Summary
The small GTPases Rab5 and Rab7 are important organisers of endosome formation and maturation. In addition, they orchestrate the trafficking of cargo through the endosomal pathway. A crucial event during maturation of endosomes is the replacement of the early organiser Rab5 with the late organiser Rab7 in a process called Rab conversion. Rab conversion is a prerequisite for late events, chief among them the fusion of matured endosomes with the lysosome. Recent work identifies members of the Sand1/Mon1 protein family as crucial factors during this process. Here, we present an analysis of the function of the Drosophila ortholog of mon1/sand1, Dmon1. We found that loss of function of Dmon1 results in an enlargement of maturing endosomes and loss of their association with Rab7. The enlarged endosomes contain Notch and other trans-membrane proteins as cargo. We report the first electron microscopy analysis of Dmon1 cells in a metazoan and extend the analysis of the endosomes in mutant cells. Our results suggest that the phenotype can be explained by the loss of function of Rab7. Moreover, the endosomes of Dmon1 cells mature normally in many aspects, despite the loss of association with Rab7. Surprisingly, we did not observe overactive or ectopic signalling through receptors such as Notch and RTKs in Dmon1 mutant cells, as would have been expected because of the accumulation of receptors in the maturing endosomes of these cells. This was the case even when receptor uptake into intraluminal vesicles was suppressed.
Key words: Mon1, Notch, Rab7, Sand1, Cell signaling, endosomal trafficking
Introduction
In eukaryotic cells a highly dynamic endomembrane system is responsible for the compartmentalisation and maintenance of several organelles as well as the transport of cargo between them (reviewed by Huotari and Helenius, 2011). During endosomal trafficking, transmembrane proteins destined for degradation are incorporated in small endocytic vesicles (EV) that have pinched off the plasma membrane during endocytosis. These EVs fuse to form the early endosome (EE). From the EE, receptors can relocate to the plasma membrane through Rab4-controlled fast or Rab11-controlled slow recycling pathways. Cargo destined for degradation remains in the maturing endosome (ME), which eventually fuses with the lysosome, where its cargo is degraded. During maturation of the endosome, vesicles bud off from its limiting membrane (LM) into its lumen at positions where cargo proteins are concentrated. The incorporation into these intraluminal vesicles (ILVs) separates transmembrane proteins from the cytosol (reviewed by Dobrowolski and De Robertis, 2012). This step is especially important for activated signalling receptors, since they remain active after their endocytosis in the EE. Examples are RTK-receptors, which are inactivated through incorporation into ILVs. The formation of the ILVs is executed by the ESCRT machinery (Henne et al., 2011).
Small GTPases of the Rab family control the fusion of membranes and trafficking of cargo (reviewed by Huotari and Helenius, 2011). These Rabs cycle between the active GTP bound and inactive GDP bound form. They are activated by guanosin exchange factors (GEFs), which exchange GDP with GTP. Upon inactivation through GTPase activating proteins (GAP), Rabs translocate from the LM into the cytosol. Rab5 controls homotypic fusion of EVs with themselves or EEs and initiates ILV formation. These processes are started through recruitment of effectors to the LM of the EE. Among the Rab5 effectors are Hrs, a component of the ESCRT-0 complex, which initiates ILV formation and EEA1, which is required for membrane fusion. Recruitment of both effectors occurs through prior recruitment of Vps34, a PI (3) Kinase, which generates PI(3)P on the cytosolic leaflet of the endosomal membrane. PI(3)P is the docking site that is recognised by the FYVE domains of Hrs and EEA1.
During late stages of endosomal maturation, Rab5 is replaced by Rab7 in a process referred to as Rab conversion (Rink et al., 2005). Rab7 organises fusion of MEs with the lysosome where its cargo is degraded. Initial tethering of both organelles is mediated by the HOPS tethering complex (reviewed by Nickerson et al., 2009). The fusion terminates the life of the ME. Thus, one critical parameter for the lifetime of the endosome is its fusion rate with the lysosome. A delay in fusion increases the lifetime and allows more homotypic fusion between MEs to occur. Hence, it results in an increase in size and numbers of MEs.
Recent work indicates that Mon1 and its C. elegans ortholog Sand-1 are required for Rab conversion in yeast, C. elegans and probably also in mammalian cells (Kinchen and Ravichandran, 2010; Poteryaev et al., 2007; Poteryaev et al., 2010). Loss of function of mon1/sand-1 results in a failure of Rab7 recruitment to the ME. Instead, Rab5 and its GEF Rabex5 accumulate on the LM of MEs. The MEs appear to be dramatically enlarged if monitored with the fluorescent microscope (Poteryaev et al., 2007; Poteryaev et al., 2010). However, an EM analysis that would confirm an enlargement is missing.
While work so far establishes an important role for SAND-1/Mon1 during Rab conversion, its precise function is not resolved. Using C. elegans and mammalian cell culture, Poteryaev et al. (Poteryaev et al., 2010) suggested that Mon1/Sand1 interrupts a positive feedback loop between Rab5 and its exchange factor Rabex5 and then recruits Rab7 to the LM of the MEs. Recent work has established that Rab conversion is also important during phagocytosis in C. elegans (Kinchen and Ravichandran, 2010). In this process, Sand1 appears to bind directly to Rab5GTP. Furthermore, Sand-1 binds to Rab7 only in complex with its binding partner Ccz1. A similar complex formation has been previously observed in yeast (Wang et al., 2002). It was proposed that Rab5GTP recruits Sand1/Mon1, which in turn recruits Rab7 to the EE. It is not clear whether the discrepancy in the suggested mechanisms of Mon1 action is due to the analysis of two different processes or due to differences in the experimental approaches. Using the yeast system, the group of Ungermann found that the Mon1–Ccz1 complex acts as a GEF of Rab7 and interacts with the HOPS subunit Vps39 (Nordmann et al., 2010). The interaction of Mon1 with Ccz1 and also Vps39 is mediated through its longin domain. According to their model, Rab5 recruits the Mon1–Ccz1 complex to the EE. The complex then inactivates Rab5 and together with Vps39, recruits and activates Rab7.
Here, we present the analysis of the function of the ortholog of mon1/sand1 in Drosophila, Dmon1. We found that loss of function of Dmon1 results in an enlargement of maturing endosomes and loss of association with Rab7. These endosomes contain Notch and other transmembrane proteins as cargo. Moreover, they appear to mature normally in all aspects tested, despite the loss of association with Rab7. We present evidence that suggest that Dmon1 act in concert with Dccz1. Moreover, we report the first EM analysis of Dmon1- and Dccz1-mutant cells in a multi-cellular organism. We further demonstrate that loss of Rab7 function results in a similar phenotype like Dmon1 and Dccz1 mutants. This suggests that the phenotype of Dmon1 mutant cells can be explained by the loss of association of Rab7 with the LM. Furthermore, we show that recruitment of Rab7 to the endosome is not dependent on the activity of vps34 and therefore probably not on the generation of PI(3)P in the endosomal membrane.
Results
Loss of mut4 and CS084 function results in the accumulation of cargo in enlarged endosomes
We isolated a mutation we named mut4 as a secondary mutation on a chromosome that bears a bib allele (see also note added at the end and supplementary material). After separation from the bib allele we found that homozygosity of mut4 causes the formation of large Notch-positive vesicles (supplementary material Fig. S1). We isolated a second allele, CS084, in a genetic screen. Both alleles are semi-lethal in homozygosity. CS084 caused a weaker phenotype than mut4, indicated by a higher number of escapers in homozygosity.
Using antibodies directed against the extracellular (NECD) and intracellular (NICD) domain of Notch, we found that the vesicles of mut4 cells contained both domains, suggesting that they contain the full-length receptor (Fig. 1A–E). They also contained the transmembrane proteins Patched (Ptc), Ser, Dl (not shown), Smoothened (Smo) and the secreted factor Wingless (Wg) (Fig. 1F–J). aPKC, a peripheral protein of the plasma membrane, was not associated with the vesicles (Fig. 1H).
Fig. 1.
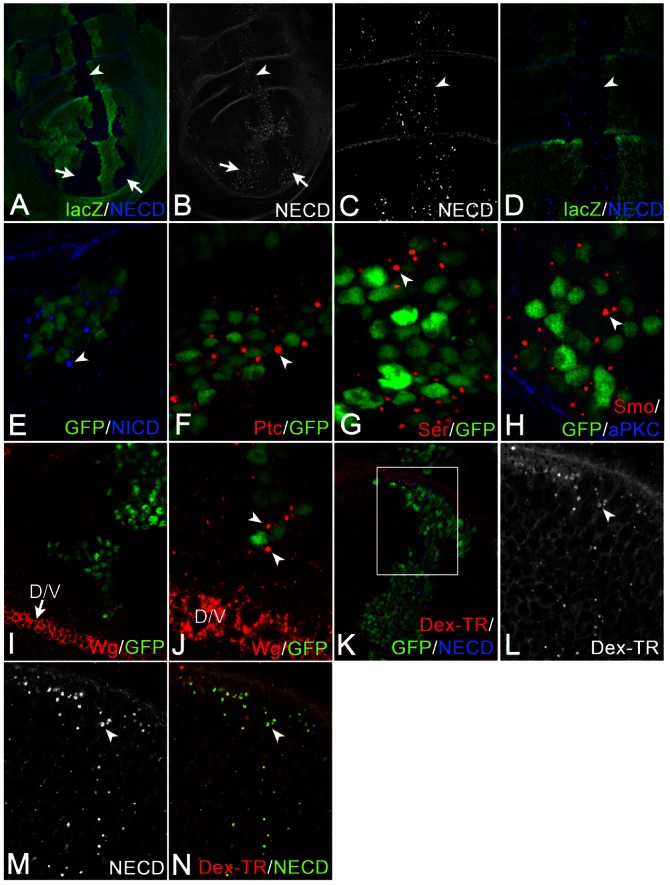
Analysis of the vesicles of mut4 cells. (A–N) mut4 cell clones were induced either by classical clonal analysis (A–D, same disc) or MARCM (E–N). (A–E) The vesicles of mut4 cells contain the extracellular (NECD) and intracellular (NICD) domain of Notch. Arrows and arrowheads in A–E highlight the clone region. The vesicles contain Ptc (F), Ser (G) and Smo (H), but not the membrane-associated aPKC (H). (I,J) Vesicles are positive for Wg, which is produced in cells at the D/V boundary (highlighted by the arrow in I). Wg can be found in large vesicles of mut4 cells that are located several cell diameters away from its source (J, arrowheads). (K–N, same disc) The Uptake experiment using an antibody directed against NECD together with dextran-Texas-Red revealed that mut4 cells contain enlarged vesicles that are positive for both markers (arrowheads). The region framed in K is shown at higher magnification in L–N.
