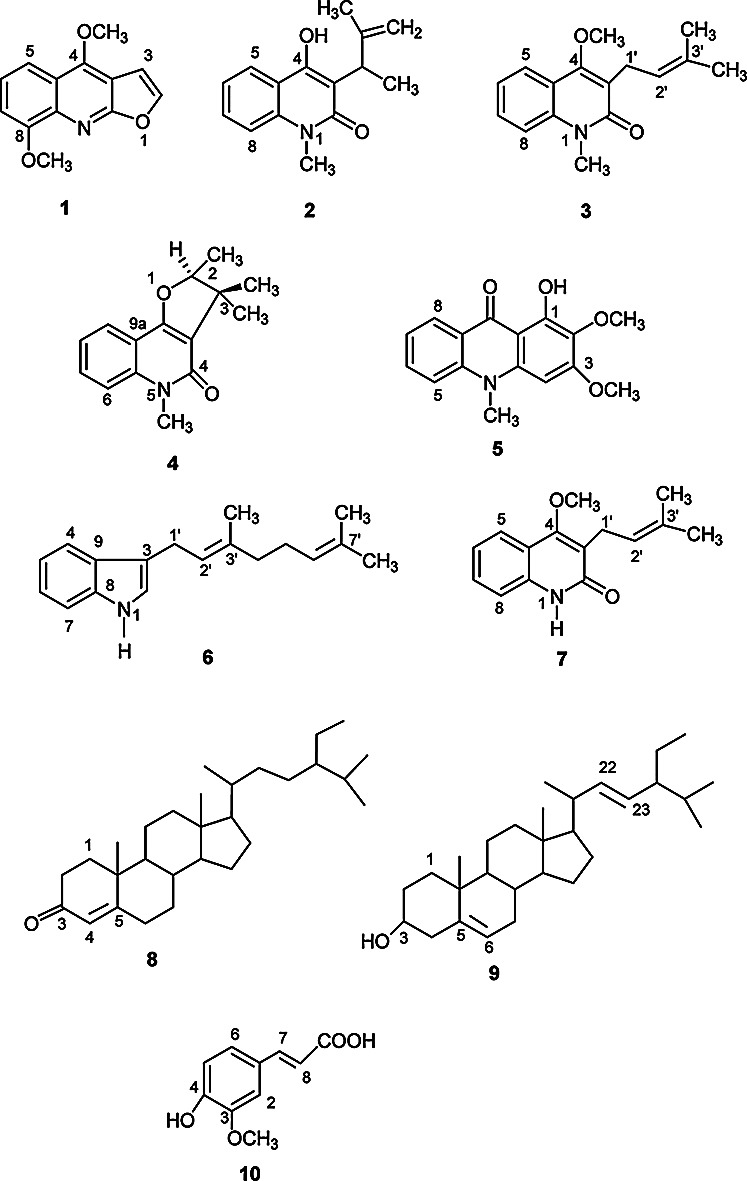
Structure of compounds 1-10 isolated from the stem of R. spectabilis
Compound 1 was obtained as a Pink gum. The structure of compound 1 was identified as γ-fagarine by comparing its 1H and 13C-NMR data with previously published report.[3,16,17] γ-fagarine was reported to have antitubercular,[8] antileishmanial,[9] and anti-HIV[10] activities. Compound 2 was obtained as a yellow gum and identified as ravenoline by comparing its 1H-NMR data with published value.[7]
Compound 3 was isolated as a yellow gum. The structure of compound 3 was established as N-methyl atanine by comparison with published paper.[18,19] Compound N-methyl atanine was isolated from this plant species for the first time.
The structure of compound 4 was confirmed as 2,3,3, 5-tetramethyl-2,3,4,5-tetrahydrofurano [3,2-c] quinolin-4-one by the mass spectrum with [M+] at m/z = 243, suggesting the molecular formula C15H17NO2 in agreement with the NMR spectra and by comparison with published article.[20] 2,3,3,5-tetramethyl-2,3,4,5-tetrahydrofurano [3,2-c] quinolin-4-one was isolated from this plant for the first time.
Compound 5 was obtained as a yellow gum. Compound 5 was identified as arborinine by comparing its 1H-NMR data with published value.[3,4,21] Arborinine showed anti-HRV-2,[11] antiplasmodial[12] and cytotoxic[13] activities.