Wg expression is restricted to a small stripe of cells at the dorso–ventral (D/V) boundary of the wing primordium (Fig. 1I, arrow). We detected large Wg-positive vesicles in mutant cells that are located several cell diameters away from its source (arrowheads in Fig. 1J). This observation suggests that Wg is endocytosed into the receiving mutant cells. Hence, the large vesicles are enlarged endosomes. To corroborate this notion, we performed uptake experiments with dextran conjugated to Texas-Red together with the NECD directed antibody (Fig. 1K–N). We found that dextran and NECD ended up in the same enlarged vesicles in mut4 cells, confirming that they are endosomes. This result indicates that endocytosis is not affected and that the defect in mut4 cells occurs during endosomal trafficking.
Loss of mut4/CS084 function results in a failure of recruitment of Rab7 to the maturing endosome
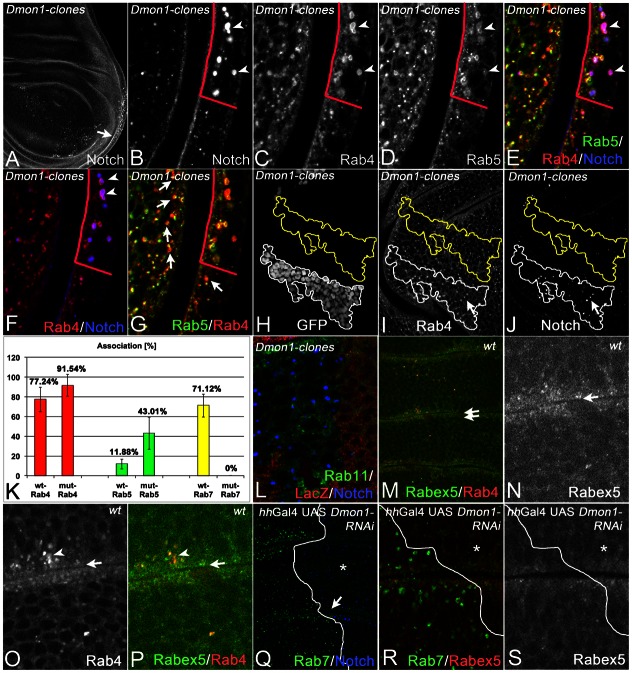
For further characterisation, we used several markers for stages of endosomal maturation (Fig. 2). We identified the enlarged endosomes in mut4 cells by co-staining with an anti-Notch antibody. Notch-positive endosomes in wild-type cells contain Rab5 as well as Rab7, indicating that they are MEs (Marois et al., 2006). We found that 43% (n = 3 clones; see later) of the Notch-positive mut4 endosomes were positive for Rab5, while this is the case in only 12% of wild-type endosomes (n = 3 clones) (Fig. 2A–C, arrows in Fig. 2B). Thus, loss of mut4 function results in an increase of association of Rab5 with endosomes. Moreover, the mut4 endosomes have lost association with Rab7 (0% association; n = 3 clones; Fig. 2A,D,E; see later). Consequently, Rab7 was located in the cytosol of mut4 cells (Fig. 2D,E).
Fig. 2.
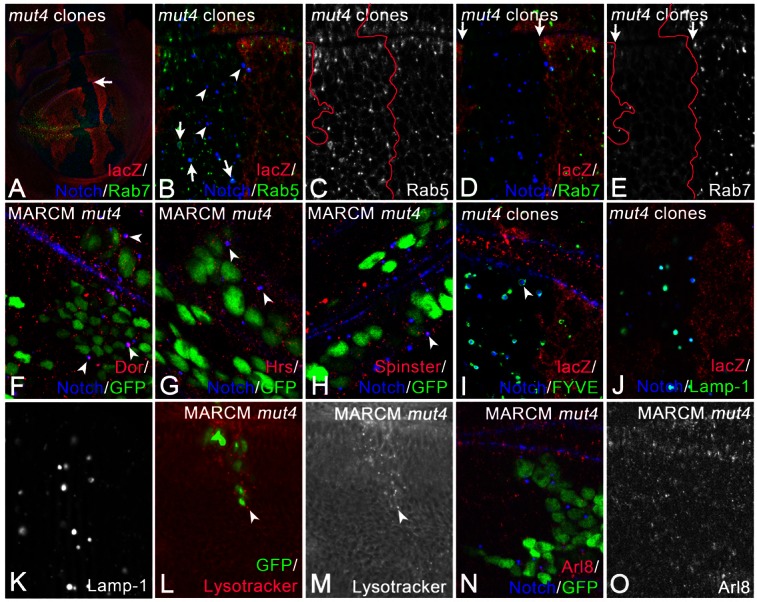
Characterisation of the enlarged endosomes of mut4 cells. (A–O) Clones were induced by classical clonal analysis (A–E, same disc; I–K) or MARCM (F–H,L–O). (A–E) The arrow in A points to the mutant area (absence of lacZ), which is shown at higher magnification in B–E. (B,C) Several of the enlarged Notch-positive endosomes of mut4 cells are associated with Rab5 (arrows in B), whereas others have lost the association (arrowheads in B). (D,E) In mutant cells, Rab7 has lost its association with endosomes and is located in the cytosol. The arrows in D and E highlight the boundaries of the clone labelled by the absence of lacZ. The enlarged endosomes are also positive for Dor (F, arrowheads), Hrs (G, arrowheads), Spinster (H, arrowhead), UAS FYVE-GFP (I, arrowhead) and tub. LAMP-1-GFP (J,K; same disc). Moreover, they can be stained with Lysotracker (L,M, arrowhead; same disc). (N,O; same disc) The distribution of the lysosomal marker Arl8 is not affected in mut4 cells.
These observations indicate that loss of the gene function affected in mut4/CS084 causes a failure of recruitment of Rab7 from the cytosol onto the endosome.
We found that Dor, a core component of the related CORVET and HOPS tethering complexes of Drosophila (Sevrioukov et al., 1999), accumulates on many enlarged endosomes of mutant cells (arrowheads in Fig. 2F). The mutant endosomes were also decorated with Hrs and Spinster, which are markers for MEs (arrowheads in Fig. 2G,H). Like MEs in wild-type cells, the mutant counterparts were labelled by the PI(3)P sensor FYVE-GFP (Fig. 2I). Thus, Rab5 mediated recruitment of Vps34 to endosomes occurs in mutant cells, indicating that Rab5 is active. The majority of the enlarged endosomes were also positive for LAMP1-GFP, a marker for MEs and lysosomes (Fig. 2J,K). However, they were not labelled with the lysosomal marker Arl8 (Hofmann and Munro, 2006). The distribution of Arl8 is unchanged in mutant compared to wild-type cells (Fig. 2N,O). Thus, lysosomes are probably not affected by the mut4 mutation, indicating that the enlarged LAMP1-positive vesicles are MEs.
During maturation, the lumen of MEs acidifies through the activity of the vacuolar ATPase (vATPase) (Huotari and Helenius, 2011). Using Lysotracker to monitor the acidification, we found that the enlarged mut4 endosomes were strongly acidified (Fig. 2L,M).
Altogether, this analysis indicates that the observed enlarged Notch containing vesicles of mut4 cells are MEs. It also reveals that these MEs appear to mature relatively normally in many aspects, although they have lost the ability to recruit Rab7.
mut4 and CS084 are alleles of the mon1 ortholog of Drosophila
Genetic mapping revealed that the locus is uncovered by the deficiencies Df(2L)Exel6010, Df(2L)Exel8012 and Df(2L)Exel9062. These deficiencies uncover the Drosophila ortholog of yeast Mon1 (here referred to as Dmon1) encoded by CG11926 (Fig. 3A). Dmon1 encodes a 528 aa long protein that possesses a longin domain, which is important for the interactions with its partner Ccz1p and also Vps39p in yeast (Nordmann et al., 2010) (Fig. 3C). Dmon1 was an obvious candidate, since loss of its function in C. elegans causes a similar phenotype (Poteryaev et al., 2007). In order to test whether mut4 is an allele of Dmon1, we conducted the following experiments: first, we tested whether loss of Dmon1 produces a similar phenotype like mut4. We depleted cells in the middle region of the wing imaginal discs of Dmon1 function by expressing an RNAi construct with ptcGal4. To achieve maximal depletion, we co-expressed UAS Dcr2, which enhances RNAi in Drosophila (Dietzl et al., 2007). We found that depleted cells contained enlarged Notch-positive endosomes, which were associated with Rab5 (Fig. 3D,E, arrowhead). Moreover, Rab7 has lost its association with endosomes (Fig. 3F). Thus, the loss of function of Dmon1 produces a phenotype similar to mut4.
Fig. 3.
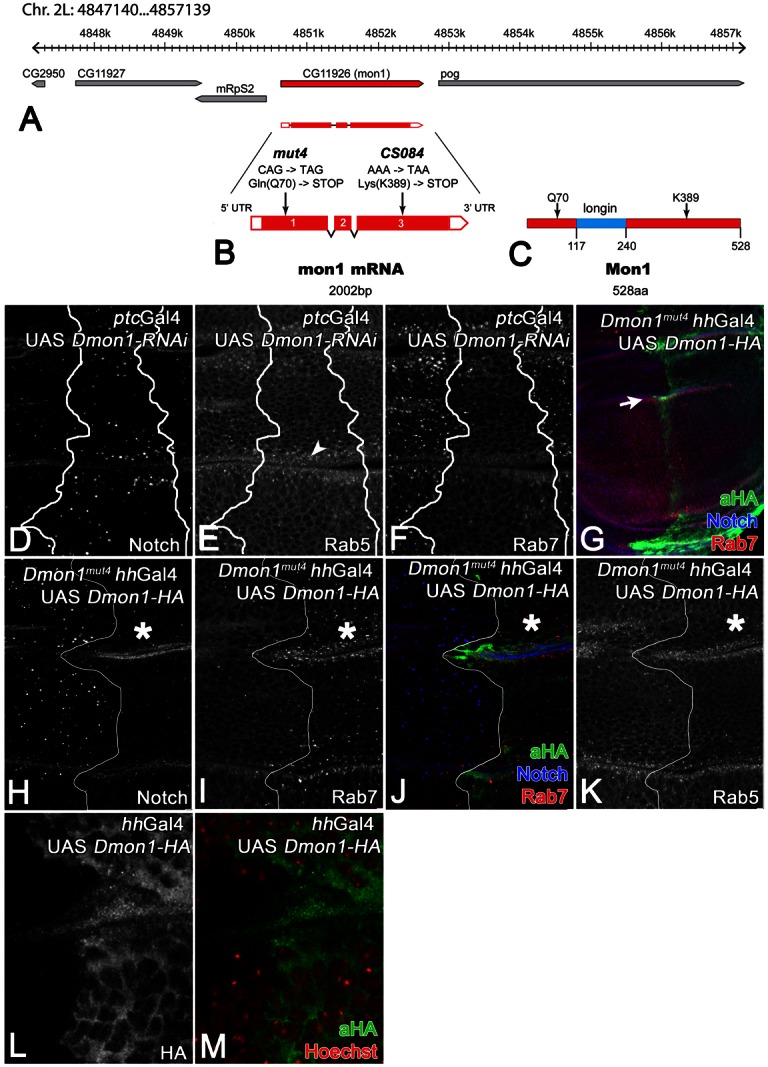
mut4 and CS084 are alleles of Dmon1. (A) The genomic region of Dmon1. (B) Mapping of the mut4 and CS084 mutations in the Dmon1 transcription unit. (C) Primary structure of Dmon1 and the variants encoded by mut4 and CS084. (D–F; same disc) Depletion of Dmon1 function by expression of an RNAi construct. The depleted cells contain enlarged Notch-positive vesicles (D), which are positive for Rab5 (E). Rab7 association with endosomes is dramatically reduced (F). (G–K; same disc) Expression of a HA-tagged UAS Dmon1 construct in the posterior compartment with hhGal4 rescues the mut4 phenotype, indicating that mut4 is an allele of Dmon1. The line demarcates the A/P compartment boundary and the asterisk the rescued posterior area. The arrow in G points to the region shown at higher magnification in H–K. In the posterior compartment, the Notch-positive vesicles have returned to a normal size (H,J) and Rab7 is associated with endosomes (I,J). (L,M; same disc) Dmon1-HA is located throughout the cytosol and is excluded from the nucleus. No obvious association with vesicles can be observed.
Next, we sequenced the Dmon1 transcription unit of mut4 and CS084 homozygous flies (Fig. 3A–C). In both of our mutants we found base changes in Dmon1. mut4 is a nonsense mutation which truncates Dmon1 after 70 aa. The truncated Dmon1 is probably not functional, since it lacks most of the protein including the longin domain (Fig. 3B,C). This notion is supported by our genetic analysis, which revealed that the phenotype of mut4 in homozygosity is similar to that in transheterozygosity with all three deficiencies (data not shown). The weaker CS084 allele bears a nonsense mutation at position K389 (Fig. 3B,C). The truncated protein includes the longin domain and might therefore have residual activity (Fig. 3C).
To unambiguously show that mut4 and CS084 are alleles of Dmon1, we generated an untagged UAS Dmon1 construct and performed rescue experiments. When expressed with hhGal4 in the posterior compartment of mut4 wing imaginal disc, UAS Dmon1 causes a normalisation of the size of the Notch-positive endosomes in mutant cells (not shown). For further characterisation, we generated a HA-tagged construct (UAS Dmon1-HA). Like the untagged version, Dmon1-HA was able to rescue the enlarged endosome phenotype of mut4 cells (Fig. 3G,H). Moreover, its expression resulted in the relocalisation of Rab7 to endosomal membranes (Fig. 3G,I,J). The distribution of Rab5 was not affected (Fig. 3K). Ubiquitous expression of Dmon1 with the daGal4 resulted in a rescue of the lethality of mut4 in homozygosity, giving rise to morphologically normal looking adult flies (not shown). Altogether, these results show that the loss of Dmon1 function is responsible for the phenotype observed in mut4 and CS084 mutants. Thus, the two mutations are alleles of Dmon1 and we renamed them Dmon1mut4 and Dmon1CS084. Since Dmon1mut4 is the stronger (null) allele, we used it for further analysis.
Anti HA staining revealed that Dmon1-HA is distributed throughout the cytosol with no obvious association with endosomes (Fig. 3L,M). The cytosolic localisation is in agreement to what has been reported for sand-1 in most cells in C. elegans (Poteryaev et al., 2007).
Further characterisation of the endosomes of Dmon1 cells
To extend the analysis of Dmon1, we monitored the distribution of key players of the two major recycling pathways in Dmon1mut4 cells. The impact of loss of Dmon1 function on these pathways has not been investigated. Rab4 is a central organiser of the fast and Rab11 for the slow recycling pathway (reviewed by Huotari and Helenius, 2011). In normal cells Rab4 and Rab5 overlap extensively on MEs (Fig. 4A–G, arrows in G). We found that Rab4 is present on most enlarged Dmon1mut4 endosomes (Fig. 4A–G). We quantified the co-localisation of Rab4 and Rab5 with Notch on wild-type and Dmon1mut4 endosomes. We therefore compared the region of a mutant cell clone with an identical wild-type region close by (see Fig. 4H–J). The analysis revealed that co-localisation of Rab4 and Rab5 with Notch-positive endosomes was increased in mutant cells, suggesting that Rab4 and Rab5 remain longer at the endosomal membranes (Fig. 4K). We found that Rab4 was the marker associated most tightly with the enlarged Notch endosomes (92%; n = 3 clones). Rab7 had lost the association with the enlarged Notch-positive endosomes of mutant cells.
Fig. 4.
Further characterisation of the enlarged endosomes of Dmon1mut4 cells. (A–G; same disc) The enlarged endosomes of Dmon1 cells are associated with Rab4. The arrow in A points to a region including a clone boundary, shown in B–G at higher magnification. The clone boundaries are highlighted by the red line in B–G. The arrowheads in B–F point to some of the enlarged Notch-positive MEs of mutant cells that are positive for Rab4 and Rab5. The line highlights the boundary between mutant and wild-type regions. (G) The same disc as in A–F. The arrows highlight some of the MEs in wild-type cells that are positive for Rab4 and Rab5. (H–K) Quantification of association of Notch-positive endosomes with the different Rabs in wild-type and Dmon1mut4 cells. (H–J, same disc) Disc that bears a MARCM Dmon1mut4 clone. The outline of the clone is used to determine a similar wild-type area close by. In both areas, all Notch-positive MEs are counted and the association with the Rabs determined. (L) Dmon1mut4 clone labelled by absence of lacZ. Rab11 is not associated with the enlarged MEs. (M–P; same disc) Distribution of Rabex5 in wild-type cells strongly overlaps with that of Rab4 on endosomes. The double arrows in M points to the region shown at higher magnification in N–P. (Q–S; same disc) Distribution of Rabex5 in cells that are depleted of Dmon1 in the posterior compartment. The arrow in Q highlights the region shown in R,S at higher magnification.
In contrast, Rab11 was not associated with the enlarged Notch-positive MEs of Dmon1mut4 cells. Moreover, the distribution of Rab11 was normal in mutant territories, suggesting that the slow recycling pathway is not affected (Fig. 4L).
To assay whether accumulation of Rabex5 occurs on the endosome of Dmon1mut4 cells as it has been observed in C. elegans (Poteryaev et al., 2010), we generated an EOS tagged Rabex5 construct driven by the tubulin promoter (tub. EOS-Rabex5). EOS-Rabex5 was located in the apical region of the cells, overlapping with Rab4 on EEVs (Fig. 4M–P). In contrast to our expectations, the distribution of Rabex5 did not change in the absence of Dmon1 (Fig. 4Q–S).