Compound 6 was found as a yellow gum. The structure of compound 6 was confirmed as 3-geranyl indole by comparison with previous published literature.[22,23] 3-Geranyl indole was reported for the first time from this plant species and second time from natural sources. We, herein, report 2D NMR for compound 6 for the first time.
Compound 7 was obtained as a yellow gum. Compound 7 was identified as atanine by comparing its 1H-NMR data with published value.[7] Atanine was found to be cytotoxic[14] and anthelmintic.[15]
The 1H NMR spectra of compound 8 were found in close agreement with those reported for sitosta-4-en-3-one.[24] It was reported from this plant species for the first time.
Compound 9 was obtained as white crystal. The structure of compound 9 was identified as stigmasterol by comparing its 1H-NMR data with published article.[3,25,26]
Compound 10 was isolated as yellow powder. According to its NMR data and a comparison with those given in the literature, the structure of 10 was identified as 3-methoxy-4-hydroxy cinnamic acid.[27] This compound was isolated from this plant for the first time.
ACKNOWLEDGEMENTS
We would like to thank Bangladesh Council of Scientific and Industrial Research (BCSIR) for NMR studies and providing laboratory facilities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Saxton JE. Recent progress in the chemistry of indole alkaloids and mould metabolites. Nat Prod Rep. 1989;6:433–74. doi: 10.1039/np9890600433. [DOI] [PubMed] [Google Scholar]
- 2.Waterman PG. Chemistry and Chemical Taxonomy of the Rutales. In: Waterman PG, Grundon MF, editors. London: Academic Press; 1983. p. 337. [Google Scholar]
- 3.Sohrab MH, Chowdhury R, Rahman KM, Hasan CM, Rashid MA. Antibacterial activity and cytotoxicity of extractives from Ravenia spectabilis. Fitoterapia. 2004;75:510–13. doi: 10.1016/j.fitote.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Alam A, Asaduzzaman M, Nasrin N. Isolation, Characterization and Evaluation of Anti-microbial Activity of Ravenia spectabilis (Rutaceae) Int J Pharmacog Phytochem Res. 2011;3:80–4. [Google Scholar]
- 5.Khan MA, Waterman PG. Constituents of Ravenia spectabilis. Fitoterapia. 1990;61:282 (Eng). [Google Scholar]
- 6.Talapatra SK, Maiti BC, Talapatra B, Das BC. Spectabiline, A new dihydrofuroquinol-4-one alkaloid from Lemonia spectabilis Lindl. Tetrahedron Lett. 1969;54:4789–90. [Google Scholar]
- 7.Paul BD, Bose PK. New quinolone alkaloids from Ravenia spectabilis Engl. J Indian Chem Soc. 1968;45:552–3. [Google Scholar]
- 8.Huang HY, Ishikawa T, Peng CF, Tsai IL, Chen IS. Constituents of the root wood of Zanthoxylum wutaiense with antitubercular activity. J Nat Prod. 2008;71:1146–51. doi: 10.1021/np700719e. [DOI] [PubMed] [Google Scholar]
- 9.Ostan I, Saglam H, Limoncu ME, Ertabaklar H, Toz SO, Ozbel Y, et al. In vitro and in vivo activities of Haplophyllum myrtifolium against Leishmania tropica. New Microbiol. 2007;30:439–45. [PubMed] [Google Scholar]