Rab7 appears to play an essential role also during maturation of autophagosomes (Jäger et al., 2004) and mon1p is required for autophagy in yeast (Wang et al., 2002). We therefore wondered whether Dmon1 has a function during this process. ATG8 is a specific marker of the autophagosomal membrane in all stages of maturation (Shpilka et al., 2011). We used ATG8-GFP to reveal autophagosomes in mut4 mutant cells, but found no effect on distribution and expression of Atg8-GFP in mutant compared to wild-type cells. Moreover, the enlarged Notch-positive endosomes were free of ATG8-GFP (supplementary material Fig. S2K–M).
Overexpression of UAS Dmon1 had no obvious effects on endosomes, neither on their morphology nor their association with Rab5, Rab7, Rabex5 or distribution of Notch (supplementary material Fig. S2A–F). This holds true even if two copies of UAS Dmon1 are expressed (supplementary material Fig. S2G–J).
The phenotype of loss of Dccz1 resembles that of Dmon1
In yeast, Mon1p functions in a complex with Ccz1p (Wang et al., 2002). An orthologous complex appears to act during phagosome maturation in C. elegans (Kinchen and Ravichandran, 2010). A function of Ccz1 during maturation of endosomes in metazoans has not been reported. We therefore analysed the consequences of loss of function of the Drosophila ortholog of ccz1 (here referred to as Dccz1), encoded by CG14980. Using RNAi, we found that the phenotype is very similar to what we observed for Dmon1 (Fig. 5A–F). Loss of Dccz1 resulted in the formation of dramatically enlarged endosomes, which are positive for Notch, Rab4 (Fig. 5A,B,E,F), and Rab5 (data not shown). Localisation of Rab7 on maturing endosomes was dramatically reduced (Fig. 5C,D,F). Like in Dmon1mut4 cells, we observe no change in the subcellular distribution of Rabex5 (Fig. 5G,H).
Fig. 5.
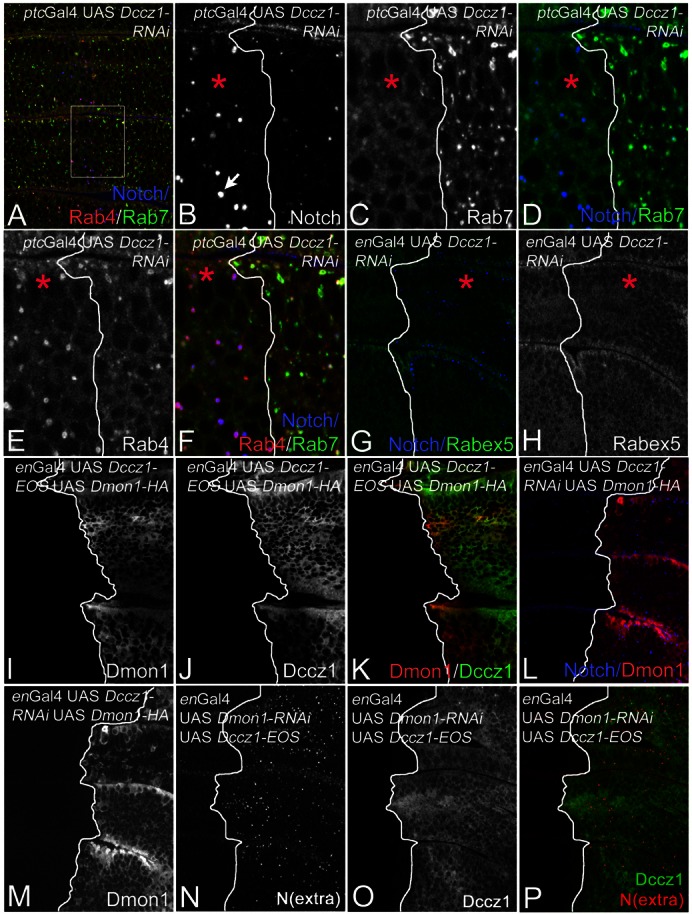
Loss of Dccz1 function causes a phenotype similar to that of loss of Dmon1 function. (A–F; same disc) Depletion of Dccz1 function by expression of UAS Dccz1-RNAi with ptcGal4. The region framed in A is shown in B–F at higher magnification. The boundary between mutant (to the left) and wild-type cells (to the right) is labelled with the line in B–F. The mutant area is also highlighted with a red asterisk in B–H. The mutant cells contain enlarged Notch-positive endosomes (arrow in B) and Rab7 has lost its association with endosomes (C,D). The mutant endosomes are positive for Rab4 (E,F). (G,H; same disc) The distribution of Rabex5 is not affected by the loss of Dccz1 function. The mutant area in G and H is to the right of the line and highlighted with the asterisk. (I–K; same disc) Subcellular distribution of Dccz1 is identical to that of Dmon1. (L,M; same disc) Depletion of Dccz1 function does not change the subcellular distribution of Dmon1. (N–P; same disc) The same is true for the converse situation.
To determine the subcellular location of Dccz1, we generated an UAS Dccz1-EOS construct. Dccz1-EOS was distributed throughout the cytosol as we have previously observed for Dmon1-HA (Fig. 5I–K). Overexpression of Dccz1 with the Gal4 system did not cause a detectable phenotype (not shown).
The Mon1–Ccz1 complex has been shown to interact with the GTP bound form of Rab5 (Rab5GTP) during phagosome maturation (Kinchen and Ravichandran, 2010). This interaction appears to be mediated by Mon1 (Kinchen and Ravichandran, 2010). We here fail to detect an association of Dmon1/Dccz1 with endosomes. One explanation is that Dmon1/Dccz1 cycles between the cytosol and the endosomal membrane. If the time of association with the endosome is short, only a small fraction of the protein is located at the endosome. This fraction might be too small to be detected. Therefore, we monitored the subcellular distribution of Dmon1 in cells depleted of Dccz1. We reasoned that if its interaction partner is missing, Dmon1 might bind to Rab5GTP at the endosome and becomes trapped there because it cannot form the necessary complex to complete its function. As a result it should accumulate on the endosome. However, we did not see any change in the distribution of Dmon1 in Dccz1 depleted cells (Fig. 5L,M). Moreover, Notch-positive endosomes were still enlarged indicating that overexpression of Dmon1 cannot compensate for the loss of Dccz1 function (Fig. 5L,M). The same is true for the converse situation (Fig. 5N–P). We next looked at the subcellular localisation of Dmon1 in situations where the endosomal trafficking is disturbed. Rab5 overexpression results in the formation of enlarged Notch-positive EE (Stenmark et al., 1994). Nevertheless, we did not observe any change in the subcellular distribution of Dmon1 (data not shown).
EM analysis of Dmon1 and Dccz1 mutant cells
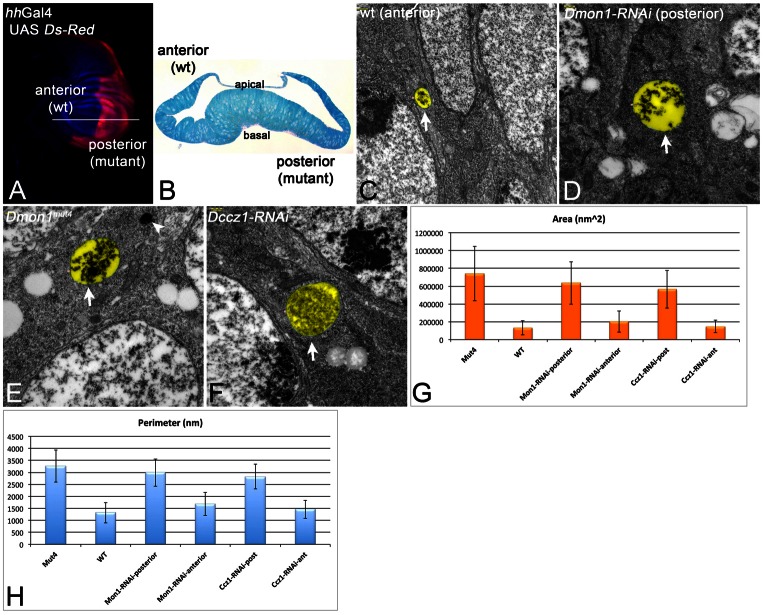
To characterise the enlarged endosomes of Dmon1 and Dccz1 cells in more detail, we conducted a transmission electron microscope (TEM) analysis (Fig. 6). We first expressed UAS Dmon1-RNAi with hhGal4 and UAS Dcr2 in the posterior compartment of the wing imaginal disc (Fig. 6A–D). This experimental design allowed us to compare (anterior) wild-type and (posterior) mutant cells within one disc. We did not observe any gross changes in epithelial morphology in mutant territories in semi-sections (Fig. 6B). However, we found an enlargement of MEs/MVBs in depleted posterior cells (Fig. 6C,D,G,H). We observed the same phenotype in Dmon1mut4 cells (Fig. 6E). This indicates that the RNAi construct is specific for Dmon1. The presence of many ILVs in mutant MVBs indicates that the activity of the ESCRT machinery is not affected, as suggested by the analysis with the fluorescence microscope. Dmon1 mutant cells contained vesicles with the typical morphology of lysosomes (arrowhead in Fig. 6E), reinforcing our conclusion that lysosome formation is not affected. The mutant cells contained more MVBs than wild-type cells: In an area of the same size, we found 30 MVBs in Dmon1mut4 discs compared to 18 in wild-type discs (see supplementary material Fig. S3). This finding suggests that the turnover of MVBs, which occurs through fusion with the lysosome, is reduced or abolished. MEs travel from the periphery of the cells to more perinuclear regions, where they fuse with the lysosome. The enlarged MVBs of Dmon1 cells were located in perinuclear regions (supplementary material Fig. S3). This suggests that their mobility is not severely affected by the loss of Rab7 association.
Fig. 6.
TEM analysis of Dmon1 and Dczz1 mutants. (A) Expression pattern of hhGal4 revealed by UAS dsRed. hhGal4 drives expression of UAS constructs in the posterior compartment. It was used to express the UAS RNAi constructs together with UAS Dcr2. The discs were sectioned at the level of the white line and the phenotypes of the posterior cells were compared to the anterior wild-type (wt) cells. (B) Semi cross-section at the level indicated by the white line in A. (C,D) Representative MVBs (arrows) of anterior wild-type (C) and posterior Dmon1-depleted (D) cells. (E) Representative MVB (arrow) of Dmon1mut4 cells. The MVBs of Dmon1 mutant cells are larger. The arrowhead in E points to a lysosome of normal appearance. (F) Representative MVB (arrow) of cells depleted of Dccz1 function. The magnification is the same in C–F. The MVBs are highlighted in pseudo-colour in C–F. (G,H) Statistical analysis of the area and perimeter of the MVBs underscores the enlargement of MVBs in mutant cells.
In order to analyse the phenotype of loss of Dccz1 function, we expressed UAS Dccz1-RNAi in the posterior compartment of the wing imaginal disc. We found similarly enlarged MVBs in the depleted posterior cells (Fig. 6F–H). This finding supports the notion that Dccz1 acts in concert with Dmon1 in Drosophila. We did not find obvious accumulation of autophagosomes in Dccz1 or Dmon1 mutant cells.
The phenotype of rab7 cells resembles that of Dmon1 and Dccz1 cells
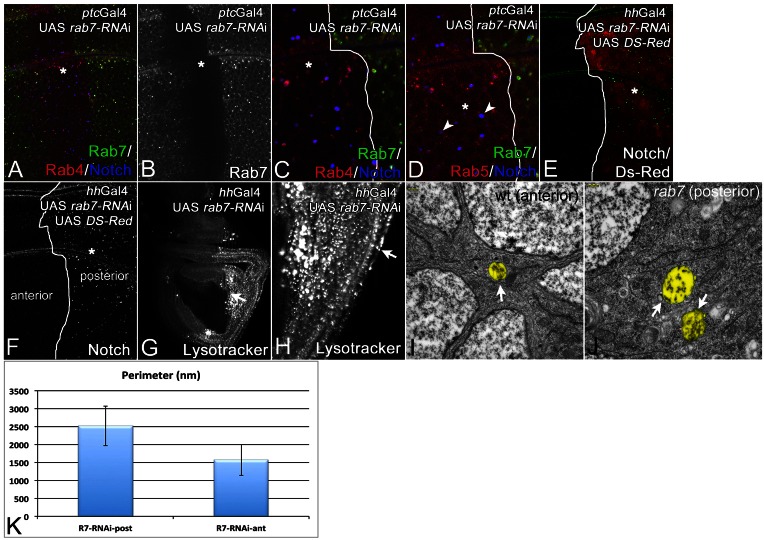
Since we observed a failure of Rab7 to be recruited to maturing endosomes in Dmon1 and Dccz1 cells, we were curious whether loss of rab7 causes a phenotype that is similar to loss of Dmon1 function. Therefore, we depleted cells of its function. The depletion of Rab7 is very efficient and reduces its expression to an undetectable level (Fig. 7A,B). The depletion caused a phenotype similar to that of Dmon1. We observed the formation of enlarged Notch- and Rab4-positive endosomes (Fig. 7A,C–F). Furthermore, the endosomes of depleted cells were acidified (Fig. 7G,H). The subcellular distribution of Dmon1 was not affected (not shown). Interestingly, we observed that many of the enlarged endosomes have lost the association with Rab5 (Fig. 7D, arrowheads; cf. Fig. 2).
Fig. 7.
Analysis of the loss of function phenotype of Rab7. (A–J) Cells were depleted of rab7 function using an UAS rab7-RNAi construct expressed with UAS Dcr2 by ptcGal4 (A–D; same disc) or hhGal4 (E–I). Asterisks in A–F highlight the region of depletion. The depletion was efficient, as shown by the loss of expression of a tub. Rab7-YFP construct in the ptc domain (asterisk). The loss of rab7 function results in the formation of enlarged Notch-positive endosomes. Several of these contained Rab4 (C) and some also Rab5 (D). Note that several of the endosomes have lost association with Rab5 (D, arrowheads). (E,F; same disc) The same enlarged Notch-positive endosomes were seen in posterior cells if UAS rab7-RNAi was expressed with hhGal4. (G,H; same disc) Lysotracker staining indicates that the Dmon1 cells contain MEs that are acidic. (H) The region highlighted with the arrow in G shown at higher magnification. The arrow in H highlights one of the many MEs that are strongly stained. (I,J) The hhGal4 UAS rab7-RNAi discs were used for TEM analysis, revealing that the enlarged endosomes of rab7-depleted cells were MVBs (arrows), which were similarly enlarged as in the case of Dmon1 and Dccz1 mutants (Fig. 6). (K) Quantification of the perimeter of the MVBs in anterior and posterior cells shown in I and J, respectively.
The EM analysis revealed that rab7 depleted cells contained enlarged MVBs as observed for Dmon1 and Dccz1 cells (compare Fig. 7I–K with Fig. 6). The results indicate that the phenotype caused by depletion of rab7 function resembles that of Dmon1 and Dccz1 mutant cells. Thus, a major function of Dmon1–Dccz1 is to recruit Rab7 to maturing endosomes.
Dmon1 and Dccz1 act in concert
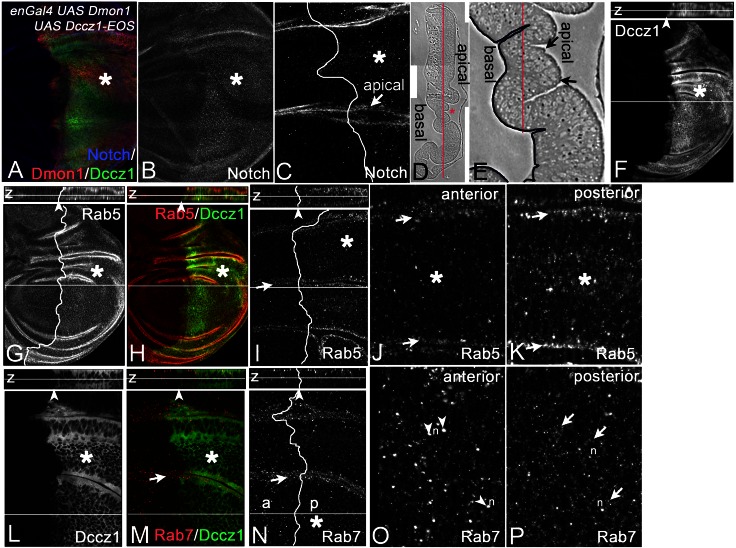
To find further evidence for a concerted action of Dccz1 and Dmon1, we co-overexpressed both together with the Gal4 system. In contrast to expression of each one alone, we observed more and slightly enlarged Notch-positive endosomes (Fig. 8A–C). In addition, the co-overexpression resulted in changes in the distribution of Rab5 and Rab7. In Drosophila wild-type cells, Rab7 partly overlaps with Rab5 on MEs, but is also located on more mature, larger endosomes, which are located deeper inside the cell, closer to the nucleus (Fig. 8N, z-section). Rab5 is located to EEVs and endosomes at the apical region of the cell (Fig. 8G,I, z-section). Upon co-overexpression of Dmon1 with Dccz1, we found a strong increase of the number of Rab5-positive endosomes at the apical region (Fig. 8G–K, arrows in J,K). Moreover, the number of Rab5-positive smaller endosomes more inside the cells is increased, resulting in a higher overall concentration of Rab5 in comparison to wild-type cells (Fig. 8G–K). The increase of Rab5 suggests that the co-overexpression of Dmon1 and Dccz1 results in the formation of more endosomes.
Fig. 8.
Consequences of co-overexpression of Dmon1 and Dccz. (A–H) Co-overexpression of UAS Dmon1 and UAS Dccz1 with enGal4 in the posterior compartment (to the right of the line, highlighted by the asterisk). (A–C; same disc) Co-overexpression of Dmon1 and Dccz1 causes the accumulation of Notch-positive vesicles in the depleted cells. (D,E) Sagittal semi-section of the wing imaginal disc to reveal its apico–basal organisation. Red asterisk in D indicates the region shown in E at higher magnification. The picture reveals the folding of the epithelium. The red lines show the level of the optical sections shown in the following. The basal side of the epithelium is highlighted by the dark line in E. (F–H, I–K; same disc) Distribution of Rab5. Rab5-positive vesicles are more abundant in the region of co-overexpression. (I–K) Optical section at the region highlighted in E more apical to the red line. The arrow in I highlights the region shown in J, K at higher magnification. The arrows in J, K,M,N show the apical sides highlighted in E with the arrows. (J) Wild-type region; (K) the region of overexpression. The comparison reveals that Rab5 is enriched in endosomes at the apical side in the regions of co-overexpression. Moreover, more Rab5-positive endosomes can be observed in more deeper regions of the cells (asterisks). (L–P; same disc) Similar analysis of the distribution of Rab7 reveals that the Rab7-positive endosomes in the region of co-overexpression are smaller inside the cells. They tend to accumulate around the nucleus (n). (O–P) Magnification of the region highlighted with the asterisk in N. (O) Wild-type region. The arrowheads point to larger Rab7-positive MEs inside the cells at the level of the nucleus. (P) Region of co-overexpression of the same optical section. The arrows highlight the regions around the nucleus where small endosomes are located. The z-sections of the discs are shown above the main images in F–I and L–N.