- 10.Cheng MJ, Lee KH, Tsai IL, Chen IS. Two new sesquiterpenoids and anti-HIV principles from the root bark of Zanthoxylum ailanthoides. Bioorg Med Chem. 2005;13:5915–20. doi: 10.1016/j.bmc.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 11.Rollinger JM, Schuster D, Danzl B, Schwaiger S, Markt P, Schmidtke M, et al. In silico target fishing for rationalized ligand discovery exemplified on constituents of Ruta graveolens. Planta Med. 2009;75:195–204. doi: 10.1055/s-0028-1088397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waffo AF, Coombes PH, Crouch NR, Mulholland DA, El Amin SM, Smith PJ. Acridone and furoquinoline alkaloids from Teclea gerrardii (Rutaceae: Toddalioideae) of southern Africa. Phytochemistry. 2007;68:663–7. doi: 10.1016/j.phytochem.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Réthy B, Zupkó I, Minorics R, Hohmann J, Ocsovszki I, Falkay G. Investigation of cytotoxic activity on human cancer cell lines of arborinine and furanoacridones isolated from Ruta graveolens. Planta Med. 2007;73:41–8. doi: 10.1055/s-2006-951747. [DOI] [PubMed] [Google Scholar]
- 14.Varamini P, Javidnia K, Soltani M, Mehdipour AR, Ghaderi A. Cytotoxic activity and cell cycle analysis of quinoline alkaloids isolated from Haplophyllum canaliculatum Boiss. Planta Med. 2009;75:1509–16. doi: 10.1055/s-0029-1185807. [DOI] [PubMed] [Google Scholar]
- 15.Perrett S, Whitfield PJ. Atanine (3-dimethylallyl-4-methoxy-2- quinolone), an alkaloid with anthelmintic activity from the Chinese medicinal plant, Evodia rutaecarpa. Planta Med. 1995;61:276–8. doi: 10.1055/s-2006-958073. [DOI] [PubMed] [Google Scholar]
- 16.Grundon MF, McCorkindale NJ. The Synthesis of Dictamnine and γ-fagarine. J Chem Soc. 1957:2177–85. [Google Scholar]
- 17.Mai HD, Van-Dufat HT, Michel S, Tillequin F, Bastien D, Sevenet T. A new diprenyl coumarin and alkaloids from the bark of Zanthoxylum dimorphophyllum. Z Naturforsch [C] 2001;56:492–94. doi: 10.1515/znc-2001-7-802. [DOI] [PubMed] [Google Scholar]
- 18.Fauvel MT, Gleye J, Moulis C, Blasco F, Stanislas E. Alkaloids and flavonoids of Melicope Indica. Phytochemistry. 1981;20:2059–60. [Google Scholar]
- 19.Ramesh M, Shanmugam P. A Convenient synthesis of the naturally occurring alkaloid N- Methylatanine. Ind J Chem Sect B: Org Chem incl Med Chem. 1984;23B:110–3. [Google Scholar]
- 20.Jurd L, Benson M, Wong RY. New Quinolinone and bis- Quinolinone Alkaloids frorn Euxylophora paraensis. Aust J Chem. l983;36:759–68. [Google Scholar]
- 21.Sultana N, Hartley TG, Waterman PG. Two novel prenylated flavones from the aerial parts of Melicope micrococci. Phytochemistry. 1999;50:1249–53. [Google Scholar]
- 22.Nkunya, Mayunga HH, Makangara JJ, Jonker SA. Prenylindoles from Tanzanian Monodora and Isolona Species. Nat Prod Res. 2004;18:253–8. doi: 10.1080/14786410310001620529. [DOI] [PubMed] [Google Scholar]
- 23.Xiuwen Z, Ganesan A. Regioselective synthesis of 3-Alkylindoles mediated by zinc tiflates. J Org Chem. 2002;67:2705–8. doi: 10.1021/jo010996b. [DOI] [PubMed] [Google Scholar]
- 24.Joshi KC, Bansal RK, Sing P. Mass and NMR spectral studies of sitost-4-en-3-one from Tabebuia rosea. Indian J Chem. 1974;12:903–4. [Google Scholar]
- 25.Khan RI. 2nd ed. N. Y., USA: Academic press; 1991. Natural product: A laboratory guide. [Google Scholar]
- 26.Xie D, Wang L, Ye H, Li G. Isolation and production of aremisinin and stigmasterol in hairy root cultures of Artemisia annua. Plant Cell Tissue Organ Culture. 2000;63:161–6. [Google Scholar]
- 27.Benzel M, Ralph J, Funk C, Steinhart H. Structural elucidation of new ferulic acid-containing phenolic dimers and trimers isolated from maize bran. Tetrahedron lett. 2005;46:5845–50. [Google Scholar]
- 28.VanWagenen BC, Larsen R, Cardellina JH, 2nd, Randazzo D, Lidert ZC, Swithenbank C. Ulosantoin, a potent insecticide from the sponge Ulosa ruetzleri. J Org Chem. 1993;58:335–7. [Google Scholar]