We further found that large Rab7-positive endosomes were located to more apical regions in regions of co-expression (Fig. 8E,L–P, arrows in M–P). Deeper inside the cells the distribution of Rab7 is more diffuse. This is due to an increase of the number of positive endosomes with smaller size located around the nucleus (Fig. 8O,P). This is the location of endosomes that are well advanced of their maturation. The reduction in size of these endosomes suggests that they have matured faster, giving less time to undergo homotypic fusion, due to an earlier fusion with the lysosome. Hence, the results suggest that Dmon1 and Dccz1 act in concert in vivo to fulfil their function and that their co-overexpression accelerates the maturation of the endosome. Since Rab7 is required for fusion, it either is recruited earlier or the mediated fusion occurs faster.
Depletion of Dccz1 in Dmon1mut4 wing imaginal discs with hhGal4 had no effect on the morphology of Notch-positive endosomes (data not shown). This finding further supports the notion that both proteins act in concert.
Rab7 localisation to the endosome does not require PI(3)P in Drosophila
Results obtained in C. elegans suggest that Mon1 might be recruited to maturing endosomes through binding to PI(3)P, which is generated by the Rab5 effector and PI(3) kinase Vps34. Thus, a failure in the generation of PI(3)P should result in a failure of Rab7 recruitment, due to a failure of Dmon1 recruitment. We tested recruitment of Rab7 in situations where the PI(3)P generation or access to it by effectors was blocked (supplementary material Fig. S4). The prediction was that if Dmon1 was dependent on PI(3)P, Rab7 recruitment to MEs should be prevented in these situations. First, we expressed an RNAi construct of vps34 and found that Rab7 is still associated with endosomes (data not shown). Secondly, we expressed up to three copies of GFP-FYVEx2 (supplementary material Fig. S4A–E). It consists of two PI(3)P binding FYVE-domains fused to GFP (Wucherpfennig et al., 2003). UAS GFP-FYVEx2 is associated with endosomes if expressed with the Gal4 system (supplementary material Fig. S4A–E). We surmised that strong expression of this construct saturates most PI(3)P binding sites on the maturing endosome and therefore should prevent binding of Dmon1. In accordance with this notion, expression of three copies of this construct (3×GFP-FYVEx2) in wing disc cells caused the formation of enlarged Notch-positive endosomes (supplementary material Fig. S4D,E). Nevertheless, we found that many are associated with Rab7 (supplementary material Fig. S4D,E). Thirdly, we suppressed the activity of Vps34 by expressing a kinase dead version, which acts in a dominant negative manner (Vps34KD). Expression of this construct results in a strong loss of GFP-FYVEx2 from endosomes (Juhász et al., 2008), supplementary material Fig. S4F,G). In addition we found that the cells contain enlarged MEs with a strongly reduced number of ILVs (arrows in supplementary material Fig. S4L). This phenotype was expected since Hrs, which initiates ILV formation, is recruited to the endosome through binding to PI(3)P through its FYVE-domain (Hurley, 2010). Altogether, these observations indicate very efficient inhibition of PI(3)P formation by Vps34KD. Expression of this construct resulted in the formation of enlarged Notch-positive endosomes, which are located apically. However, they were still associated with Rab7 (supplementary material Fig. S4H–K). Last, we monitored the distribution of Rab7 in cells mutant for the null allele vps34Δm22 (supplementary material Fig. S4M–O). Again, we observed enlarged Rab7-positive endosomes in mutant cells (supplementary material Fig. S4M–O, arrows in N,O). These results suggest that formation of PI(3)P is not necessary for the Dmon1 mediated recruitment of Rab7 onto the endosome in Drosophila. Interestingly, the loss of function of vps34 does not result in the ectopic activation of Notch, although the formation of ILVs is suppressed (not shown).
Accumulation of signalling receptors combined with a failure of ILV formation does not result in activation of signalling pathways
Given the importance of the endosomal pathway in regulation of signalling pathways, it was surprising for us that the few Dmon1mut4 homozygous flies that escaped to the adult stage did not display any obvious patterning defects. This suggests that cell signalling during pattern formation is not affected. This conclusion was confirmed when we monitored the expression of target genes of several signalling pathways in Dmon1 wing discs (supplementary material Fig. S5). We monitored the activity of the EGF-, Wg-, Dpp-, and Notch pathways using the expression of argos-lacZ, Dfz3-lacZ, omb-lacZ and Gbe+Su(H)-GFP, respectively. None of these markers were affected in their expression. Moreover, we did not observed any change of the markers if ILV formation was suppressed in Dmon1 cells by concomitant removal of hrs function (supplementary material Fig. S5). These results indicate that incorporation into ILVs is not the only mode of inactivation of active signalling receptors in these cells.
Discussion
Here, we report the characterisation of the Drosophila ortholog of Mon1/Sand-1, Dmon1, and its partner Dccz1. We show that Dmon1 is required for the recruitment of Rab7 to the maturing endosome as has been previously observed for the orthologs in C. elegans and yeast. Our experiments support the notion that Dmon1 and Dccz1 act in concert to fulfil their function in metazoans. This is in agreement with the finding that both proteins act in concert in yeast and during phagosome maturation in C. elegans (Kinchen and Ravichandran, 2010). We here provide evidence that this is true also during endosomal trafficking of cargo in metazoans. We present the first EM analysis of Dmon1 and Dccz1 in a metazoan and show that the loss of function of each gene results in the same phenotype, the formation of more and dramatically enlarged MEs with a high number of ILVs. We extended the characterisation of the endosomes in mon1 mutants using additional markers. We found that the endosomes acidify and the adapter lipid PI(3)P is generated in their limiting membranes, indicating that Rab5 is active and its effectors can be recruited to the endosome. In agreement with this notion, we observe association of Hrs and FYVE-GFP with the enlarged endosomes. These markers are recruited to the LM through binding to PI(3)P. The recruitment of the ESCRT-0 component suggests that ILV formation is initiated. This conclusion was confirmed by our EM analysis where we found that the enlarged MEs of Dmon1 cells are MVBs with many ILVs. In our eyes these results are remarkable, since it indicates that the main aspects of endosome maturation occur in absence of Rab7 function and thus, appear not to be coordinated with the processes mediated by Dmon1 and Rab7.
Based on experiments in mammalian cell culture, it has been proposed that RILP, an effector of Rab7, is involved in ILV formation and interacts with ESCRT-II besides its established function in mediating the movement of the ME to perinuclear regions (Progida et al., 2007). Our results indicate that ILV formation is not significantly affected in Dmon1 or Rab7 mutant cells. Thus, the function of RILP during ILV formation is either independent of Rab7, or RILP has no such function in an integral tissue or in cells in Drosophila. Furthermore, RILP has been shown to be required for endosomal motility in mammalian cell culture cells (Cantalupo et al., 2001). We observed that, like in the wild type, the MEs of Dmon1 cells move to a more perinuclear region. Thus, they can move at least to a certain degree in the absence of RILP recruitment by Rab7. Either endosomes can move in an alternative manner in cells or a functional tissue or the mobility is regulated differently in Drosophila. In contrast, the enlarged endosomes in cells depleted of vps34 function were located close to the apical cell membrane, indicating that their transport to the perinuclear region is severely affected. Thus, it appears that the function of Vps34 is required for the movement.
Our TEM analysis shows that depletion of Rab7 results in a similar defect like loss of Dmon1 function. Hence, the failure to localise Rab7 to the endosome explains the phenotypes of Dmon1 and Dccz1 cells: Rab7 is required for fusion of MEs with the lysosome (Huotari and Helenius, 2011). Consequently, loss of Rab7 function leads to a loss of ME turnover and, hence, to an increase of the number of MVBs in a cell. Moreover, the extended lifetime of MEs allows more Rab5 mediated homotypic fusions to occur, which result in the enlargement of MEs. We observe all these phenotypes in our EM analysis of rab7, Dmon1 and Dccz1 cells. The similarity of the phenotypes of these cells also indicates that the recruitment of Rab7 to the endosomal membrane is pivotal for its function.
While many of our findings are in agreement with previous reports, there are also differences. We did not find evidence for a role of PI(3)P for the recruitment of Dmon1 to the endosome, as has been proposed for C. elegans (Poteryaev et al., 2010). We observed that the loss of function of Vps34 and therefore loss of PI(3)P does not prevent recruitment of Rab7 to MEs. Thus, the formation of PI(3)P is probably not involved in its recruitment. This is in agreement with previous work in mammalian cell culture where the role of PI(3)P in Rab7 recruitment to maturing phagosomes was investigated. In this case treatment with PI(3) Kinase inhibitors failed to prevent Rab7 recruitment onto phagosomes (Vieira et al., 2003).
We observed that Rab5 does not accumulate on endosomes in the absence of Dmon1 or Dccz1. Rather, we find that Rab5 is eventually lost from a fraction of the enlarged endosomes. Nevertheless, the fact that we observed enlarged endosomes in cells mutant for Dmon1 or Dccz1, suggests that more fusion events between endosomes occur. Assuming that these fusions are mediated by Rab5, a potential explanation for the enlarged endosomes is that the transient association of Rab5 with the endosomal membrane is prolonged in mutant cells. This is supported by our finding that the fraction of Rab5-positive endosomes is increased in Dmon1mut4 cells. Thus, a crucial common parameter in C. elegans and Drosophila is the longer perdurance of Rab5 on the ME, which occurs in both species. The accumulation of Rab5/Rabex5 might be a species-specific effect.
We found that co-overexpression of Dmon1 and Dccz1 results in an increase of Rab5 associated endosomes. Thus, in contrast to C. elegans, the association of Rab5 with endosomes is maintained rather than weakened, as would be expected if the complex were a negative regulator of the Rab5/Rabex5 loop. Thus, this finding does not support a role of the Dmon1–Dccz1 complex in inactivation of the Rabex5/Rab5 loop.
The observed differences in Drosophila versus C. elegans can be explained by the analysis of different species and/or analysis of different tissues. However, this explanation would imply different mechanisms of Dmon1–Dccz1 action in different species. Another possibility is that the differences in the results reflect the analysis of different types of tissues. We analysed the function in an epithelium whereas the analysis in C. elegans were largely done in oocytes and coelomocytes. This notion is supported by the differences in results obtained in C. elegans if oocytes and coelomocytes on the one hand and phagocytes on the other are analysed (see Introduction). More work is required to clarify these issues.
To our surprise, autophagy appears not to be affected by loss of Dmon1, in marked contrast to the yeast Mon1p mutant phenotype (Wang et al., 2002). Thus, if Rab7 is involved in autophagosomal maturation in Drosophila, it must be recruited to the autophagosome in a Dmon1 independent manner. It is possible that the few weakly Rab7-positive vesicles we occasional observe in Dmon1 cells are autophagosomes. The function of yeast Mon1p appears to differ in certain aspects from Mon1 of metazoans, since Sand1 can only partially rescue the yeast Mon1p mutant (Poteryaev et al., 2007). In addition yeast Mon1p is not able to rescue Dmon1 mutants of Drosophila (J.Y. and T.K., unpublished results). In this light it is not surprising to find differences in the function of Dmon1 compared to Mon1p.
Activation of signalling pathways during derailed endosomal trafficking
Loss of function of ESCRT complexes I to III results in over-activation of several signalling pathways, among them the Notch, RTK and Dpp pathways (Huotari and Helenius, 2011). A major function of these complexes is to incorporate the activated signalling receptors into ILVs of MEs. This function is thought to attenuate signalling since the intracellular domain of activated receptors becomes sequestered from the cytosol (Dobrowolski and De Robertis, 2012). Thus, one explanation of how loss of ESCRT function leads to unregulated signalling is that the activated receptors are not sequestered from the cytosol through the failure of ILV formation. In the case of RTK and Dpp signalling this could lead to prolonged signalling. In the case of Notch, perdurance of the receptor in the limiting membrane could lead to activation through alternative ecto-domain shedding mediated by the activated proteases in the ME. We tested this explanation by monitoring situations where ILV formation and endosome turnover is suppressed. We failed to observe ectopic activation or over-activation of signalling pathways in Dmon1 cells, even when ILV formation was disturbed. Thus, the activated receptors still become sequestered from the cytosol despite their accumulation. We were surprised that the concomitant loss of Dmon1 and hrs function fails to affect cell signalling. While loss of hrs function does not result in Notch activation, it has been shown to activate RTK signalling in several tissues (Jékely et al., 2005; Jékely and Rorth, 2003; Lloyd et al., 2002). We expected this effect to be enhanced in cells where the lifetime of the endosomes is dramatically prolonged and receptors accumulate to higher levels than normal. However, we do not observe ectopic or stronger activation of the RTK signalling, neither upon hrs, nor Dmon1 hrs loss of function. In addition, we did not observe an effect on the activity of the other pathways tested, such as the Dpp and Notch pathway, which have been shown to become activated upon inactivation of ESCRT-I–III complexes (Moberg et al., 2005; Thompson et al., 2005; Vaccari and Bilder, 2005). Likewise, we did not observe ectopic activation upon loss of vps34 function. In this case we observe a strong reduction of formation of ILVs, similar to loss of hrs function (Lloyd et al., 2002). Altogether, the results suggest that the failure of incorporation of activated receptors in ILVs is not sufficient to explain the uncontrolled signalling of various pathways observed in ESCRT mutants. Additional mechanisms must be involved.
Note
It should be noted that the Dmon1mut4 allele is a background mutation present on some bib1 chromosomes, and it accounts for the aberrant protein trafficking phenotype that was erroneously attributed to bib1 in a previous study (Kanwar and Fortini, 2008). The attribution of this phenotype to bib1 was initially made based on rescue of the phenotype by a 16.5 kb bib+ wild-type genomic transgene. However, subsequent retesting of this bib+ rescue fragment established that it does not rescue the protein trafficking phenotype (R. Kanwar and M.F., unpublished data), which instead is caused by the unlinked Dmon1mut4 mutation as reported in the current study.
Materials and Methods
Drosophila genetics
UAS lines used were: UAS Dmon1-RNAi (#VDRC 38600, 103378), UAS Ccz1-RNAi (#VDRC 18479), UAS Vps34-RNAi (#VDRC 100269).UAS-Mon1, UAS-Mon1HA, tubRab4-mCherry were generated in this work. Gal4 lines used in this work: hhGal4 (Tanimoto et al., 2000), ptcGal4 (Speicher et al., 1994), hsGal4 (Bloomington Stock Center), enGal4 (Bloomington Stock Center). Other lines used: Gbe+Su(H)-GFP (de Navascués et al., 2012), tub.lamp1 (kindly provided by Helmut Kramer), hrsD28 (Lloyd et al., 2002), UAS vps34KD (Juhász et al., 2008), UAS FYVE-GFP (Wucherpfennig et al., 2003), HG.Rab11-GFP (Dollar et al., 2002), Df(2L)Exel6010, Df(2L)Exel8012, Df(2L)Exel9062 (Bloomington Stock Center), tub.rab5-CFP and tub.rab7-YFP (Marois et al., 2006), tub.rab4-RFP (this work).
Clonal analysis
Clones were generated with the Flp/FRT system (Xu and Rubin, 1993) and induced in first instar larvae (24–48 hours after egg laying) by applying a 70-minute heat shock (37°C). Dmon1mut4 MARCM clones were induced with hsFlp1.22 tubGal4 UAS nlsGFP; FRT40A Gal80/Dmon1mut4 FRT40A larvae.
Uptake assay
Uptake assays were performed as described (Le Borgne et al., 2005) (Texas Red dextran, MW3000, Molecular Probes, D-3328).
Immunostaining and microscopy
Antibody staining was performed according to standard protocols. Discs were stained in addition with the nuclear marker Hoechst 33258. anti-Wg antibody (4D4), the mouse Notch antibodies against the extracellular (C458.2H) and intracellular (C17.9C6) domains, anti-Dl, anti-Smo and anti-Ptc were obtained from DSHB Iowa; anti-Rab7 is a gift from Akira Nakamura (Tanaka and Nakamura, 2008), anti Rab5 (a gift of Marcos Gonzalez-Gaitan); and anti-HA (Roche); anti-Hrs (Lloyd et al., 2002); anti Ser (a gift from Elisabeth Knust). Fluorochrome-conjugated secondary antibodies were purchased from Invitrogen/Molecular Probes. Images were obtained with a Zeiss AxioImager Z1 Microscope equipped with a Zeiss Apotome.
Constructs
All PCR-amplifications were performed with the Expand HF-PCR Kit (Roche). PCR-products were extracted from agarose gel by E.Z.N.A. Gel Extraction Kit (Omega Bio-Tek Inc., USA). The UAS Dmon1HA construct was generated by PCR using a Dmon1-cDNA (NCBI Ref. Seq. No. NM_135024.1, synthesized by GenScript USA Inc.). Amplified DNA-fragments were cloned into the pUAS-pattB-Vector (Bischof et al., 2007) using the NotI-site. UAS Dmon1 was generated by NotI cloning into the pattB-UAS vector.
UAS Dccz1-EOS (EOS-tag at C-terminus) construct was generated by splice over extension PCR (Horton et al., 1990) using Gold-Clone RH02365 (DGRC) and EOS. ccz1-EOS was cloned into pattB-UAS vector by NotI. tub.EOS-rabex5 (EOS-tag at the N-terminus) construct was generated by splice over extension PCR from Gold-Clone SD03358 (DGRC) and EOS. EOS-rabex5 was then cloned into pCasp-tubPromoter-3′UTR.
tub.rab4-mCherry (mCherry-tag at N-terminus) construct was generated by splice over extension PCR from EST-Clone RE40706 (DGRC) and mCherry-cDNA (pAB118-mCherry) and cloned into pCasp-tubPromoter-3′UTR.
EM analysis
Wing discs were fixed in 2.5% glutaraldehyde in 100 mM phosphate buffer washed in 100 mM phosphate buffer and postfixed in 2% osminum tetroxide in phosphate buffer for 1 hour on ice. After contrasting en bloc in 2% uranyl acetate, the specimens were dehydrated in EtOH and embedded in araldite using acetone as an intermediate solvent. Thin sections were stained with 2% uranyl acetate and lead citrate. Sections were observed under an EM 902 (Zeiss) microscope at 80 KV.
Supplementary Material
Acknowledgments
We thank D. Ready, S. Bray, A. Martinez-Arias, H. Krämer, S. Eaton, G. Davies, H. Bellen, M. Gonzalez-Gaitan and A. Nakamura for supplying fly stocks and reagents used in this work. We are especially grateful to A. Bachmann for critical reading and comments on the manuscript and S. Schnorrenberg and B. Schnute for help during some of the experiments. Thanks to R. Jaekel for essential help in several experiments. We thank S. Tannebaum and G. Helbig for excellent technical assistance. The Bloomington Stock Center, VDRC and the Developmental Studies Hybridoma Bank supplied fly stocks and reagents.
Footnotes
Author contributions
J.Y. was involved in all experiments (performed the major works); T.T. provided the control experiments for the Rab7 analysis. F.G. helped with the electron microscopy. M.F. and T.S. provided the Dmon1 allele CS084 T.K. is the supervisor of the project.
Funding
The work was supported by the DFG [grant number SFB 590]. This research was also supported by the National Institutes of Health (NIH) [grant number R01 GM087650], specifically the National Institute of General Medical Sciences (NIGMS) and the Intramural Research Program of the NIH, National Cancer Institute (NCI), Center for Cancer Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS, NCI or NIH. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.114934/-/DC1
References
- Bischof J., Maeda R. K., Hediger M., Karch F., Basler K. (2007). An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104, 3312–3317 10.1073/pnas.0611511104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo G., Alifano P., Roberti V., Bruni C. B., Bucci C. (2001). Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 20, 683–693 10.1093/emboj/20.4.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Navascués J., Perdigoto C. N., Bian Y., Schneider M. H., Bardin A. J., Martínez-Arias A., Simons B. D. (2012). Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J. 31, 2473–2485 10.1038/emboj.2012.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K-C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S. et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156 10.1038/nature05954 [DOI] [PubMed] [Google Scholar]
- Dobrowolski R., De Robertis E. M. (2012). Endocytic control of growth factor signalling: multivesicular bodies as signalling organelles. Nat. Rev. Mol. Cell Biol. 13, 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollar G., Struckhoff E., Michaud J., Cohen R. S. (2002). Rab11 polarization of the Drosophila oocyte: a novel link between membrane trafficking, microtubule organization, and oskar mRNA localization and translation. Development 129, 517–526 [DOI] [PubMed] [Google Scholar]
- Henne W. M., Buchkovich N. J., Emr S. D. (2011). The ESCRT pathway. Dev. Cell 21, 77–91 10.1016/j.devcel.2011.05.015 [DOI] [PubMed] [Google Scholar]
- Hofmann I., Munro S. (2006). An N-terminally acetylated Arf-like GTPase is localised to lysosomes and affects their motility. J. Cell Sci. 119, 1494–1503 10.1242/jcs.02958 [DOI] [PubMed] [Google Scholar]
- Horton R. M., Cai Z. L., Ho S. N., Pease L. R. (1990). Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 8, 528–535 [PubMed] [Google Scholar]
- Huotari J., Helenius A. (2011). Endosome maturation. EMBO J. 30, 3481–3500 10.1038/emboj.2011.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. H. (2010). The ESCRT complexes. Crit. Rev. Biochem. Mol. Biol. 45, 463–487 10.3109/10409238.2010.502516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger S., Bucci C., Tanida I., Ueno T., Kominami E., Saftig P., Eskelinen E. -L. (2004). Role for Rab7 in maturation of late autophagic vacuoles. J. Cell Sci. 117, 4837–4848 10.1242/jcs.01370 [DOI] [PubMed] [Google Scholar]
- Jékely G., Rorth P. (2003). Hrs mediates downregulation of multiple signalling receptors in Drosophila. EMBO Rep. 4, 1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jékely G., Sung H. H., Luque C. M., Rørth P. (2005). Regulators of endocytosis maintain localized receptor tyrosine kinase signaling in guided migration. Dev. Cell 9, 197–207http://dx.doi.org/10.1242/jcs.01370 10.1016/j.devcel.2005.06.004 [DOI] [PubMed] [Google Scholar]
- Juhász G., Hill J. H., Yan Y., Sass M., Baehrecke E. H., Backer J. M., Neufeld T. P. (2008). The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J. Cell Biol. 181, 655–666 10.1083/jcb.200712051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar R., Fortini M. E. (2008). The big brain aquaporin is required for endosome maturation and notch receptor trafficking. Cell 133, 852–863 10.1016/j.cell.2008.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kinchen J. M., Ravichandran K. S. (2010). Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature 464, 778–782 10.1038/nature08853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R., Remaud S., Hamel S., Schweisguth F. (2005). Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS Biol. 3, e96 10.1371/journal.pbio.0030096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd T. E., Atkinson R., Wu M. N., Zhou Y., Pennetta G., Bellen H. J. (2002). Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108, 261–269 10.1016/S0092-8674(02)00611-6 [DOI] [PubMed] [Google Scholar]
- Marois E., Mahmoud A., Eaton S. (2006). The endocytic pathway and formation of the Wingless morphogen gradient. Development 133, 307–317 10.1242/dev.02197 [DOI] [PubMed] [Google Scholar]
- Moberg K. H., Schelble S., Burdick S. K., Hariharan I. K. (2005). Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev. Cell 9, 699–710 10.1016/j.devcel.2005.09.018 [DOI] [PubMed] [Google Scholar]
- Nickerson D. P., Brett C. L., Merz A. J. (2009). Vps-C complexes: gatekeepers of endolysosomal traffic. Curr. Opin. Cell Biol. 21, 543–551 10.1016/j.ceb.2009.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann M., Cabrera M., Perz A., Bröcker C., Ostrowicz C., Engelbrecht-Vandré S., Ungermann C. (2010). The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr. Biol. 20, 1654–1659 [DOI] [PubMed] [Google Scholar]
- Poteryaev D., Fares H., Bowerman B., Spang A. (2007). Caenorhabditis elegans SAND-1 is essential for RAB-7 function in endosomal traffic. EMBO J. 26, 301–312 10.1038/sj.emboj.7601498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteryaev D., Datta S., Ackema K., Zerial M., Spang A. (2010). Identification of the switch in early-to-late endosome transition. Cell 141, 497–508 10.1016/j.cell.2010.03.011 [DOI] [PubMed] [Google Scholar]
- Progida C., Malerød L., Stuffers S., Brech A., Bucci C., S H. (2007). RILP is required for the proper morphology and function of late endosomes. J. Cell Sci. 120, 3729–3737 [DOI] [PubMed] [Google Scholar]
- Rink J., Ghigo E., Kalaidzidis Y., Zerial M. (2005). Rab conversion as a mechanism of progression from early to late endosomes. Cell 122, 735–749 10.1016/j.cell.2005.06.043 [DOI] [PubMed] [Google Scholar]
- Sevrioukov E. A., He J-P., Moghrabi N., Sunio A., Krämer H. (1999). A role for the deep orange and carnation eye color genes in lysosomal delivery in Drosophila. Mol. Cell 4, 479–486 10.1016/S1097-2765(00)80199-9 [DOI] [PubMed] [Google Scholar]
- Shpilka T., Weidberg H., Pietrokovski S., Elazar Z. (2011). Atg8: an autophagy-related ubiquitin-like protein family. Genome Biol. 12, 226 10.1186/gb-2011-12-7-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speicher S. A., Thomas U., Hinz U., Knust E. (1994). The Serrate locus of Drosophila and its role in morphogenesis of the wing imaginal discs: control of cell proliferation. Development 120, 535–544 [DOI] [PubMed] [Google Scholar]
- Stenmark H., Parton R. G., Steele-Mortimer O., Lütcke A., Gruenberg J., Zerial M. (1994). Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 13, 1287–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Nakamura A. (2008). The endocytic pathway acts downstream of Oskar in Drosophila germ plasm assembly. Development 135, 1107–1117 10.1242/dev.017293 [DOI] [PubMed] [Google Scholar]
- Tanimoto H., Itoh S., ten Dijke P., Tabata T. (2000). Hedgehog creates a gradient of DPP activity in Drosophila wing imaginal discs. Mol. Cell 5, 59–71 10.1016/S1097-2765(00)80403-7 [DOI] [PubMed] [Google Scholar]
- Thompson B. J., Mathieu J., Sung H. H., Loeser E., Rørth P., Cohen S. M. (2005). Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev. Cell 9, 711–720 10.1016/j.devcel.2005.09.020 [DOI] [PubMed] [Google Scholar]
- Vaccari T., Bilder D. (2005). The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev. Cell 9, 687–698 10.1016/j.devcel.2005.09.019 [DOI] [PubMed] [Google Scholar]
- Vieira O. V., Bucci C., Harrison R. E., Trimble W. S., Lanzetti L., Gruenberg J., Schreiber A. D., Stahl P. D., Grinstein S. (2003). Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol. Cell. Biol. 23, 2501–2514 10.1128/MCB.23.7.2501-2514.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. W., Stromhaug P. E., Shima J., Klionsky D. J. (2002). The Ccz1-Mon1 protein complex is required for the late step of multiple vacuole delivery pathways. J. Biol. Chem. 277, 47917–47927 10.1074/jbc.M208191200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig T., Wilsch-Bräuninger M., González-Gaitán M. (2003). Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Biol. 161, 609–624 10.1083/jcb.200211087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Rubin G. M. (1993). Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.